SYNOPSIS
Neonatal bacterial meningitis is an uncommon but devastating infection. Although the incidence and mortality have declined over the last several decades, morbidity among survivors remains high. The types and distribution of causative pathogens are related to birth gestational age, postnatal age, and geographic region. Confirming the diagnosis of meningitis can be difficult. Clinical signs are often subtle, and the lumbar puncture is frequently deferred in clinically unstable infants. When obtained, confirmatory testing with cerebrospinal fluid (CSF) culture is often compromised by antepartum or postnatal antibiotic exposure. While blood cultures and CSF parameters may be helpful in cases where the diagnosis is uncertain, bacterial meningitis occurs in infants without bacteremia and with normal CSF parameters. Newer tests such as the polymerase chain reaction are promising but require further study. Prompt treatment with appropriate antibiotics is essential to optimize outcomes. Successful efforts to prevent meningitis in infants have included the use of intrapartum antibiotic prophylaxis against Group B Streptococcus (GBS). Clinical trials investigating the use of a GBS vaccine for the prevention of neonatal GBS disease are ongoing.
Keywords: Neonatal bacterial meningitis, Very low birth weight, Lumbar puncture, Cerebrospinal fluid, Antibiotics, Vaccine
INTRODUCTION
Bacterial meningitis is a devastating infection associated with high mortality and morbidity in the neonatal population. Prompt diagnosis and treatment are essential to achieving good outcomes in affected infants. While overall incidence and mortality have declined over the last several decades, morbidity associated with neonatal meningitis remains virtually unchanged.1, 2 Prevention strategies, adjunctive therapies, and improved diagnostic strategies have been the focus of recent research seeking to improve the outcomes.3
DESCRIPTION OF THE DISEASE
Meningitis is the acute inflammation of the meninges, subarachnoid space, and brain vasculature resulting from infection.4 Neonatal meningitis is categorized as early and late onset, which is defined by the presence of signs of infection and organism isolation from cerebrospinal fluid (CSF) cultures at ≤72 hours and >72 hours of life, respectively.3, 5–7
EPIDEMIOLOGY
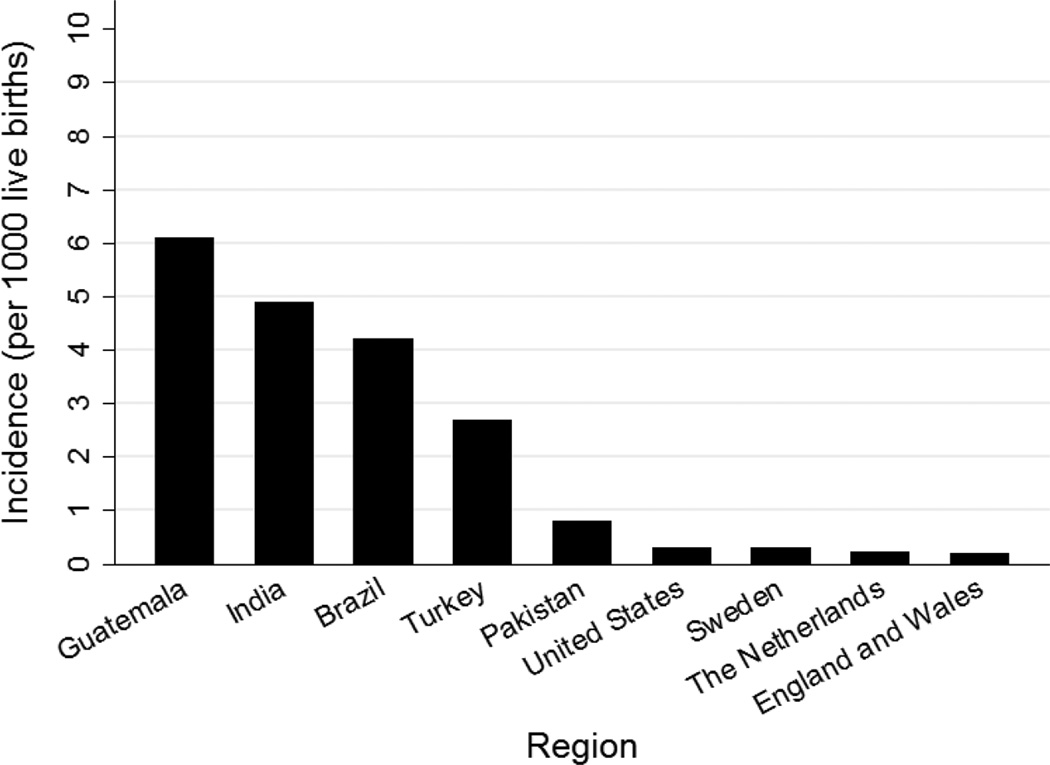
The incidence of neonatal meningitis varies by geographic location (Figure 1).8–10 Compared with older age groups, the incidence of meningitis is highest during the neonatal period.9, 16
Figure 1.
Incidence of neonatal meningitis across the world. Data from references 2, 11–15.
Developed Countries
The incidence of culture-proven neonatal meningitis is estimated at 0.3 per 1000 live births in developed countries. 2, 10, 16 This is likely an underestimation of the true incidence, however. For infants in the intensive care nursery who are evaluated for sepsis, 30–50% do not have a lumbar puncture (LP) performed.6, 17 When an LP is performed, more than 75% of the time it occurs after the initiation of antibiotics, possibly biasing CSF culture results.6, 17, 18.
