Abstract
While motivated behavior involves multiple neurochemical systems, few studies have focused on the role of glutamate, the brain’s excitatory neurotransmitter, and glucose, the energetic substrate of neural activity in reward-related neural processes. Here, we used high-speed amperometry with enzyme-based substrate-sensitive and control, enzyme-free biosensors to examine second-scale fluctuations in the extracellular levels of these substances in the nucleus accumbens shell during glucose-drinking behavior in trained rats. Glutamate rose rapidly after the presentation of a glucose-containing cup and before the initiation of drinking (reward seeking), decreased more slowly to levels below baseline during consumption (sensory reward), and returned to baseline when the ingested glucose reached the brain (metabolic reward). When water was substituted for glucose, glutamate rapidly increased with cup presentation and in contrast to glucose drinking, increased above baseline after rats tasted the water and refused to drink further. Therefore, extracellular glutamate show distinct changes associated with key events of motivated drinking behavior and opposite dynamics during sensory and metabolic components of reward. In contrast to glutamate, glucose increased at each stimulus and behavioral event, showing a sustained elevation during the entire behavior and a robust post-ingestion rise that correlated with the gradual return of glutamate levels to their baseline. By comparing active drinking with passive intra-gastric glucose delivery, we revealed that fluctuations in extracellular glucose are highly dynamic, reflecting a balance between rapid delivery due to neural activity, intense metabolism, and the influence of ingested glucose reaching the brain.
Keywords: glutamate, glucose, electrochemistry, motivated behavior, sensory reward, metabolic reward, brain metabolism
INTRODUCTION
Although multiple neurochemical systems are involved in the organization and regulation of motivated behavior, most work has focused on dopamine (DA) as a critical substrate of reward. While initial evidence was obtained in behavioral experiments (Wise and Bozarth 1987; Le Moal and Simon 1991; Koob 1996; Salamone and Correa 2012), the development of high-speed voltammetry made it possible to evaluate dynamic changes in DA levels during different behaviors, thus clarifying the role of DA in motivational and reward-related neural processes (Phillips et al. 2003; Heien and Wightman 2006; Cacciapaglia et al. 2011). Yet glutamate is the major excitatory neurotransmitter in the CNS and its importance for the development and performance of motivated behavior is supported by neuropharmacological (Robbins and Everitt 1996; Kelley et al. 2005; Berridge et al. 2010) and electrophysiological evidence (Calabresi et al. 1997; Moult 2009; Britt et al. 2012). However, much less is known about physiological fluctuations in glutamate release and extracellular glutamate levels, their behavioral correlates, and their possible role in natural motivated behavior.
This is partially due to technical limitations: while glutamate is a rapid neurotransmitter, microdialysis is limited by its low temporal resolution (1-20 min) and classic electrochemistry based on oxidation/reduction is not possible for this non-electroactive substance. Although enzyme-based glutamate sensors provide second-scale resolution, their use in behavioral experiments has been limited and data remain controversial (Rutherford et al. 2007; John et al. 2008; Wassum et al. 2012). Using high-speed amperometry with enzyme-based glutamate microsensors and resolving significant methodological challenges associated with their use in freely moving rats, we recently characterized rapid fluctuations in nucleus accumbens (NAc) extracellular glutamate elicited by natural arousing stimuli (Wakabayashi and Kiyatkin 2012; Kiyatkin et al. 2013). Here, we employed this approach to examine second-scale fluctuations in extracellular glutamate in the NAc, a critical structure involved in sensorimotor integration and reinforcement (Mogenson et al. 1980; Wise 2002), during motivated glucose-drinking behavior in trained rats.
While high-speed glutamate measurements could provide new important information on the regulation of impulse activity of NAc neurons, we also assessed fluctuations in extracellular glucose, the primary energetic source for brain metabolism. In contrast to glutamate, which is synthesized in the brain and released by neural and glial cells, glucose enters neural tissue from arterial blood via rapid gradient-dependent facilitated diffusion via GLUT-1 transporters (Fellows and Boutelle 1993; Silver and Ericinske 1994; de Vries et al. 2003) and is continuously consumed by brain cells. By using glucose biosensors, we showed that fluctuations in NAc extracellular glucose in freely moving rats are highly dynamic, exhibiting rapid increases after natural arousing stimuli (Kiyatkin and Lenoir 2012) that also excite accumbal cells due to phasic glutamate release (Kiyatkin and Rebec 1999). These findings and consistent increases in glucose levels induced by intra-NAc glutamate microinjections (Kiyatkin and Lenoir 2012) suggest that glucose rapidly enters the extracellular space due to local neuronal activation. Hence, direct monitoring of NAc extracellular glucose allowed us to examine how this metabolic substrate for neural activity enters brain tissue during motivated behavior. Additionally, we were able to discern when ingested glucose reached the brain, thus providing a behavior-independent measure of metabolic reward. Finally, by measuring glutamate and glucose together, we examined the relationship between these two parameters reflecting different aspects of neural activity.
Therefore, we examined the pattern of dynamic fluctuations in NAc extracellular glutamate and glucose associated with key events of a glucose-drinking behavior in trained rats to evaluate their functional significance in motivated behavior and their relation to reward. To clarify the specificity of these fluctuations and their underlying mechanisms, we also conducted additional control tests: water substitution for glucose, exposure to sensory stimuli with other or no behavioral consequences, and passive intra-gastric glucose delivery.
METHODS
Due to size limits, this section highlights key methods used in this study. An in-depth version together with illustrative materials is presented in the Supplemental Appendix S1.
Subjects
Data from 23 male Long-Evans rats (Charles River Laboratories, Raleigh, NC, USA) weighing 440±40 g at the time of surgery were used in this study. Rats were housed individually in a climate-controlled vivarium maintained on a 12-12 hour light-dark cycle (lights on at 8:00AM), with food and water available ad libitum. All experiments complied with the “Guide for the Care and Use of Laboratory Animals” (8th edition, 2011, US National Research Council) and experimental protocols were approved by the Intramural Research Program (NIDA) Animal Care and Use Committee and the UK ARRIVE guidelines.
Motivated drinking behavior
In this study, we used a naturalistic motivated glucose-drinking behavior that required minimal training and was relatively uniform and stable in all rats. Rats were pre-trained with 5mL of 10% glucose solution presented in a plastic cup for 2 hours twice per 6-hr session for 4 days. All training and subsequent recordings were performed inside a Plexiglas chamber. A camera permitted in-cage viewing and video recording of animal behavior, which was used to synchronize behavior with electrochemical measurements.
Surgeries
Surgical procedures for electrochemical experiments have been described in detail elsewhere (Wakabayashi and Kiyatkin 2012; see also Appendix S1). Briefly, under general anesthesia, each rat was unilaterally implanted with a BASi guide cannula (Bioanalytical Systems, West Lafayette, IN, USA) for later insertion of the electrochemical sensor in the right NAc shell, a critical brain structure involved in sensorimotor integration, behavioral regulation and reward (Mogenson et al. 1980; Wise and Bozarth 1987; Salamone and Correa 2012; Di Chiara 2002). Target coordinates were: AP +1.2 mm, ML ±0.8 mm, and DV +7.6 mm from the skull surface, according to coordinates of Paxinos and Watson (1998). The cannula was secured with dental acrylic in a head mount anchored to the skull. After a minimum of 4 days recovery, rats underwent an additional 6-hr drinking session prior to the electrochemical recording on the next day.
Three rats were also equipped with a chronic intragastric catheter during the same surgery. The catheter was implanted into the forestomach (the upper area with minimal vascularization) and tightly fixed to the stomach wall. After closure of the abdominal muscular wall, the catheter was fed subcutaneously to an injection port on the head mount. During recovery, the catheter was flushed daily with water to maintain patency.
Electrochemical sensors
Commercially produced glutamate oxidase-based glutamate, glucose oxidase-based glucose, and enzyme-free Null sensors (Pinnacle Technology, Lawrence, KS, USA) were used in this study. Each sensor is prepared from platinum-iridium wire (180μm diameter), with a ~1mm sensing cavity at the tip and a sensing area of ~0.56 mm2. The active electrode is incorporated with an integrated Ag/AgCl reference electrode. On the active surface of glutamate sensors, glutamate oxidase converts glutamate to α-ketoglutarate and hydrogen peroxide (H2O2), which is detected as an amperometric oxidation current generated by a +0.6 V applied potential (Hu et al. 1994). Similarly, glucose oxidase on the sensor’s active surface converts glucose to glucono-1,5-lactone and H2O2 on the active surface of glucose sensors, which is likewise detected as an oxidation current at +0.6 V (Hu and Wilson 1997). In both glutamate and glucose sensors, the contribution of ascorbate to the measured current is competitively reduced by co-localizing ascorbic acid oxidase on the active surface of the sensor to convert electroactive ascorbate to non-electroactive dehydroascorbate and water. A negatively charged Nafion polymer layer under the enzyme layer further helps to exclude endogenous anionic compounds. The currents from all sensors were passed to a computer via a potentiostat (Model 3104, Pinnacle Technology), and all electrochemical data were sampled at 1 Hz using the PAL software utility (Version 1.5.0, Pinnacle Technology).