In developed countries, mortality from neonatal meningitis ranges from 10–15%.2, 8 In a prospective study including 444 cases of confirmed meningitis from 2001–2007, mortality in premature infants compared with term infants was >2-fold higher (26% vs. 10%, p < 0.01).8 Up to 50% of infants with a history of meningitis will be neurologically impaired, with 25% having severe disability.2, 8, 19 With advances in medical practices, the incidence and mortality associated with meningitis have declined over the past 40 years; however, morbidity remains unchanged.19
Developing Countries
In developing countries, the reported incidence of neonatal meningitis is much higher at 0.8–6.1 per 1000 live births, with a mortality of 40–58%.9, 11 True values may actually be higher because of underreporting in regions with limited resources, diagnostic testing, and access to health care.9
ETIOLOGY
The types and distribution of organisms commonly observed in neonatal meningitis depend on postnatal age, location, and gestational age. The distribution of organisms seen in neonatal meningitis is similar to neonatal sepsis (Table 1.).1, 6, 16
Table 1.
Common pathogens of meningitis and commonly used empiric antibiotics
| Type | Major Pathogens | Empiric Antibiotics |
|---|---|---|
| Early-onset | Group B Streptococcus | Ampicillin |
| E. coli | Gentamicin | |
| L. monocytogenes | Cefotaxime | |
| S. pneumoniae | ||
| Late-onset | Coagulase-negative Staphylococcus | Vancomycin |
| S. aureus | Gentamicin | |
| E. coli | Cefotaxime | |
| Klebsiella sp. | Ampicillin | |
| Enterococcus sp. | ||
| Enterobacter sp. | ||
| Pseudomonas sp. | ||
| Group B Streptococcus | ||
Early-Onset Meningitis
Despite the institution of maternal intrapartum prophylaxis, Group B Streptococcus (GBS) has remained the most common cause of neonatal sepsis and meningitis since the early 1980s, responsible for >40% of all early-onset infections.2, 6, 20 Escherichia coli (E. coli) is the second most common pathogen and is isolated in 30% of all early-onset infections.6 Since the 1990s, E. coli has emerged as the most common cause of early-onset sepsis and meningitis among very low birth weight (VLBW, <1500 g birth weight) infants.21–24
Late-Onset Meningitis
Late-onset meningitis is predominantly seen in premature infants, and the incidence is directly related to decreasing birth gestational age and weight.25 Surveillance of 6956 VLBW infants from 1998–2000 found coagulase-negative staphylococci (48%) and Staphylococcus aureus (8%) to be the first and second most common pathogens, respectively.7 E. coli (5%) and Klebsiella (4%) spp. were the most common gram-negative causes of late-onset infections.5, 7 Although GBS (2%) was less common in this cohort, other studies found that infants were more likely to have confirmed meningitis with late-onset GBS sepsis compared with early-onset GBS sepsis (Table 2).7, 26, 27
Table 2.
Infants with late-onset vs. early-onset group B Streptococcus (GBS) sepsis complicated by meningitis
| N (ref) | Years | Study Population | Proportion of Infants with Late-Onset GBS Sepsis Complicated by Meningitis, n (%) |
Proportion of Infants with Early-Onset GBS Sepsis Complicated by Meningitis, n (%) |
P |
|---|---|---|---|---|---|
| 347 (26) | 2001–2003 | 23–43 weeks GA ≤90 days PNA Confirmed GBS sepsis |
84/136 (62) | 33/206 (16) | <0.001 |
| 179 (27) | 1997–2004 | 31–40 weeks GA Confirmed GBS sepsis |
13/24 (54) | 22/155 (14) | <0.01 |
GA, gestational age; PNA, postnatal age.
PATHOGENESIS
While several mechanisms in the development of neonatal meningitis have been described, primary bloodstream infection with secondary hematogenous distribution to the central nervous system (CNS) is the most common (Box 1).16 For this reason, the epidemiology and microbiology of neonatal meningitis is similar to neonatal sepsis.1
Mechanisms for Development of Neonatal Meningitis.
|
Early-Onset Infection
Organisms present in the maternal genitourinary tract ascend from the vagina and can infect the amniotic fluid through disruptions in the amniotic membranes, which the infant then aspirates.28 Organisms can also colonize exposed neonatal skin and mucosa during passage through the birth canal and invade through barrier disruptions.1 Organisms such as Listeria monocytogenes can also be transmitted transplacentally.16 In rare cases, hematogenous transmission of GBS from maternal bacteremia has been reported as a cause of early-onset GBS infections in infants.29
Late-Onset Infection
Organisms can be acquired from the colonized mother, as seen with GBS.30, 31 Poor hand hygiene among caregivers and hospital staff can result in the transfer of organisms between infected and uninfected infants.32 Foreign, invasive devices such as ventricular reservoirs, ventricular shunts, endotracheal tubes, venous or arterial catheters, urinary catheters, and feeding tubes can also introduce pathogens to the infant.16. Exposure to prolonged courses of empirical antibiotics for suspected infections can also result in increased risk for late-onset infections.33 Among 365 VLBW infants ≤32 weeks gestational age, infants exposed to empirical antibiotics ≥5 days had increased odds of developing late-onset sepsis (OR, 2.45 [95% CI, 1.28–4.67]).34
Infection of the CNS
After attaching to the endothelium of the cerebral microvasculature and choroid plexus, bacteria can enter the CSF by several mechanisms (Box 2).1, 4, 35 Inflammatory mediators are then released into the CSF in response to the presence of bacterial products, resulting in meningitis and increased permeability of the blood-brain barrier.1, 31
Bacterial Mechanisms for Entry into the Cerebrospinal Fluid.
|
RISK FACTORS
Risk factors for the development of neonatal meningitis are similar to neonatal sepsis (Box 3).5, 10, 36 Immaturity of the neonatal immune system, impaired phagocytic ability of neutrophils and monocytes, and diminishing maternal antibodies all contribute to increased risk of infections in both term and preterm infants.4, 24 Because most maternal immunoglobulins do not cross the placenta before 32 weeks gestation, infants born extremely preterm are at significantly higher risk for infections.10 Early initiation of breastfeeding may be protective against infections, however, due to transfer of immunoglobulin A.37
Risk Factors for Neonatal Meningitis.
|
CLINICAL PRESENTATION
The clinical signs of neonatal meningitis can be subtle and nonspecific (Box 4).16, 19, 38 Meningitic signs such as convulsions, irritability, bulging fontanel, and nuchal rigidity are often late findings that are associated with poor outcomes.2, 10, 38
Clinical Signs of Neonatal Meningitis.
|
Exposure to intrapartum antibiotic prophylaxis (IAP) against GBS has led to concerns that the signs of neonatal infections could be delayed or masked. Several studies have determined no significant difference in the clinical presentation of early-onset GBS disease between infected infants with and without prior exposure to IAP.20 Signs of early-onset sepsis manifested in 90% of infected infants within the first 24 hours of life.20
DIAGNOSIS
To confirm the diagnosis of neonatal meningitis, an LP is needed to collect CSF. Positive growth on the CSF culture provides identification of the offending organism and enables refinement of therapy.2, 39 The LP is frequently deferred during the septic workup due to concerns of exacerbating clinical deterioration in the sick infant.39–41
Performing or Deferring the LP
Because the LP is an invasive procedure with risks, it is difficult to determine which infant should receive one as part of the septic workup.40, 41 Among infants with positive blood cultures, up to 30% will have a concurrent positive CSF culture.42 However, in infants with confirmed meningitis, 15–38% will have a negative blood culture.27, 43–45 In rare cases, the blood and CSF cultures can be discordant.44 Approaches in which only infants with confirmed bacteremia are evaluated for meningitis will result in missed diagnoses of meningitis.
The incidence of meningitis among asymptomatic infants with risk factors is very low (<1%).46, 47 When clinical signs can be attributed to noninfectious causes, such as respiratory distress syndrome or transient tachypnea of the newborn, clinical judgment is required in deciding when to perform an LP.43 Among 238 infants admitted for respiratory distress without other symptoms, 17/238 (7%) infants had a positive blood culture, and none were found to have meningitis.48
The current recommendation is to perform an LP on all clinically stable infants suspected to have early- or late-onset sepsis and who are exhibiting signs of infection.20, 39, 43 Whenever possible, the LP should be performed prior to the administration of antibiotics.
Interpreting CSF Parameters
Infants are often exposed to intrapartum or empiric antibiotics prior to receiving an LP, which can result in falsely negative CSF cultures in those with meningitis.18 In these cases, CSF parameters are used to help determine the likelihood of meningitis.
CSF indexes vary according to age, with normal values in infants poorly defined.16, 44, 45 Common practice states that a CSF leukocyte count ≥20/mm3 is suggestive of bacterial meningitis in the infant.16 A study involving 1064 infants demonstrated that infants <28 days old without bacterial meningitis had a median CSF leukocyte count of 3/mm3 and 95th percentile value of 19/mm3.49 However, in a study evaluating 9111 infants ≥34 weeks gestational age, the use of 20/mm3 as a cutoff resulted in a missed diagnosis in 13% of infants with confirmed meningitis.44 Several infants with confirmed meningitis had normal CSF parameters without bacteremia. CSF protein and glucose were considered poor predictors of meningitis due to considerable overlap of values between infants with and without confirmed meningitis. A study of 4632 infants <34 weeks gestational age reached similar conclusions in the preterm population.45 There is great difficulty in predicting the diagnosis of meningitis solely based on CSF parameters, suggesting that CSF culture continues to be the gold standard for diagnosis.
Ancillary Tests
The polymerase chain reaction (PCR) has been explored as a diagnostic tool for meningitis. In addition to improved sensitivity and specificity, PCR also allows quicker detection of pathogens compared with traditional cultures.50 A real-time PCR assay designed to detect multiple pathogens including Streptococcus pneumonia, E. coli, GBS, S. aureus, and L. monocytogenes had an overall higher detection rate of any CSF pathogen compared with traditional cultures (72% vs. 48%).51 Among patients exposed to antibiotics before collection of CSF, PCR had a higher detection rate compared with culturing (58% vs. 29%).51 Further testing is needed before PCR can be used routinely in the diagnosis of bacterial meningitis.52
Other tests used to aid in clinical decision-making include the complete blood count with differential and C-reactive protein. Studies examining the usefulness of these tests in the diagnosis of neonatal bacterial meningitis are limited (Table 3).
Table 3.
Available studies on biomarkers for neonatal meningitis
| Biomarker | N (ref) | Study Population | Notable Findings |
|---|---|---|---|
| Complete blood count differential ratio |
72 (53) | Term <4 weeks PNA |
Complete blood count differential ratio <1.5 as cutoff for predicting bacterial meningitis, test achieved the following: sensitivity=100% specificity=67% positive predictive value=47% negative predictive value=100% |
| 40 (54) | Term <28 days PNA |
Used same cutoff values as above study on different cohort achieved the following: sensitivity=70% specificity=54% positive predictive value=39% negative predictive value=81% |
|
| Peripheral white blood cell count | 8312 (44) | ≥34 weeks GA 0–150 days PNA |
Use as predictor for bacterial meningitis had positive likelihood ratio <1.0 |
| Neither sensitive nor specific | |||
| C-reactive protein, cerebrospinal fluid | 23 (55) | 28–41 weeks GA 0–6 weeks PNA |
1/7 infants without infection, 0/5 infants with sepsis without meningitis, and 2/11 infants with meningitis had C-reactive protein >1 mg/dl |
| Levels do not distinguish between infants with meningitis from those with no confirmed infection | |||
GA, gestational age; PNA, postnatal age.