As shown in our previous studies (Wakabayashi and Kiyatkin 2012, 2014; Kiyatkin and Lenoir 2012; Kiyatkin et al., 2013), electrochemical currents detected in vivo by enzyme-based glutamate and, to a lesser degree, by glucose sensors used in this study are affected by a) other cationic and anionic electroactive species that are present in the extracellular space and oxidized by the same applied potentials; b) naturally occurring fluctuations in brain temperature; and (c) a consistent downward drift in basal currents occurring during long-term recording. To reduce these non-specific influences, we used Null sensors, which are similarly constructed but lack an active enzyme and, when used in the same type of in vivo experiment, are exposed to the same physical and chemical environment as substrate-sensitive sensors. As shown previously and confirmed in this study, Null sensors are fully insensitive to either glutamate or glucose, but have similar temperature sensitivity, a similar downward current drift during long-term in vitro recordings, and a comparable sensitivity to ascorbate and DA, two possible chemical interferents to the electrochemical currents generated by glutamate and glucose sensors in vivo. Therefore, the difference in currents detected by the substrate-sensitive and Null sensors provides a useful method of revealing actual changes in glutamate and glucose levels with these sensors. Electrochemical recordings employing different sensors were performed in separate rats to completely eliminate the possibility of electrical crosstalk between sensors during in vivo recording (Kiyatkin et al. 2013).
Immediately before and after each in vivo experiment, all sensors were calibrated in vitro in PBS solution (pH 7.3, t°=22-23°C) to determine their substrate sensitivity and selectivity against ascorbate. In selected electrodes of each type we also determined their sensitivity to DA and temperature dependence (see Appendix S1 for additional technical details). Since the current response to glutamate and glucose directly depends upon temperature and this dependence is very stable across multiple in vitro tests for different substrate-sensitive and Null sensors (Kiyatkin et al. 2013), all sensitivity values were corrected for 37°C (+84% for glutamate and +96% for glucose).
The glutamate sensors used in this study varied slightly in their glutamate sensitivity (mean: 0.54±0.11 nA/μM), producing incremental, highly linear (r=0.99) increases in current with increases in glutamate concentration ([glutamate]) during pre-recording calibrations (see Fig. S1a,c). The in vitro detection limit of these sensors was 0.02±0.002 nA, thus allowing us to detect ~38 nM glutamate after a single test, although the precision of glutamate detection increases (as √n) with the averaging of repeated tests. The response time measured in vitro following rapid glutamate delivery was ~1-3 s. Glutamate sensors used in this study also showed current changes with addition of ascorbate (mean 0.07±0.02 nA/25 μM; mean selectivity ratio 1:104) and DA (0.24±0.01 nA/1 μM). While the latter value is comparable to that of glutamate, current changes within the range of basal DA levels and its physiological increases (~5-25 nM and ~30-70 nM, respectively) are within the background noise (~12 and 24 pA for 50 and 100 nM DA change, respectively). The temperature sensitivity of glutamate sensors determined in vitro was 0.14±0.04 nA/1.0°C. Post-recording calibrations of glutamate sensors revealed an approximately two-fold decrease in their sensitivity to both glutamate (0.13±0.03 nA/1 μM) and ascorbate (0.034±0.009 nA/25 μM), with virtually unchanged glutamate-ascorbate selectivity ratio (1:94). This decrease in sensitivity is consistent with other studies using sensors of similar design (Behrend et al. 2009, Naylor et al. 2011) and may be due in part to fouling of the active surface (Kulagina et al. 1999) or disruption of the enzyme layer during sensor removal from the brain.
The glucose sensors used in this study also showed incremental, highly linear current increases with increases in glucose concentration, with an averaged sensitivity 11.98 nA/1 mM (Fig. S1b-d). Similar to glutamate sensors, glucose sensors showed very small changes in current with addition of ascorbate (mean 0.03±0.01 nA/25 μM), lower sensitivity to DA (~0.1 nA/1 μM) but they were equally temperature-sensitive (0.13±0.03 nA/1.0°C). The mean post-recording calibration curve for glucose sensors was almost identical to the pre-recording curve, and sensitivity and selectivity remained virtually unchanged after 7-8 hours of in vivo recording (see Fig. S1).
As expected, Null sensors were fully insensitive to both glutamate and glucose (Fig. S1c-d) and they showed comparable but slightly weaker current responses to both ascorbate (0.02 nA/25 μM) and DA (0.03-0.15 nA/1 μM) than substrate-sensitive probes. As shown previously, Null sensors were equally temperature-sensitive (0.19±0.02 nA/1.0°C) as both glutamate and glucose sensors and they showed a similar downward trend in baseline currents following long-term in vitro and in vivo recordings.
Experimental protocol
On the day of electrochemical recording, rats were minimally anesthetized with isoflurane and a calibrated sensor (glutamate, Null, or glucose) was inserted into the NAc through the guide cannula. The rat was then placed in the testing chamber and the sensor was connected to the potentiostat via an electrically shielded cable and a multi-channel electrical swivel. Testing began a minimum of 150 min after sensor insertion to allow the baseline current to stabilize. First, to verify sensor functionality and the specificity of drinking-induced glucose responses, rats were exposed to two sensory stimuli that were not related to our behavioral task: a brief auditory stimulus (75 dB, 0.25 s) that induces rapid, transient EEG desynchronization and EMG activation, with no evident behavioral changes (Kiyatkin and Smirnov 2010) and a 3-min tail-touch, a mild arousing stimulus that induces behavioral activation and phasic release of NAc glutamate and glucose (Wakabayashi and Kiyatkin 2012, Kiyatkin and Lenoir 2012). These control tests were important for evaluating the contribution of sensory input and arousal to changes in [glucose] during drinking behavior. One hour after sensory controls, rats were presented with a cup containing 5 mL of 10% glucose solution (warmed to 37°C), and the elapsed time from cup presentation to the beginning and end of drinking (after the entire 5-mL volume was fully consumed) was confirmed visually by two observers and by video recording. Removal of the empty cup from the cage occurred a minimum of 40 min after its presentation and the second glucose cup was presented a minimum of 30 min after cup removal. After two presentations of 10% glucose, an identical volume of water (also warmed to 37°C) was presented as a comparison condition. During this test, although rats approached the cup normally and tasted water, they quickly refused to consume more.
To distinguish the component of the glucose response related to behavior and reveal the contribution of behavior-associated metabolic use of glucose, in three rats equipped with chronic intra-gastric catheters, we examined changes in NAc glucose currents during stress- and cue-free intra-gastric delivery of glucose at the same volume, concentration and rate (5 mL of 10% solution, warmed to 37°C and delivered over ~200 s) as consumed in behavioral experiments. Each rat typically received one water and two glucose injections at 120-min inter-injection intervals.
At the end of the recording, rats were removed from the cage, lightly anesthetized with isoflurane (<2 min), and the biosensor was removed for post-recording calibration. Then rats were deeply anesthetized with and transcardially perfused with PBS followed by 10% formalin. Sensor placements were verified on 45 μm brain slices using the stereotaxic atlas of Paxinos and Watson (1998). Results of sensor placement verification for glutamate, glucose, and Null sensors are shown in Fig. S1e.
Data analysis
Basal levels of extracellular glutamate and glucose in the NAc were determined by taking the difference in mean absolute currents detected by substrate-sensitive and Null sensors before a cup presentation (quiet resting conditions), and then calculating the concentration based on the sensor’s substrate sensitivity corrected for 37°C.
Since equally trained rats showed variable latencies to initiate drinking after cup presentation and exhibited different durations of drinking, we analyzed relative changes in electrochemical currents preceding and following key behavioral events (peri-event analysis). For the glucose-drinking test, these events were: glucose cup presentation, initiation and end of drinking, and empty cup removal. Since rats did not drink significant amounts of water, when it was substituted for the glucose, only two behavioral events (water cup presentation and initiation of drinking) were analyzed and compared with the analogous events during the glucose-drinking test. For these analyses, we determined relative changes in current (vs. baseline=0 nA) detected by substrate-sensitive and Null sensors and, based on their difference, determined relative changes in [glutamate] and [glucose] preceding and following the events of interest. Different quantification bins (2-60 s, indicated in figure legends) and different sample durations were used for individual data analyses to accurately represent and properly statistically evaluate both rapid and slow changes in electrochemical currents and analyte concentrations.
Two-way repeated-measures (RM) ANOVAs were initially used for evaluating differences in the substrate-sensitive and Null sensor currents. Since currents were analyzed as a change from 0 nA baseline, the length of the effect was determined as the duration when either the substrate-specific currents were different from Null (main effect) or when the substrate-specific currents were changing with respect to the Null current (Current × Time interaction). We then evaluated the pattern and magnitude of changes in [glutamate] and [glucose] associated with these events by subtracting substrate-specific currents from the mean Null sensor current, and transforming this value into concentrations. These data were further analyzed using one-way (RM) ANOVA. If a significant effect of time was detected, individual data points were compared against the peri-event baseline point using Fisher post-hoc test to detect the latency and duration of this change.
After conducting these individual peri-event analyses and determining mean basal values of [glutamate] and [glucose] immediately preceding each behavioral event, we evaluated the pattern of fluctuations in these neurochemical parameters during the entire glucose-drinking behavior taking into account their slow changes and essential variability of natural animal behavior. Linear regression was then used to assess the relationship between [glutamate] and [glucose] during the entire behavior. For text clarity, most in-depth statistical results are shown in figure captions.
To evaluate the possible influence of ingested glucose on the extracellular glucose levels detected in the NAc, changes during a glucose-drinking session were compared to those occurring after passive intra-gastric delivery of glucose at the same volume. These data were also compared to those obtained with control intra-gastric water injections at the same volumes.
RESULTS
All rats included in this data sample (n=18; 37 glucose-drinking tests during 18 recording sessions), showed consistent drinking behavior following each of the two glucose presentations per recording session. While the latencies to start drinking varied in each of two tests (mean 175±40 s; median 65 s), the duration of drinking (time until the entire cup volume was consumed) was less variable: between 113 and 296 s (mean 192±10 s) for the first drink and between 117 s and 273 s (mean 193±9 s) for the second drink. These differences were not significant (t18=1.05, p=0.17), thus allowing us to combine all glucose-drinking tests into one group (start of drinking: mean 175±40 s, median 65 s; duration of drinking: mean 224±24 s, median 185 s).
Basal levels of glutamate and glucose
The mean basal currents (quiet rest prior to the test) significantly differed for each type of sensor; these values were highest for glucose, lower for glutamate, and minimal for Null sensors. By subtracting currents detected by Null sensors from those of the active sensors and transforming the current difference into concentrations based on calibration values, the mean basal levels of glutamate and glucose in the NAc shell were 1.16±0.18 μM (n=14) and 663.67±170.45 μM (n=12), respectively. These concentrations are similar to those obtained with no-net microdialysis (Hershey and Kennedy 2013, McNay and Gold 2002, Dunn-Meynell et al. 2009), suggesting the validity of this subtraction approach.
Rapid and slow fluctuations in NAc [glutamate] and [glucose] associated with key events of glucose-drinking behavior
In order to reveal the patterns of glutamate and glucose fluctuations associated with specific behavioral events as they occurred with different latencies and durations within each drinking test, we next examined changes in currents and concentrations associated with glucose-filled cup presentation, initiation and end of drinking, and removal of the empty cup 40-60 min after the rat finished drinking. These peri-event analyses represent relative changes in currents and concentrations from the “basal” values immediately preceding the events of interest.
Presentation of glucose-containing cup and approach behavior
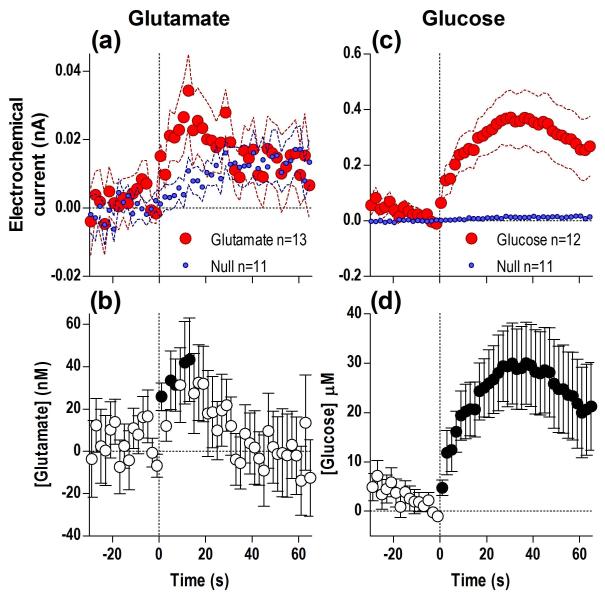
When trained rats were presented with a glucose-containing cup, there was a significant difference in NAc glutamate (Fig. 1a) and glucose currents (c) for the duration of the analysis window (65 s) when compared to currents detected by the Null sensors (detailed statistical results are presented in figure legends). Based on these differences, there was a significant increase in [glutamate] (b) and [glucose] (d) from the quiet rest baseline preceding this stimulus. The glutamate increase began with ~ 5-s latency and peaked (~43 nM, or ~4% above baseline) at ~13 s after cup presentation, slowly decreasing afterward when rats began to drink (b). Similar to glutamate, glucose levels began to increase immediately after cup presentation (within the first 2-s bin), peaked at ~30 s (~30 μM, or ~5% above baseline), and remained elevated after most rats had already begun drinking (d). Compared to the glutamate response, this change was greater, more rapid, and more prolonged.
Fig. 1. Presentation of a glucose-containing cup results in rapid increases in NAc glutamate (a, b) and glucose (c, d).
Top graphs (a, c) show changes in active (red) and null (blue) electrochemical currents and bottom graphs (b, d) show changes in [glutamate] and [glucose], all at 2-s bins. In each case, a significant difference in substrate-sensitive versus Null currents (glutamate: Current × Time interaction F33,726=4.49; glucose: main effect F1,21=11.87, Current × Time interaction F33,693=3.03, all p<0.05) resulted in significant changes in concentrations vs. baseline (glutamate: F12,441=2.24, glucose: F11,407=3.39, both p<0.05). n defines number of tests in each group and filled symbols in b and d show values significantly different from the initial, quiet-rest baseline (Fisher LSD post-hoc test; p<0.05).
Drinking
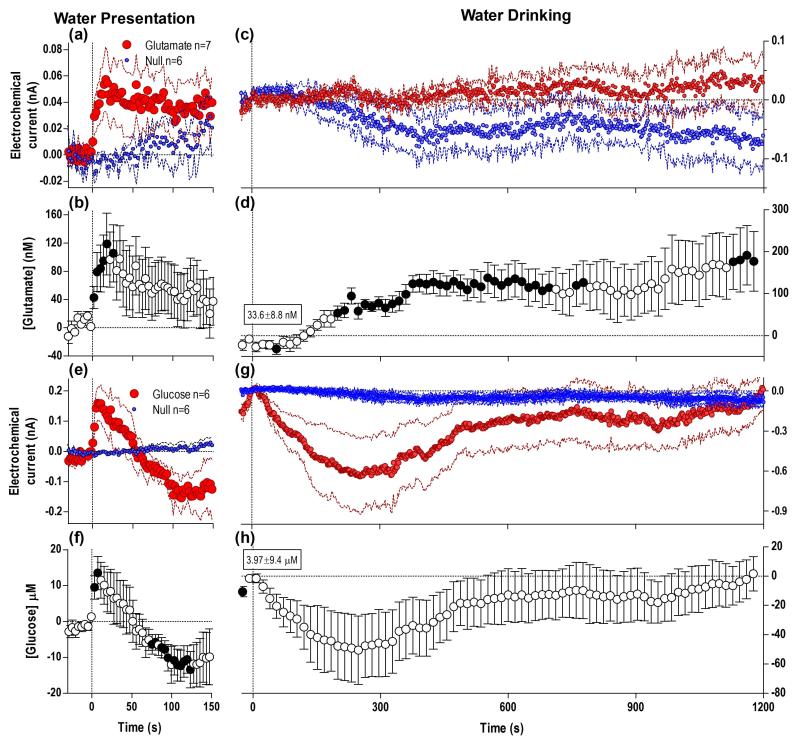
When rats initiated drinking, glutamate levels were higher than the initial pre-test baseline (6.85±10.21 nM) but this change did not reach statistical significance. After drinking began, glutamate currents dynamically fell for ~113 s with respect to Null currents (Fig. 2a). Glutamate levels evaluated based on differences between glutamate and Null currents from the pre-drinking baseline slightly jumped at the start of drinking but this increase was not significant (b). Soon after the initiation of drinking, [glutamate] began to decrease steadily, reaching significance at ~70 s from the start of drinking. At the moment when rats completed drinking, mean [glutamate] decreased 77.94±26.29 nM below the initial baseline (F1,23=8.79, p<0.02) and continued to decrease rapidly upon the cessation of drinking (c-d), reaching a nadir (~50 nM) at ~150 s after the end of drinking and slowly returning to baseline within ~330 s after the end of drinking. Interestingly, this post-consumption drop in [glutamate] (d) was nominally hidden in the active glutamate current because of a concomitant slow rise in Null currents during this period (c). Due to this overall glutamate decrease that began during drinking and continued for some time after its completion, glutamate levels at its final nadir were significantly lower vs. the initial, pre-test baseline (~120 nM or −10%).