Repeating the LP
The need for a repeat LP during treatment in an infant with confirmed meningitis has been debated. Some experts recommend routinely repeating an LP in all patients at 48 hours, whereas others suggest repeating an LP only if clinical conditions are not improved by 24–72 hours after beginning therapy.56–59
In a retrospective study of 14,018 infants, 221 infants were identified as having culture-positive meningitis, with 118 infants (53%) receiving ≥2 LPs during the treatment course.56 Among infants with available mortality data receiving ≥2 LPs, 6/23 (26%) infants with repeat positive cultures died compared with 6/81 (7%) infants with repeat negative cultures (p=0.02). No significant difference in mortality was seen among the 81 (7%) infants with a repeat negative culture compared with the 90 (12%) infants with meningitis with no repeat LP (p=0.32). A survey of 109 pediatricians and neonatologists across northwest England found that 89 (82%) practitioners did not routinely repeat the LP in infants with bacterial meningitis unless clinically indicated.57
TREATMENT
Antimicrobial Therapy
Prompt initiation of antibiotics is critical. Delays in treatment are associated with increased mortality and morbidity.60 Empiric antimicrobials used in suspected meningitis require adequate CSF penetration and sensitivity against the most probable pathogens.10, 60 Upon identification of the pathogen and its susceptibilities, antimicrobial coverage should be adjusted accordingly (Table 4).
Table 4.
Common antibiotics used to treat neonatal meningitis
| Antibiotic (ref) | Susceptible Bacteria | Notes |
|---|---|---|
| Penicillin G (61) | GBS | Monotherapy acceptable if GBS confirmed by culture and clinical improvement is observed |
| Ampicillin (2, 6, 62, 63) | GBS L. monocytogenes Enterococcus sp. |
17–78% of E. coli isolates resistant Poor CNS penetration Requires higher doses for meningitis |
| Gentamicin (2, 62, 64) |
E. coli Klebsiella sp. Enterobacter sp. Pseudomonas sp. Citrobacter sp. Serratia sp. |
Poor CNS penetration Synergistic effect with ampicillin in treatment of L. monocytogenes Pseudomonas sp. may require combination therapy with a second agent Requires therapeutic drug monitoring |
| Cefotaxime (24, 62, 64) |
E. coli Klebsiella sp. Enterobacter sp. Citrobacter sp. Serratia sp. |
Good CNS penetration Used instead of gentamicin in cases of suspected or confirmed meningitis Not active against L. monocytogenes or Enterococcus sp. |
| Meropenem (64, 65) |
E. coli Klebsiella sp. Enterobacter sp. Citrobacter sp. Serratia sp. Pseudomonas sp. |
Good CNS penetration Limit use to multidrug resistant organisms (e.g., extended-spectrum beta-lactamase-producing organisms) |
| Vancomycin (62, 66) | Coagulase-negative staphylococci S. aureus Enterococcus sp. |
Variable CNS penetration Effective against methicillin-resistant S. aureus Requires therapeutic drug monitoring |
| Nafcillin (66, 67) | Methicillin-sensitive S. aureus | Good CNS penetration Superior to vancomycin for treatment of methicillin-sensitive S. aureus |
GA, gestational age (weeks); PNA, postnatal age (days); SCr, serum creatinine (mg/dL).
Duration of Antimicrobial Therapy
For uncomplicated meningitis, the minimum recommended treatment durations are the following:10, 61, 64
14 days for GBS, L. monocytogenes, and S. pneumonia
21 days for Pseudomonas and gram-negative enteric bacteria such as E. coli
Longer treatment courses are recommended for infants with meningitis with delayed clinical improvement after beginning therapy or with complications such as brain abscesses, ventriculitis, or brain infarctions.62
Adjunctive Therapy
In an effort to improve outcomes in infants with meningitis, several adjunctive therapies have been explored, including the use of intraventricular antibiotics, dexamethasone, intravenous immunoglobulins, granulocyte or granulocyte macrophage colony stimulating factor, and oral glycerol.2 At present, none of the proposed adjunctive therapies are used in routine practice.
LONG-TERM OUTCOMES
Survivors of neonatal meningitis are at considerable risk for long-term neurologic impairment.68, 69 A prospective study that followed 1717 survivors of neonatal meningitis through 5 years of age found that those who had neonatal meningitis were 10 times more likely to have moderate or severe disability than children who never had meningitis.68 Certain characteristics can help identify infected infants at the highest risk for a poor outcome (Box 5).70, 71
Predictors of Poor Neurologic Outcomes in Survivors of Bacterial Meningitis.
|
PREVENTING NEONATAL MENINGITIS
Intrapartum Antibiotic Prophylaxis
Since the adoption of IAP use in 1996 and universal antenatal screening for GBS colonization in 2002, the incidence of early-onset GBS infections has decreased significantly from 1.8 cases per 1000 live births in 1990 to 0.26 cases per 1000 live births in 2010.72 The incidence of late-onset GBS disease remains unaffected by IAP use.30, 72
IAP use has also been implicated in the increased proportion of non-GBS early-onset infections seen in VLBW infants. Since the late 1990s, E. coli has surpassed GBS as the predominant pathogen observed in early-onset infections among VLBW infants.6, 21–23 This likely reflects the success of IAP in reducing the incidence of early-onset GBS infections rather than an increase in the incidence of non-GBS infections.73 This change in proportion of non-GBS early-onset infections has not been observed in term infants.6
Reports from single-center studies have associated frequent use of ampicillin for IAP with an increase in ampicillin-resistant E. coli infections, particularly in premature infants.22, 74 However, rates of ampicillin-resistant E. coli have also increased in the general community.20 Furthermore, a multicenter trial involving 389 infants with confirmed early-onset sepsis found that, when comparing infants exposed and not exposed to IAP, frequencies of ampicillin-resistant E. coli infections were not significantly different.6 Although GBS has become increasingly resistant to clindamycin and erythromycin, sensitivity to ampicillin remains unchanged.75.