Fig. 2. Glucose drinking results in robust but differential changes in NAc glutamate and glucose.
Top graphs show changes in electrochemical currents detected by glutamate (a, c) and glucose (e, g) sensors analyzed at 2-s bins with respect to the start (a, e) and end of drinking (c, g). Each graph also show changes detected by Null sensors under identical conditions (blue). Bottom graphs show changes in [glutamate] (b, d) and [glucose] (f, h) at the start (4-s bins) and end (10-s bins) of drinking. Inset in (h) shows a rapid, but non-significant decrease in [glucose] immediately after the end of drinking (2-s bins). n defines number of tests in each group. While glutamate currents fell with respect to Null currents during drinking (Current × Time interaction F71,1562=2.60, p<0.05), relative glutamate currents remained significantly lower than Null currents after drinking behavior (Current × Time interaction F90,1710=2.53, p<0.05). Both behavioral events resulted in a significant decrease in [glutamate] during (F12,623=4.68, p<0.05) and for 300-400 s post-drinking (F9,909=6.48, p<0.05). Glucose currents significantly differed from Null currents during the same events (start of drinking, main effect F1,21=7.14, Current × Time interaction F71,1491=2.41; end of drinking, main effect F1,21=5.97, Current × Time interaction F90,1890=8.48; all p<0.05), resulting in significant increases in [glucose] at the start of drinking for 73.5 s (F11,575=2.66, p<0.05), that slightly decreased after its end, and began to increase robustly again from ~150 s after the end of glucose consumption (F11,1091=9.31, p<0.05). n defines number of tests in each group, change in concentration from pre-test baseline shown in boxes, and filled symbols in b, d, f, h show values significantly different from the initial, quiet-rest baseline (p<0.05).
Baseline glucose levels when the rats began drinking were significantly higher than the initial pre-test baseline (13.3±3.36 μM; F1,19=15.67, p<0.05 vs. baseline) and, in contrast to glutamate, they showed a rapid and significant rise after drinking began (Fig. 2e). In terms of concentrations, this phasic increase was relatively small (~20 μM) but was maintained during glucose consumption (f), with another much weaker, non-significant peak at the end of drinking (g-h and inset). While [glucose] at the end of drinking was significantly greater compared to pre-test baseline (31.78±11.20 μM, F1,19=8.08, p<0.05), and was higher than at the start of drinking (13.31±3.36 μM), the increase between the start and end of drinking only approached significance (p=0.08) due to between-subject variability. After the rats finished drinking, glucose levels slightly dropped for ~100 s (at this lowest point, they were virtually identical to pre-test baseline), but began to steadily increase from ~120 s after the end of drinking (or ~5 min after the start of drinking). This late increase in glucose was much larger in magnitude and longer-lasting (>10 min) when compared to behavior-related fluctuations, and was the result of an increase in the glucose current over the Null (h).
Removal of empty cup
When the empty cup was removed from the cage, changes in glutamate and Null currents were minimal and identical, and the [glutamate] remained unchanged (Fig. 3a-b). However, the same event evoked a dynamic change in glucose versus Null currents (c), which resulted in a rapid (~5-s latency), significant but relatively small and transient (~15 μM for ~25 s) increase in [glucose] (d). While the rise in glucose during cup presentation and removal was equally rapid, glucose levels did not show a sustained elevation after cup removal, but rather a downward rebound pattern more typical of that induced by simple sensory stimuli (see below). While glucose cup presentation and empty cup removal are associated with similar environmental changes, both glutamate and glucose responses were significantly less when the empty cup was removed (see Fig. S2a-b), suggesting that the motivational value of the stimulus may have modulated both neurochemical responses.
Fig. 3. Removal of an empty cup does not affect NAc glutamate levels but still induces a rapid rise in glucose.
Top graphs show mean (±SEM) changes in currents (2-s bins) detected by glutamate, glucose and Null sensors (a, c) and bottom graphs show changes in [glutamate] (b) and [glucose] (d). While the changes in the glutamate versus Null currents and therefore [glutamate] were not significant (F11,1103=1.26; n.s.), glucose currents significantly differed from Null currents (Current × Time interaction F91,1911= 4.82; p<0.05) resulting in significant changes in [glucose] (F11,1103=5.32; p<0.05). Note that glucose rise was rapid, unimodal, transient, and followed by a robust decrease in its concentration below baseline. n defines number of tests in each group and filled symbols in b and d show values significantly different from the initial, quiet-rest baseline (p<0.05).
Fluctuations in NAc [glutamate] and [glucose] during substitution of water for glucose
When rats were presented with an identical cup containing water, glutamate currents rose with respect to Null currents (Fig. 4a) resulting in a rise in [glutamate] (b) that initially resembled that of a glucose-containing cup (see Fig. 2a). Glucose currents also rapidly changed (e), indicating that [glucose] also rapidly increased within the first 4-s bin (e-f) but peaked quickly (~8 s) and dropped below baseline thereafter, resembling the glucose response to empty cup removal and other simple sensory stimuli (see below), perhaps reflecting the difference in behavioral response to a glucose versus water-containing cup. All rats approached the water-containing cup (median latency 51 s) and tasted the water, but the duration of water drinking was much shorter than that of glucose (mean 24.2 s, median 19 s vs. 224±24 s) and no rat consumed significant amounts of water (duration of drinking is shown as lines in c). Glutamate levels (d) changed biphasically during this extended period after water tasting, first showing a very slight but significant decrease (60-130 s) at the time when rats tasted the water, followed by a more pronounced, sustained and significant increase, reaching ~200 nM (or ~17% above baseline) by the end of the 20-min analysis window. Interestingly, this glutamate concentration change was the result of dynamic changes in both glutamate and Null currents (c) where the glutamate current weakly increased concurrently with a more pronounced decrease in Null current.
Fig. 4. Substitution with water to rats trained to drink glucose results in refusal of drinking and different dynamics of NAc glutamate and glucose.
Graphs in the left column show mean (±SEM) changes in currents detected by glutamate, Null and glucose sensors (a, e; 2-s bins) and mean (±SEM) changes in [glutamate] and [glucose] (b, f; 4-s bins) after presentation of the water-filled cup. Graphs in right columns show mean (±SEM) changes in currents detected by glutamate, Null and glucose sensors (c, g; 2-s bins) and mean (±SEM) changes in glucose and glucose concentrations (d, h; 16-s bins) after the start of drinking. Water presentation resulted in a difference between glutamate and Null currents (Current × Time interaction F38,418=1.87 p<0.05), indicating a rapid and significant rise in [glutamate] (b, F6,272=4.10, p<0.05). Glucose currents also rapidly changed with water presentation (Current × Time interaction, F38,380=3.27 p<0.05) showing a rapid, significant, and unimodal rise in [glucose] (f, F5,233=2.83, p<0.05). After the rats tasted the water, glutamate currents dynamically changed (Current × Time interaction F76,836=1.96 p<0.05) indicating [glutamate] levels began to increase steadily (d, F6,531=3.12 p<0.05), reaching levels <200 μM 20 min after this event. Concurrently, the glucose current changed versus the Null (Current × Time interaction F76,760=2.11 p<0.05), signifying a significant [glucose] change (h, F5,455=2.18, p<0.05), initially showing a greater and more persistent decrease when compared to glucose drinking, although due to variability no individual bin was significantly different from baseline. Water drinking also lacked the robust tonic increase seen after glucose consumption. n defines number of tests in each group and filled symbols in b and d show values significantly different from the initial, quiet-rest baseline (p<0.05).
In contrast to [glutamate], [glucose] phasically increased during cup approach behavior just prior to the start of water drinking (Fig. 4e-f), but then showed a sustained decrease after tasting the water, reaching a nadir at ~280 s before slowly returning to baseline (g-h). In this case, the concentration change resulted from the robust decrease in glucose currents with respect to changes in the Null currents (h).
Although glutamate and glucose responses to the presentation of the water- and glucose-containing cups were initially similar (see Fig. S2 a,b), the changes in both parameters later diverged one from another due to differences in behaviors elicited by these stimuli (Fig. S2c,d).