GBS Vaccine
Vaccines against GBS can reduce the number of missed opportunities for screening and IAP administration due to false-negative screens, precipitous deliveries, or extremely preterm births.6, 72 Maternal immunity to the most common serotypes of GBS can be transferred passively to the infant and protect against early- and late-onset infections.76 A trivalent GBS vaccine has shown promise in phase I and II trials, with another phase II trial currently recruiting participants (Table 5).76
Table 5.
Group B Streptococcus (GBS) vaccine clinical trials
|
ClinicalTrials.gov Identifier (ref) |
Phase | Objectives | Vaccine | Subjects | N | Design | Study Period |
|---|---|---|---|---|---|---|---|
| NCT00645346 (77) | I | Safety, tolerability, and immunogenicity | GBS glycoconjugate | Healthy non-pregnant women ages 18–40 years | 130 | Randomized, single-center, single-blind, placebo-controlled | 2008–2009 |
| NCT01193920 (78) | Ib/II | Safety and immunogenicity | Trivalent GBS | Healthy non-pregnant and pregnant women ages 18–40 years | 380 | Randomized, single-center, single-blind, placebo-controlled | 2010–2012 |
| NCT01446289 (79) | II | Immune response; amount of vaccine-induced antibody transferred to infant | Trivalent GBS | Healthy pregnant women ages 18–40 years | 86 | Randomized, multicenter, single-blind, placebo-controlled | 2011–2013 |
| NCT02046148 (80) | II | Safety and immunogenicity; placental transfer of GBS antibodies; levels of GBS antibodies in infants; levels of GBS antibodies in breast milk | Trivalent GBS | Healthy pregnant women ages 18–40 years | 75 | Randomized, multicenter, double-blind, placebo-controlled | 2014–2015 |
SUMMARY
Neonatal meningitis is a devastating disease that requires a high index of suspicion, prompt diagnosis, and rapid treatment. While the incidence and mortality have declined with improved neonatal intensive care practices and universal adoption of preventative screening and prophylaxis programs, the associated morbidity remains unchanged. Performing an LP to collect CSF is critical to confirming the diagnosis, determining the causative pathogen, and refining antimicrobial therapy. Through better diagnostic practices and development of vaccines, there is great hope that we may further reduce the burden of this devastating disease.
KEY POINTS.
Neonatal bacterial meningitis is uncommon but associated with high mortality and morbidity.
Group B Streptococcus (GBS) is the most common cause of neonatal meningitis.
Escherichia coli has recently become the most common pathogen isolated from very-low-birth-weight infants with meningitis.
Infants with culture-proven meningitis can have negative blood cultures and normal cerebrospinal fluid parameters.
All infants exhibiting signs of infection and with suspected early- or late-onset sepsis should undergo a lumbar puncture.
A GBS vaccine for the prevention of neonatal GBS disease including meningitis is currently under development.
Best Practices Box.
What is the current practice?
Neonatal Bacterial Meningitis
Best Practice/Guideline/Care Path Objective(s)
Promptly diagnose with performance of lumbar puncture
Begin antibiotics without delay
Reduce mortality and prevent long-term neurodevelopmental impairment
What changes in current practice are likely to improve outcomes?
Collection of CSF in all stable, symptomatic infants suspected of early- or late-onset infections with additional signs beyond respiratory distress
Increased vigilance in identifying and treating mothers with GBS colonization
Introduction of an effective GBS vaccine in the prevention of neonatal GBS disease
Is there a clinical algorithm?
Major Recommendations
Perform a lumbar puncture on all clinically stable infants with suspected sepsis and meningitis (Grade 1B)
-
Begin empiric antibiotics in all cases of suspected sepsis and meningitis (Grade 1A)
-
○
For suspected early-onset meningitis, consider ampicillin and gentamicin or cefotaxime in infants
-
○
For suspected late-onset meningitis, consider vancomycin in addition to ampicillin and gentamicin or cefotaxime
-
○
Repeat the lumbar puncture in infants who fail to demonstrate clinical improvement 24–48 hours after initiation of antibiotics (Grade 1B)
Repeat lumbar punctures are unnecessary in infants who demonstrate rapid clinical improvement after initiation of antibiotics and at end of successful therapy (Grade 1B)
All infants with a history of bacterial meningitis should be followed long-term for development of neurological sequelae (Grade 1A)
Bibliographic Source(s)
Heath, P.T., I.O. Okike, and C. Oeser, Neonatal meningitis: can we do better? Adv Exp Med Biol. 2011;719:11–24.
Heath, P.T. and I.O. Okike, Neonatal bacterial meningitis: an update. Paediatr Child Health. 2010;20:526–530.
Verani, J.R., L. McGee, and S.J. Schrag, Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59(Rr-10):1–36.
Summary statement
A high-index of suspicion, prompt diagnosis, and rapid initiation of antibiotics are essential to reducing mortality and morbidity associated with neonatal bacterial meningitis.
Acknowledgments
Funding support: Dr. Ku receives research support from the National Institute of Child Health and Human Development (5T32GM086330-03 [PIs: Brouwer, Benjamin, Watkins]). Dr. Boggess receives research support from the National Institute of Child Health and Human Development (5T32HD040672-13 [PI: Boggess]; 5K12HD001441 [PI: Orringer}; 1R01HD064729-01A1 [PI: Tita]), the National Center for Advancing Translational Sciences of the National Institutes of Health (1UL1TR001111), and Research Point Corporation, Inc. Dr. Cohen-Wolkowiez receives support for research from the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR001117), the Food and Drug Administration (1U01FD004858-01), the Biomedical Advanced Research and Development Authority (BARDA) (HHSO100201300009C), the nonprofit organization Thrasher Research Fund (www.thrasherresearch.org), and from industry for drug development in adults and children (www.dcri.duke.edu/research/coi.jsp).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Polin RA, Harris MC. Neonatal bacterial meningitis. Semin Neonatol. 2001;6:157–172. doi: 10.1053/siny.2001.0045. [DOI] [PubMed] [Google Scholar]
- 2.Heath PT, Okike IO, Oeser C. Neonatal meningitis: can we do better? Adv Exp Med Biol. 2011;719:11–24. doi: 10.1007/978-1-4614-0204-6_2. [DOI] [PubMed] [Google Scholar]
- 3.Shane AL, Stoll BJ. Recent developments and current issues in the epidemiology, diagnosis, and management of bacterial and fungal neonatal sepsis. Am J Perinatol. 2013;30:131–141. doi: 10.1055/s-0032-1333413. [DOI] [PubMed] [Google Scholar]
- 4.Barichello T, Fagundes GD, Generoso JS, et al. Pathophysiology of neonatal acute bacterial meningitis. J Med Microbiol. 2013;62:1781–1789. doi: 10.1099/jmm.0.059840-0. [DOI] [PubMed] [Google Scholar]
- 5.Cohen-Wolkowiez M, Moran C, Benjamin DK, et al. Early and late onset sepsis in late preterm infants. Pediatr Infect Dis J. 2009;28:1052–1056. doi: 10.1097/inf.0b013e3181acf6bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoll BJ, Hansen NI, Sanchez PJ, et al. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics. 2011;127:817–826. doi: 10.1542/peds.2010-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 8.Gaschignard J, Levy C, Romain O, et al. Neonatal Bacterial Meningitis, 444 Cases in 7 Years. Pediatr Infect Dis J. 2011;30:212–217. doi: 10.1097/inf.0b013e3181fab1e7. [DOI] [PubMed] [Google Scholar]
- 9.Furyk JS, Swann O, Molyneux E. Systematic review: neonatal meningitis in the developing world. Trop Med Int Health. 2011;16:672–679. doi: 10.1111/j.1365-3156.2011.02750.x. [DOI] [PubMed] [Google Scholar]
- 10.Heath PT, Okike IO. Neonatal bacterial meningitis: an update. Paediatrics and Child Health. 2010;20:526–530. [Google Scholar]
- 11.Thaver D, Zaidi AK. Burden of neonatal infections in developing countries: a review of evidence from community-based studies. Pediatr Infect Dis J. 2009;28:S3–S9. doi: 10.1097/INF.0b013e3181958755. [DOI] [PubMed] [Google Scholar]
- 12.Okike IO, Johnson AP, Henderson KL, et al. Incidence, Aetiology and Outcome of Bacterial Meningitis in Infants Aged <90 days in the UK and Republic of Ireland: prospective, enhanced, national population-based surveillance. Clin Infect Dis. 2014 doi: 10.1093/cid/ciu514. [DOI] [PubMed] [Google Scholar]
- 13.Mulder CJ, Zanen HC. A study of 280 cases of neonatal meningitis in The Netherlands. J Infect. 1984;9:177–184. doi: 10.1016/s0163-4453(84)91351-3. [DOI] [PubMed] [Google Scholar]
- 14.Kavuncuoglu S, Gursoy S, Turel O, et al. Neonatal bacterial meningitis in Turkey: epidemiology, risk factors, and prognosis. J Infect Dev Ctries. 2013;7:73–81. doi: 10.3855/jidc.2652. [DOI] [PubMed] [Google Scholar]
- 15.Persson E, Trollfors B, Brandberg LL, et al. Septicaemia and meningitis in neonates and during early infancy in the Goteborg area of Sweden. Acta Paediatr. 2002;91:1087–1092. doi: 10.1080/080352502760311593. [DOI] [PubMed] [Google Scholar]
- 16.Edwards M. Postnatal Bacterial Infections. In: Martin RJ, Fanaroff AA, Walsh MC, editors. Fanaroff and Martin's neonatal-perinatal medicine : diseases of the fetus and infant. 9th edition. Philadelphia: Saunders/Elsevier; 2011. pp. 793–830. [Google Scholar]
- 17.May M, Daley AJ, Donath S, et al. Early onset neonatal meningitis in Australia and New Zealand: 1992–2002. Arch Dis Child Fetal Neonatal Ed. 2005;90:F324–F327. doi: 10.1136/adc.2004.066134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanegaye JT, Soliemanzadeh P, Bradley JS. Lumbar puncture in pediatric bacterial meningitis: defining the time interval for recovery of cerebrospinal fluid pathogens after parenteral antibiotic pretreatment. Pediatrics. 2001;108:1169–1174. [PubMed] [Google Scholar]
- 19.Galiza EP, Heath PT. Improving the outcome of neonatal meningitis. Curr Opin Infect Dis. 2009;22:229–234. doi: 10.1097/QCO.0b013e32832ad49e. [DOI] [PubMed] [Google Scholar]
- 20.Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59:1–36. [PubMed] [Google Scholar]
- 21.Stoll BJ, Hansen NI, Higgins RD, et al. Very low birth weight preterm infants with early onset neonatal sepsis: the predominance of gram-negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002–2003. Pediatr Infect Dis J. 2005;24:635–639. doi: 10.1097/01.inf.0000168749.82105.64. [DOI] [PubMed] [Google Scholar]