The pattern of rapid and slow fluctuations in NAc [glutamate] and [glucose] during the entire glucose-drinking behavior
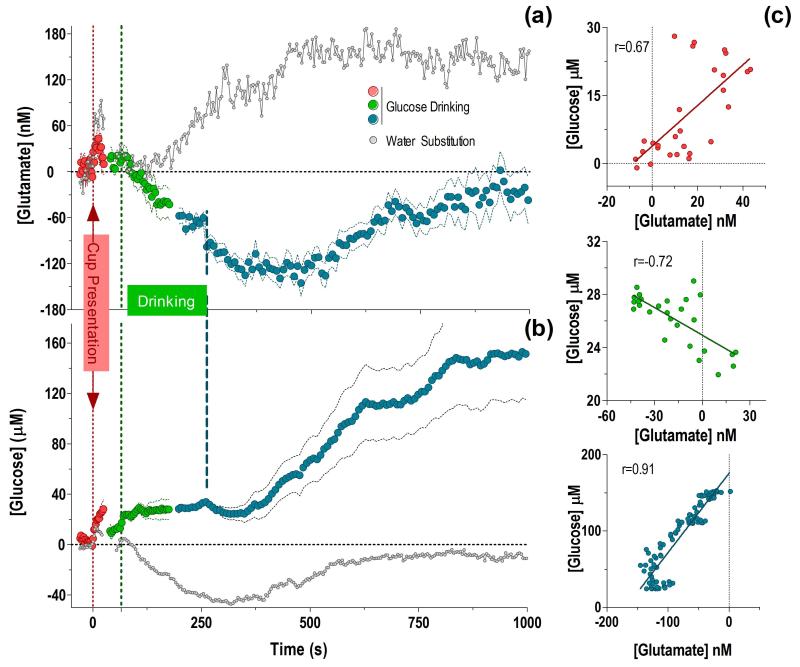
After analyzing rapid changes in [glutamate] and [glucose] relative to each individual behavioral event, we combined these data taking into account the tonic changes in concentrations compared to the overall pre-test baseline, and also considering the inherent variability of natural behavior (Fig. 5).
Fig. 5. Dynamic fluctuations in NAc [glutamate] (a) and [glucose] (b) during motivated glucose-drinking behavior relative to the overall pre-test baseline.
Data are shown in term of concentrations with respect to the initial pre-test baseline (=0), and are combined in to one common time-scale. The vertical dashed lines show the moment of cup presentation (0 s), median latency of the start of drinking (65 s), and median end of drinking (250 s), respectively. To better account for variability in behavioral responses, concentration changes following cup presentation (2-s bins, red) are shown for +25 s, an interval where 75% of rats had not yet started drinking. Likewise, changes preceding drinking (4-s bins, green) are shown for the same length of time (−25 s). Changes during drinking are shown for +113 s, the minimum length of time all rats spent drinking. Changes associated with the end of drinking (8-s bins) are shown in blue for −56 s preceding this event (a time interval when all rats were engaged in drinking), and for +945 s following this event. Changes in both neurochemical measures during water substitution for similar events and time intervals are shown in gray for comparison. [Glutamate] and [glucose] positively correlated for cup presentation, inversely correlated during drinking, and positively correlated in the post-ingestion period (c).
Viewed from this perspective, both [glutamate] and [glucose] rapidly rose when the cup was introduced in the cage and the rat was engaged in seeking behavior. After peaking, the concentrations of both substances decreased but their absolute values at the moment when the rat began drinking remained larger than the initial baseline. These initial changes in both substances correlated (c; r= 0.67), suggesting a common underlying mechanism. While [glucose] maintained a sustained increase during drinking, [glutamate] consistently decreased, falling well below the initial baseline at its end, and continuing to decrease further for ~180 s after drinking was completed. Thus, during drinking [glutamate] and [glucose] inversely correlated (r=−0.72). After reaching a nadir at 420 s (~130 nM), [glutamate] began to return slowly to its baseline levels. In contrast, [glucose] slightly decreased after the end of drinking but began to increase slowly but robustly from ~420 s. Importantly, when seen together on the same time-scale, this post-drinking return of [glutamate] to its basal values occurred over the same time period as the large rise in NAc [glucose]. These changes highly correlated (r=0.91).
Taken together, the pattern of neurochemical fluctuations during glucose-drinking behavior differed profoundly from those occurring with water substitution (a). While the [glutamate] response to the liquid-filled cup was similar in both experimental conditions, once the rat tasted the water and refused to drink more, the [glutamate] response diverged markedly from that seen with a glucose-filled cup and began to increase steadily, remaining elevated well passed the duration of the analysis window. During the water cup presentation, [glucose] also initially showed similar phasic increases to the cup presentation regardless of its contents, but once the rat tasted the water, [glucose] fell below the initial baseline and slowly returned to basal levels ~600 seconds later (b). These differences possibly reflect the behavioral and physiological consequences of reward omission.
NAc glucose dynamics after passive intra-gastric glucose delivery
In addition to active, neural activity-driven entry of glucose into the extracellular space, its levels could also be affected by changes in glucose levels in the peripheral blood (Kiyatkin and Lenoir, 2012). Since consumption of glucose should result in its increase in the blood, this mechanism could be responsible for the large elevation in NAc glucose occurring minutes after glucose drinking (see Figs. 2h, 5b). To substantiate this mechanism and distinguish the behavior-associated component of the glucose response, we examined how NAc [glucose] would be affected by its passive infusion into the stomach via an intra-gastric catheter.
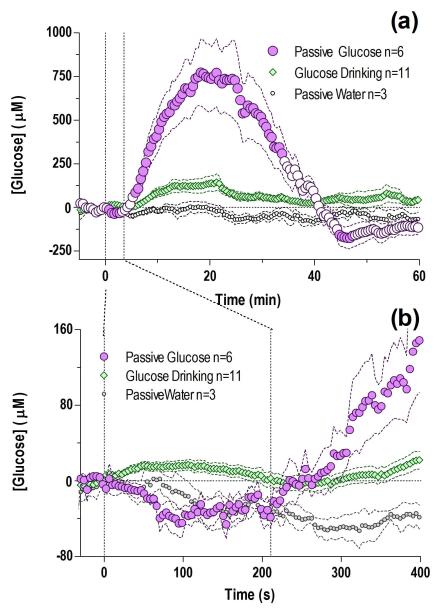
When the same amount and volume of glucose consumed during the drinking test (10%, 5 ml) was passively infused at the same rate directly into the stomach, NAc glucose levels rose markedly to a maximal concentration of ~750 μM (i.e. twice the basal levels) peaking at ~20 min after the infusion onset (Fig. 6a). This increase, evaluated with 30-s bins, became significant at 6 min after the start of the infusion and was followed by a weak negative rebound at ~45 min. In contrast, passive delivery of an equal volume of water did not affect glucose currents, and the difference versus passive glucose delivery was highly significant (a). Surprisingly, the rise in brain glucose levels seen after an intra-gastric injection was more than five times greater in amplitude than the increase occurring during drinking of the same volume of glucose. When analyzed with greater temporal resolution (b, 4-s bins), the latency to the first significant increase in glucose levels was 247 s after the start of the passive glucose infusion. When compared to the initiation of drinking, which was associated with a significant increase in [glucose], its levels slightly decreased at the start of intra-gastric delivery. However, after ~230 s, an interval similar to the average duration of glucose drinking, NAc glucose levels in both passively delivered and actively consumed groups became equal. Thereafter, brain glucose in passively delivered rats rapidly outpaced levels seen in rats that actively consumed the same amount of glucose.
Fig. 6. Passive intra-gastric glucose delivery results in a robust, greater tonic increase in NAc glucose levels than that seen after active glucose consumption.
(a) Mean (±SEM) changes in NAc glucose induced by intra-gastric glucose delivery under quiet resting conditions analyzed at a slow time-scale (30-s bins for 60 min; F5,623=13.3 p<0.05). Differences between intra-gastric versus active glucose consumption (main effect F1,15=9.53, Delivery × Time interaction F120,1800=18.38) and water injection (F1,7=7.21, Infusion × Time interaction F120,840=6.59) were highly significant (all p<0.05). (b) Changes in the same parameters shown for the initial 400 s following the infusion start analyzed with high temporal resolution (4-s bin); a highly significant rise was seen in glucose levels versus baseline in the NAc during this time period (F5,605=8.77, p<0.05), which was absent when an equal volume of water is delivered. When compared to active drinking, intra-gastric glucose resulted in significantly different glucose levels for the first 230 s (main effect F1,15=16.67, p<0.05, Delivery × Time interaction F58,870=3.69, p<0.05). Vertical dashed lines show mean interval of intra-gastric injection (210 s). n defines number of tests in each group and filled symbols in b and d show values significantly different from the initial, quiet-rest baseline (p<0.05).