- 22.Bizzarro MJ, Dembry LM, Baltimore RS, et al. Changing patterns in neonatal Escherichia coli sepsis and ampicillin resistance in the era of intrapartum antibiotic prophylaxis. Pediatrics. 2008;121:689–696. doi: 10.1542/peds.2007-2171. [DOI] [PubMed] [Google Scholar]
- 23.Stoll BJ, Hansen N, Fanaroff AA, et al. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med. 2002;347:240–247. doi: 10.1056/NEJMoa012657. [DOI] [PubMed] [Google Scholar]
- 24.Camacho-Gonzalez A, Spearman PW, Stoll BJ. Neonatal infectious diseases: evaluation of neonatal sepsis. Pediatr Clin North Am. 2013;60:367–389. doi: 10.1016/j.pcl.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fanaroff AA, Korones SB, Wright LL, et al. Incidence, presenting features, risk factors and significance of late onset septicemia in very low birth weight infants. The National Institute of Child Health and Human Development Neonatal Research Network. Pediatr Infect Dis J. 1998;17:593–598. doi: 10.1097/00006454-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Fluegge K, Siedler A, Heinrich B, et al. Incidence and clinical presentation of invasive neonatal group B streptococcal infections in Germany. Pediatrics. 2006;117:e1139–e1145. doi: 10.1542/peds.2005-2481. [DOI] [PubMed] [Google Scholar]
- 27.Ansong AK, Smith PB, Benjamin DK, et al. Group B streptococcal meningitis: cerebrospinal fluid parameters in the era of intrapartum antibiotic prophylaxis. Early Hum Dev. 2009;85:S5–S7. doi: 10.1016/j.earlhumdev.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polin RA Committee on F, Newborn. Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics. 2012;129:1006–1015. doi: 10.1542/peds.2012-0541. [DOI] [PubMed] [Google Scholar]
- 29.Ferrieri P, Wallen LD. Neonatal Bacterial Sepsis. In: Gleason CA, Devaskar SU, editors. Avery's Diseases of the Newborn. 9th edition. Philadelphia: Saunders/Elsevier; 2012. pp. 538–550. [Google Scholar]
- 30.Berardi A, Rossi C, Lugli L, et al. Group B streptococcus late-onset disease: 2003–2010. Pediatrics. 2013;131:e361–e368. doi: 10.1542/peds.2012-1231. [DOI] [PubMed] [Google Scholar]
- 31.Pong A, Bradley JS. Bacterial meningitis and the newborn infant. Infect Dis Clin North Am. 1999;13:711–733. doi: 10.1016/s0891-5520(05)70102-1. viii. [DOI] [PubMed] [Google Scholar]
- 32.Ng PC, Wong HL, Lyon DJ, et al. Combined use of alcohol hand rub and gloves reduces the incidence of late onset infection in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 2004;89:F336–F340. doi: 10.1136/adc.2003.031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cotten CM, Smith PB. Duration of empirical antibiotic therapy for infants suspected of early-onset sepsis. Curr Opin Pediatr. 2013;25:167–171. doi: 10.1097/MOP.0b013e32835e01f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuppala VS, Meinzen-Derr J, Morrow AL, et al. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr. 2011;159:720–725. doi: 10.1016/j.jpeds.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim KS. Acute bacterial meningitis in infants and children. Lancet Infect Dis. 2010;10:32–42. doi: 10.1016/S1473-3099(09)70306-8. [DOI] [PubMed] [Google Scholar]
- 36.Mukhopadhyay S, Puopolo KM. Risk assessment in neonatal early onset sepsis. Semin Perinatol. 2012;36:408–415. doi: 10.1053/j.semperi.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Debes AK, Kohli A, Walker N, et al. Time to initiation of breastfeeding and neonatal mortality and morbidity: a systematic review. BMC Public Health. 2013;13(Suppl 3):S19. doi: 10.1186/1471-2458-13-S3-S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nizet V, Klein J. Bacterial sepsis and meningitis. In: Remington J, Klein J, Wilson C, et al., editors. Infectious diseases of the fetus and newborn infant. 7th edition. Philadelphia, PA: Saunders/Elsevier; 2011. pp. 222–275. [Google Scholar]
- 39.Stoll BJ, Hansen N, Fanaroff AA, et al. To tap or not to tap: high likelihood of meningitis without sepsis among very low birth weight infants. Pediatrics. 2004;113:1181–1186. doi: 10.1542/peds.113.5.1181. [DOI] [PubMed] [Google Scholar]
- 40.Speidel BD. Adverse effects of routine procedures on preterm infants. Lancet. 1978;1:864–866. doi: 10.1016/s0140-6736(78)90204-0. [DOI] [PubMed] [Google Scholar]
- 41.Weisman LE, Merenstein GB, Steenbarger JR. The effect of lumbar puncture position in sick neonates. Am J Dis Child. 1983;137:1077–1079. doi: 10.1001/archpedi.1983.02140370037012. [DOI] [PubMed] [Google Scholar]
- 42.Visser VE, Hall RT. Lumbar puncture in the evaluation of suspected neonatal sepsis. J Pediatr. 1980;96:1063–1067. doi: 10.1016/s0022-3476(80)80643-3. [DOI] [PubMed] [Google Scholar]
- 43.Newborn, et al. Committee on Infectious D, Committee on F. Policy statement-Recommendations for the prevention of perinatal group B streptococcal (GBS) disease. Pediatrics. 2011;128:611–616. doi: 10.1542/peds.2011-1466. [DOI] [PubMed] [Google Scholar]
- 44.Garges HP, Moody MA, Cotten CM, et al. Neonatal meningitis: what is the correlation among cerebrospinal fluid cultures, blood cultures, and cerebrospinal fluid parameters? Pediatrics. 2006;117:1094–1000. doi: 10.1542/peds.2005-1132. [DOI] [PubMed] [Google Scholar]