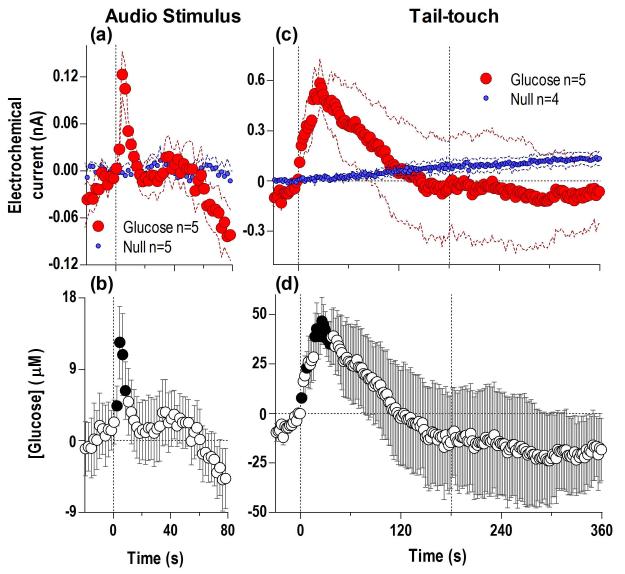
Rapid increases in NAc [glucose] induced by sensory stimuli
To better understand the mechanisms underlying the fluctuations in NAc [glucose] during the glucose-drinking test, we examined how this parameter is affected by two sensory stimuli unrelated to our behavioral task. Following a brief auditory signal, glucose currents rapidly increased compared to Null currents (Fig. 7a), reflecting a rapid rise in [glucose] (latency ~2 s) to a unimodal peak, followed by a sharp rebound-like decrease below baseline (b). A similarly rapid increase occurred at the start of a 3-min tail-touch, but this effect was larger in magnitude and more prolonged (d). After peaking (~45 μM) at ~23 s after the onset of the tail-touch, glucose levels steadily decreased, falling below the pre-stimulus baseline at the end of the tail-touch and during the post-stimulation period. This rapid change in [glucose] resulted from a robust increase in the glucose current; Null currents showed a slow, tonic increase that peaked well after the end of tail-touch (c).
Fig. 7. Simple and complex sensory stimuli induce rapid, transient increases in NAc glucose levels.
Top graphs show changes in glucose and Null currents induced by a brief auditory stimulus (a) and 3-min tail-touch (c) and bottom graphs show changes in [glucose] (b, d). Both stimuli induced significant changes in glucose versus Null currents (auditory stimulus: Current × Time interaction F40,320=3.65; tail-touch, Current × Time interaction F180,1260=2.33, both p<0.05) indicating increases in glucose concentrations (auditory stimulus: F4,204 =4.05, tail-touch:F4,499=2.50, both p<0.05). Filled symbols on b and d show individual 2-s bins significantly different from baseline (p<0.05).
Changes in electrochemical currents detected by Null sensors
While critical for excluding non-specific contributions to electrochemical currents generated by glutamate and glucose sensors, currents detected by the Null sensors also showed robust changes during glucose-drinking behavior (Fig. S3a). To identify the major contributor to these changes, we directly compared Null current dynamics with NAc temperature changes previously recorded during a similar behavioral paradigm (b) (Smirnov and Kiyatkin 2010).
Changes in Null currents and NAc temperatures were mostly parallel. Both parameters increased after the cup presentation and during approach behavior, remained relatively stable during drinking, and increased again after drinking (a-b). These parameters tightly correlated (r=0.917); this correlation was exceptionally high during the period between cup presentation and initiation of drinking and during the post-ingestion period (r= 0.913 and 0.939, respectively; both p<0.001) when both parameters increased (c). However, there was no correlation during drinking per se, when both parameters fluctuated at relatively stable levels. Therefore, brain temperature fluctuations greatly contribute to the slow current changes detected by the Null sensors under these behavioral conditions. Since this temperature influence is equally valid for substrate-sensitive and Null sensors, subtracting null currents from active currents greatly diminishes this non-specific influence, thus allowing to reveal more subtle but highly phasic fluctuations in [glucose], and especially [glutamate].
DISCUSSION
To our knowledge, this is the first study to examine second-scale fluctuations in extracellular glutamate and glucose in the NAc during motivated glucose-drinking behavior in trained rats. This project is an extension of our previous work, where we resolved basic methodological issues related to the reliability of electrochemical measurements of glutamate and glucose in freely moving rats and established the importance of Null sensor recordings for reducing non-specific chemical and physical interferents with sensors of this design. Here, we combined glutamate and glucose measurements during the performance of a glucose-drinking task to evaluate the functional significance of these neuroactive substances in motivated behavior and their relationship with reward.
Neurochemical correlates of motivated drinking behavior
While motivated behavior can be analyzed from different approaches, in this study it was analyzed with respect to three key behavioral events. While the presentation of a glucose-containing cup is the initial event for all subsequent behavioral and neurochemical changes, it could also be viewed as a motivationally significant, appetitive stimulus that triggers approach behavior. After cup presentation, NAc glutamate and glucose rapidly increased, then slightly fell but remained elevated above baseline until the rat began drinking. This response pattern differs from those induced by simple sensory stimuli, which induce equally rapid but transient up-down fluctuations in both glutamate and glucose (Wakabayashi and Kiyatkin 2012, 2014; Kiyatkin and Lenoir 2012, this study). Importantly, a similar environmental change associated with the removal of an empty cup after drinking had minimal behavioral consequences and induced weaker neurochemical responses. Here, the initial rise in [glucose] was comparable in magnitude to that seen with full-cup presentation, indicating its link to sensory stimulation, but instead of a sustained increase seen with the full-cup presentation, [glucose] rapidly rebounded in this case. In contrast to a phasic glutamate rise triggered by full-cup presentation, the glutamate response to the empty cup removal was virtually absent. Therefore, glutamate is rapidly released and glucose rapidly enters the brain after exposure to a motivationally significant appetitive stimulus that triggers seeking and approach behavior to the cup for glucose consumption.
Glucose drinking constitutes the consummatory phase of this motivated behavior. While [glucose] rapidly increased at the start of drinking and remained elevated during the entire period of consumption, [glutamate] decreased steadily, falling at the end of drinking well below the initial baseline. Glutamate levels continued to decrease for some time during the post-ingestion period, reaching a nadir (~11% below baseline) at ~300 s from the start of drinking and slowly returning to baseline thereafter. This return coincides with the rise of NAc [glucose] that, as shown with passive glucose delivery, results from ingested glucose entering the brain. Therefore, this slow decrease in NAc extracellular [glutamate] that occurs during glucose drinking and continues for some time after drinking may represent a single effect induced by the sensorimotor aspects of glucose consumption.
Since glucose consumption in trained rats engaged in motivated behavior could be conceptually viewed as “reward”, this tonic drop in NAc extracellular *glutamate+ could be viewed as its local correlate. Importantly, this robust change appears to be related to the sensory (or possibly sensorimotor) experience of glucose consumption (sensory or hedonic component of reward) but not its brain metabolic consequences (metabolic component of reward), which begin to occur minutes later when the consumed glucose directly enters the brain and interacts with multiple glucose-sensitive neurons (Levin et al. 1999; Levin 2001). This post-consummatory rise in NAc glucose tightly correlates with the reversal of the decrease in [glutamate] and its gradual return to baseline, suggesting a possible relationship between these neural effects. This slow decrease in glutamate could be also viewed as a correlate of the consummatory (satisfying) phase of motivated behavior, which follows its activation-related, seeking phase, which is associated with a rapid rise in [glutamate]. When glucose was substituted for water, glutamate initially increased in a pattern similar to glucose-cup presentation, but after the rats tasted the water without drinking a significant amount, [glutamate] steadily increased, reaching relatively large values (200 nM or ~17% increase) at 20 min after water-cup presentation. Therefore, the lack of expected glucose and no sensory stimulation associated with its consumption (no sensory reward or reward omission) is accompanied by an opposite change in NAc [glutamate].
Mechanisms underlying behavior-related fluctuations in NAc extracellular [glutamate] and [glucose]
It is challenging to interpret the mechanisms underlying biphasic fluctuations in [glutamate] during the glucose-drinking test. The rapid and relatively small increases occurring after cup presentation could be an echo of phasic neuronal release and overflow of synaptic glutamate into the extracellular space, where its basal concentrations are maintained at relatively low levels (~1 M). Although glutamate is phasically released in the NAc by axon terminals of nerve cells in response to sensory stimulation and motor activity (Parent and Hazrati 1995; Wilson and Kawaguchi 1996; Rebec 1998) and its concentrations inside of glutamate synapses are high, increases in extracellular [glutamate] in this study were relatively weak in magnitude (40-50 μM or ~5% over baseline). This could be related to the relatively large sensor’s size, the known rapidity of glutamate reuptake, and quantification bins that greatly exceeded the timing of synaptic events; all these factors can dampen the detected response magnitude. However, by this logic it is more difficult to explain why [glutamate] tonically decreases during glucose drinking. This drop in glutamate could result from cessation of release associated with the prior seeking phase of behavior, but it remains unclear how the cessation of relatively weak phasic glutamate release could result in such a robust, tonic drop in extracellular [glutamate]. However, this pattern of glutamate activity is consistent with electrophysiological findings, which revealed both phasic activations of accumbal cells induced by reward-predicting sensory cues and tonic decreases in neuronal activity during drinking (Roitman et al. 2005; Nicola et al. 2004a, 2004b; Taha and Fields 2005; Krause et al. 2010). Moreover, decreases in neuronal activity associated with glucose drinking also have been suggested in fMRI studies in humans (Smeets et al. 2007; Page et al. 2013). Finally, decreases in NAc [glutamate] had been reported in rats during free feeding using rapid-sampling microdialysis (Rada et al. 1997).
Although the mechanisms underlying synaptic release of glutamate, its interaction with postsynaptic glutamate receptors and its removal by glutamate transporters are well-understood (Clements 1996; Bergles et al. 1999), much less is known about the regulation and functional relevance of extrasynaptic glutamate fluctuations. In addition to synaptic release, glutamate is also released by neurons at extrasynaptic sites and by glial cells, interacting with high-affinity glutamate receptors located on both neural and glial cells and activated by relatively small-magnitude glutamate concentrations (Barbour and Hausser 1997; Vizi et al. 2010). Moreover, glial cells are primarly responsible for glutamate clearance from the extracellular space via high-affinity glutamate transporters (Danbolt 2001; Haydon and Carmignoto 2006). Therefore, although the initial rapid increases in glutamate may be explained by neuronal release, glial mechanisms involving both the release and uptake could be involved in mediating its slower, more pronounced, fluctuations (Fillenz 1995; Timmerman and Westerink 1997). Clearly, the mechanisms underlying tonic decreases in extracellular glutamate found in this study remain unclear and need to be clarified by future work.
Consistent with tight relationships between ultra-fast increases in NAc glucose and sensory stimulation (Kiyatkin and Lenoir 2012), [glucose] rapidly increased after cup presentation, at the start and end of drinking, and after removal of an empty cup, and remained consistently elevated during the entire duration of drinking, slowly returning toward baseline for ~150 s after the end of consumption. At this point, glucose levels began to increase again, correlating with the timing of glucose entry into the brain from the stomach. Therefore, discreet sensory stimuli appear to induce transient, up-down glucose fluctuations, while complex stimuli with behavioral consequences induce equally rapid but more prolonged, sustained increases in extracellular glucose levels. When water was substituted for glucose, NAc [glucose] phasically rose after cup presentation and preceding water tasting, but then decreased below baseline resembling the pattern seen with sensory stimuli.
In contrast to glutamate, which is synthetized in the brain and is released by both neuronal and glial cells, glucose enters neural tissue from the peripheral circulation and is continuously consumed by brain cells for their metabolism. Since accumbal neurons are uniformly excited by sensory stimuli due to phasic glutamate release (Kiyatkin and Rebec 1999) and [glucose] rise following intra-NAc glutamate microinjections (Kiyatkin and Lenoir 2012), phasic increases in extracellular [glucose] appear to be triggered by neuronal activation, which results in rapid increases in cerebral blood flow and accelerated transporter-mediated glucose entry glucose via the blood-brain barrier. Unexpectedly, NAc [glucose] increased more during passive, intra-gastric glucose delivery than after behavior-induced glucose consumption. This seemingly paradoxical finding could be explained by considering the second, usually hidden, force affecting extracellular [glucose]: its decrease due to cellular metabolism. Therefore, the weaker post-ingestion glucose rise could be attributed to the behavior-associated glucose use for cellular metabolism. Despite intense glucose consumption that tends to decrease its levels in the extracellular space, this process is opposed by larger glucose entry from the peripheral blood, thus maintaining a positive balance (i.e. ready glucose supply) throughout the entire behavioral cycle. This finding underscores the importance of active, neural activity-regulated glucose entry in the brain, which is essential to anticipate its future use for cellular metabolism.
Perspectives and significance
While numerous studies suggest that glutamate is involved in motivated behaviors, this study is the first, where glutamate fluctuations were actually measured at high temporal resolution during glucose-drinking behavior, thus allowing to reveal the highly dynamic nature of NAc extracellular glutamate and establish the relationships between its fluctuations and key events of appetitive motivated behavior. By supplementing these measurements with direct monitoring of NAc glucose, we elucidated the relationship between changes in glutamate and glucose levels, the principal components of this behavior, and three basic reward-related processes: reward seeking, sensory reward and metabolic reward. Further studies should clarify to what extent the glutamate changes seen in the NAc are typical to other brain structures, which glutamate afferents to the NAc or other local non-neural mechanisms mediate these changes, and how glutamate responses will change in more complex, operant models of motivated behavior.
Supplementary Material
Acknowledgements
This study was supported by the Intramural Research Program of NIDA-IRP.
Footnotes
Author contribution
EAK and KTW designed the experiments and wrote the manuscript. KTW, SEM and EAK ran the experiments, collected and analyzed the data.
EAK supervised the project, KTW, SEM and EAK performed experiments, wrote the manuscript and have approved the submission of this version.
Conflict of interests
The Authors declare no competitive interests in relation to the work described.
REFERENCES
- Barbour B, Hausser M. Intersynaptic diffusion of neurotransmitter. Trends Neurosci. 1997;20:377–384. doi: 10.1016/s0166-2236(96)20050-5. [DOI] [PubMed] [Google Scholar]
- Behrend CE, Cassim SM, Pallone MJ, Daubenspeck JA, Hartov A, Roberts DW, Leiter JC. Toward feedback controlled deep brain stimulation: dynamics of glutamate release in the subthalamic nucleus in rats. J. Neurosci. Methods. 2009;180:278–289. doi: 10.1016/j.jneumeth.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Diamond JS, Jahr CE. Clearance of glutamate inside the synapse and beyond. Curr. Opin. Neurobiol. 1999;9:293–298. doi: 10.1016/s0959-4388(99)80043-9. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76:790–803. doi: 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciapaglia F, Wightman RM, Carelli RM. Rapid dopamine signaling differentially modulates distinct microcircuits within the nucleus accumbens during sucrose-directed behavior. J. Neurosci. 2011;31:13860–13869. doi: 10.1523/JNEUROSCI.1340-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Centonze D, Bernardi G. Synaptic plasticity and physiological interactions between dopamine and glutamate in the striatum. Neurosci. Biobehav. Rev. 1997;21:519–523. doi: 10.1016/s0149-7634(96)00029-2. [DOI] [PubMed] [Google Scholar]
- Clements JD. Transmitter timecourse in the synaptic cleft: its role in central synaptic function. Trends Neurosci. 1996;19:163–171. doi: 10.1016/s0166-2236(96)10024-2. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog. Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- De Vries MG, Arseneau LM, Lawson ME, Beverly JL. Extracellular glucose in rat ventromedial hypothalamus during acute and recurrent hypoglycemia. Diabetes. 2003;52:2767–2773. doi: 10.2337/diabetes.52.11.2767. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav. Brain Res. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Dunn-Meynell AA, Sanders NM, Compton D, Becker TC, Eiki J, Zhang BB, Levin BE. Relationship among brain and blood glucose levels and spontaneous and glucoprivic feeding. J. Neurosci. 2009;29:7015–7022. doi: 10.1523/JNEUROSCI.0334-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows LK, Boutelle MG, Fillenz M. Physiological stimulation increases nonoxidative glucose metabolism in the brain of the freely moving rat. J. Neurochem. 1993;60:1258–1263. doi: 10.1111/j.1471-4159.1993.tb03285.x. [DOI] [PubMed] [Google Scholar]
- Fillenz M. Physiological release of excityatory amino acids. Behav. Brain Res. 1995;71:51–67. doi: 10.1016/0166-4328(95)00045-3. [DOI] [PubMed] [Google Scholar]
- Heien ML, Wightman RM. Phasic dopamine signaling during behavior, reward, and disease states. CNS Neurol. Disord. Drug Targets. 2006;5:99–108. doi: 10.2174/187152706784111605. [DOI] [PubMed] [Google Scholar]
- Hershey ND, Kennedy RT. In vivo calibration of microdialysis using infusion of stable-isotope labeled neurotransmitters. ACS Chem. Neurosci. 2013;4:729–736. doi: 10.1021/cn300199m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocite control of synaptic ytransmission and neurovascular couling. Physiol. Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Hu Y, Mitchell KM, Albahadily FN, Michaelis EK, Wilson GS. Direct measurement of glutamate release in the brain using a dual enzyme-based electrochemical sensor. Brain Res. 1994;659:117–125. doi: 10.1016/0006-8993(94)90870-2. [DOI] [PubMed] [Google Scholar]
- Hu Y, Wilson GS. Rapid changes in local extracellular rat brain glucose observed with an in vivo glucose sensor. J. Neurochem. 1997;68:1745–1752. doi: 10.1046/j.1471-4159.1997.68041745.x. [DOI] [PubMed] [Google Scholar]
- John J, Ramanathan L, Siegel JM. Rapid changes in glutamate levels in the posterior hypothalamus across sleep-wake states in freely behaving rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R2041–2049. doi: 10.1152/ajpregu.90541.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol. Behav. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Lenoir M. Rapid fluctuations in extracellular brain glucose levels induced by natural arousing stimuli and intravenous cocaine: fueling the brain during neural activation. J. Neurophysiol. 2012;108:1669–1684. doi: 10.1152/jn.00521.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, Rebec GV. Modulation of striatal neuronal activity by glutamate and GABA: iontophoresis in awake, unrestrained rats. Brain Res. 1999;822:88–106. doi: 10.1016/s0006-8993(99)01093-8. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Smirnov MS. Rapid EEG desynchronization and EMG activation induced by intravenous cocaine in freely moving rats: a peripheral, nondopamine neural triggering. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298:R285–300. doi: 10.1152/ajpregu.00628.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, Wakabayashi KT, Lenoir M. Physiological fluctuations in brain temperature as a factor affecting electrochemical evaluations of extracellular glutamate and glucose in behavioral experiments. ACS Chem. Neurosci. 2013;4:652–665. doi: 10.1021/cn300232m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Hedonic valence, dopamine and motivation. Mol. Psychiatry. 1996;1:186–189. [PubMed] [Google Scholar]
- Krause M, German PW, Taha SA, Fields HL. A pause in nucleus accumbens neuron firing is required to initiate and maintain feeding. J. Neurosci. 2010;30:4746–4756. doi: 10.1523/JNEUROSCI.0197-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulagina NV, Shankar L, Michael AC. Monitoring glutamate and ascorbate in the extracellular space of brain tissue with electrochemical microsensors. Anal. Chem. 1999;71:5093–5100. doi: 10.1021/ac990636c. [DOI] [PubMed] [Google Scholar]
- Le Moal M, Simon H. Mesocorticolimbic dopaminergic network: functional and regulatory roles. Physiol. Rev. 1991;71:155–234. doi: 10.1152/physrev.1991.71.1.155. [DOI] [PubMed] [Google Scholar]
- Levin BE. Glucosensing neurons do more than just sense glucose. Int. J. Obes. Relat. Metab. Disord. 2001;25(Suppl 5):S68–72. doi: 10.1038/sj.ijo.0801916. [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA, Routh VH. Brain glucose sensing and body energy homeostasis: role in obesity and diabetes. Am. J. Physiol. 1999;276:R1223–1231. doi: 10.1152/ajpregu.1999.276.5.R1223. [DOI] [PubMed] [Google Scholar]
- McNay EC, Gold PE. Food for thought: fluctuations in brain extracellular glucose provide insight into the mechanisms of memory modulation. Behav. Cogn. Neurosci. Rev. 2002;1:264–280. doi: 10.1177/1534582302238337. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog. Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Moult PR. Neuronal glutamate and GABAA receptor function in health and disease. Biochem. Soc. Trans. 2009;37:1317–1322. doi: 10.1042/BST0371317. [DOI] [PubMed] [Google Scholar]
- Naylor E, Aillon DV, Gabbert S, Harmon H, Johnson DA, Wilson GS, Petillo PA. Simultaneous real-time measurement of EEG/EMG and l-glutamate in mice: A biosensor study of neuronal activity during sleep. J. Electroanal. Chem. 2011;656:106–113. doi: 10.1016/j.jelechem.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Yun IA, Wakabayashi KT, Fields HL. Cue-evoked firing of nucleus accumbens neurons encodes motivational significance during a discriminative stimulus task. J. Neurophysiol. 2004a;91:1840–1865. doi: 10.1152/jn.00657.2003. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Yun IA, Wakabayashi KT, Fields HL. Firing of nucleus accumbens neurons during the consummatory phase of a discriminative stimulus task depends on previous reward predictive cues. J. Neurophysiol. 2004b;91:1866–1882. doi: 10.1152/jn.00658.2003. [DOI] [PubMed] [Google Scholar]
- Page KA, Chan O, Arora J, et al. Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA. 2013;309:63–70. doi: 10.1001/jama.2012.116975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res. Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Justice JB., Jr. Extracellular concentration and in vivo recovery of dopamine in the nucleus accumbens using microdialysis. J. Neurochem. 1992;58:212–218. doi: 10.1111/j.1471-4159.1992.tb09298.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th Edition Academic Press, Inc.; San Diego: 1998. [Google Scholar]
- Phillips PE, Robinson DL, Stuber GD, Carelli RM, Wightman RM. Real-time measurements of phasic changes in extracellular dopamine concentration in freely moving rats by fast-scan cyclic voltammetry. Methods Mol. Med. 2003;79:443–464. doi: 10.1385/1-59259-358-5:443. [DOI] [PubMed] [Google Scholar]
- Rada P, Tucci S, Murzi E, Hernandez L. Extracellular glutamate increases in the lateral hypothalamus and decreases in the nucleus accumbens during feeding. Brain Res. 1997;768:338–340. doi: 10.1016/s0006-8993(97)00788-9. [DOI] [PubMed] [Google Scholar]
- Rebec GV. Dopamine, glutamate, and behavioral correlates of striatal neuronal activity. Adv. Pharmacol. 1998;42:737–740. doi: 10.1016/s1054-3589(08)60853-4. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr. Opin. Neurobiol. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Carelli RM. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron. 2005;45:587–597. doi: 10.1016/j.neuron.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Rutherford EC, Pomerleau F, Huettl P, Stromberg I, Gerhardt GA. Chronic second-by-second measures of L-glutamate in the central nervous system of freely moving rats. J. Neurochem. 2007;102:712–722. doi: 10.1111/j.1471-4159.2007.04596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76:470–485. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver IA, Erecinska M. Extracellular glucose concentration in mammalian brain: continuous monitoring of changes during increased neuronal activity and upon limitation of oxygen supply in normo-, hypo-, and hyperglycemic animals. J. Neurosci. 1994;14:5068–5076. doi: 10.1523/JNEUROSCI.14-08-05068.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets PA, Vidarsdottir S, de Graaf C, Stafleu A, van Osch MJ, Viergever MA, Pijl H, van der Grond J. Oral glucose intake inhibits hypothalamic neuronal activity more effectively than glucose infusion. Am. J. Physiol. Endocrinol. Metab. 2007;293:E754–758. doi: 10.1152/ajpendo.00231.2007. [DOI] [PubMed] [Google Scholar]
- Smirnov MS, Kiyatkin EA. Phasic and tonic fluctuations in brain, muscle, and skin temperatures during motivated drinking behavior in rats: physiological correlates of motivation and reward. Brain Res. 2010;1310:87–102. doi: 10.1016/j.brainres.2009.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha SA, Fields HL. Encoding of palatability and appetitive behaviors by distinct neuronal populations in the nucleus accumbens. J. Neurosci. 2005;25:1193–1202. doi: 10.1523/JNEUROSCI.3975-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman W, Westerink BH. Brain microdialysis of GABA and glutamate: what does it signify? Synapse. 1997;27:242–261. doi: 10.1002/(SICI)1098-2396(199711)27:3<242::AID-SYN9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Vizi ES, Fekete A, Karoly R, Mike A. Non-synaptic receptors and transporters involved in brain functions and targets of drug treatment. Br. J. Pharmacol. 2010;160:785–809. doi: 10.1111/j.1476-5381.2009.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi KT, Kiyatkin EA. Rapid changes in extracellular glutamate induced by natural arousing stimuli and intravenous cocaine in the nucleus accumbens shell and core. J. Neurophysiol. 2012;108:285–299. doi: 10.1152/jn.01167.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi KT, Kiyatkin EA. Critical role of peripheral drug actions in experience-dependent changes in nucleus accumbens glutamate release induced by intravenous cocaine. J. Neurochem. 2014;128:672–685. doi: 10.1111/jnc.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Tolosa VM, Tseng TC, Balleine BW, Monbouquette HG, Maidment NT. Transient extracellular glutamate events in the basolateral amygdala track reward-seeking actions. J. Neurosci. 2012;32:2734–2746. doi: 10.1523/JNEUROSCI.5780-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J. Neurosci. 1996;16:2397–2410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol. Rev. 1987;94:469–492. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.