- 45.Smith PB, Garges HP, Cotton CM, et al. Meningitis in preterm neonates: importance of cerebrospinal fluid parameters. Am J Perinatol. 2008;25:421–426. doi: 10.1055/s-0028-1083839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson CE, Whitwell JK, Pethe K, et al. Term Newborns Who Are at Risk for Sepsis: Are Lumbar Punctures Necessary? Pediatrics. 1997;99:e10–e10. doi: 10.1542/peds.99.4.e10. [DOI] [PubMed] [Google Scholar]
- 47.Fielkow S, Reuter S, Gotoff SP. Cerebrospinal fluid examination in symptom-free infants with risk factors for infection. J Pediatr. 1991;119:971–973. doi: 10.1016/s0022-3476(05)83058-6. [DOI] [PubMed] [Google Scholar]
- 48.Eldadah M, Frenkel LD, Hiatt IM, et al. Evaluation of routine lumbar punctures in newborn infants with respiratory distress syndrome. Pediatr Infect Dis J. 1987;6:243–246. doi: 10.1097/00006454-198703000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Kestenbaum LA, Ebberson J, Zorc JJ, et al. Defining cerebrospinal fluid white blood cell count reference values in neonates and young infants. Pediatrics. 2010;125:257–264. doi: 10.1542/peds.2009-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Backman A, Lantz P, Radstrom P, et al. Evaluation of an extended diagnostic PCR assay for detection and verification of the common causes of bacterial meningitis in CSF and other biological samples. Mol Cell Probes. 1999;13:49–60. doi: 10.1006/mcpr.1998.0218. [DOI] [PubMed] [Google Scholar]
- 51.Chiba N, Murayama SY, Morozumi M, et al. Rapid detection of eight causative pathogens for the diagnosis of bacterial meningitis by real-time PCR. J Infect Chemother. 2009;15:92–98. doi: 10.1007/s10156-009-0670-3. [DOI] [PubMed] [Google Scholar]
- 52.Liu CL, Ai HW, Wang WP, et al. Comparison of 16S rRNA gene PCR and blood culture for diagnosis of neonatal sepsis. Arch Pediatr. 2014;21:162–169. doi: 10.1016/j.arcped.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 53.Bonadio WA, Smith DS. CBC differential profile in distinguishing etiology of neonatal meningitis. Pediatr Emerg Care. 1989;5:94–96. doi: 10.1097/00006565-198906000-00005. [DOI] [PubMed] [Google Scholar]
- 54.Metrou M, Crain EF. The complete blood count differential ratio in the assessment of febrile infants with meningitis. Pediatr Infect Dis J. 1991;10:334–335. doi: 10.1097/00006454-199104000-00014. [DOI] [PubMed] [Google Scholar]
- 55.Philip AGS, Baker CJ. Cerebrospinal-Fluid C-Reactive Protein in Neonatal Meningitis. Journal of Pediatrics. 1983;102:715–717. doi: 10.1016/s0022-3476(83)80241-8. [DOI] [PubMed] [Google Scholar]
- 56.Greenberg RG, Benjamin DK, Jr, Cohen-Wolkowiez M, et al. Repeat lumbar punctures in infants with meningitis in the neonatal intensive care unit. J Perinatol. 2011;31:425–429. doi: 10.1038/jp.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Agarwal R, Emmerson AJ. Should repeat lumbar punctures be routinely done in neonates with bacterial meningitis?. Results of a survey into clinical practice. Arch Dis Child. 2001;84:451–452. doi: 10.1136/adc.84.5.450d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heath PT, Nik Yusoff NK, Baker CJ. Neonatal meningitis. Arch Dis Child Fetal Neonatal Ed. 2003;88:F173–F178. doi: 10.1136/fn.88.3.F173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klein JO, Feigin RD, McCracken GH., Jr Report of the Task Force on Diagnosis and Management of Meningitis. Pediatrics. 1986;78:959–982. [PubMed] [Google Scholar]
- 60.Weisfelt M, de Gans J, van de Beek D. Bacterial meningitis: a review of effective pharmacotherapy. Expert Opin Pharmacother. 2007;8:1493–1504. doi: 10.1517/14656566.8.10.1493. [DOI] [PubMed] [Google Scholar]
- 61.Pickering L, editor. Group B Streptococcal Infections. Red Book: 2012 Report of the Committee on Infectious Disease. 29th edition. Elk Grove: American Academy of Pediatrics; 2012. pp. 680–685. [Google Scholar]
- 62.Stockmann C, Spigarelli MG, Campbell SC, et al. Considerations in the pharmacologic treatment and prevention of neonatal sepsis. Paediatr Drugs. 2014;16:67–81. doi: 10.1007/s40272-013-0057-x. [DOI] [PubMed] [Google Scholar]
- 63.Tadesse DA, Zhao S, Tong E, et al. Antimicrobial drug resistance in Escherichia coli from humans and food animals, United States, 1950–2002. Emerg Infect Dis. 2012;18:741–749. doi: 10.3201/eid1805.111153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pickering L, editor. Escherichia coli and Other Gram-Negative Bacilli. Red Book: 2012 Report of the Committee on Infectious Disease. 29th edition. Elk Grove: American Academy of Pediatrics; 2012. pp. 321–324. [Google Scholar]
- 65.Smith PB, Cohen-Wolkowiez M, Castro LM, et al. Population pharmacokinetics of meropenem in plasma and cerebrospinal fluid of infants with suspected or complicated intra-abdominal infections. Pediatr Infect Dis J. 2011;30:844–849. doi: 10.1097/INF.0b013e31822e8b0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pickering L, editor. Staphylococcal Infections. Red Book: 2012 Report of the Committee on Infectious Disease. 29th edition. Elk Grove: American Academy of Pediatrics; 2012. pp. 653–668. [Google Scholar]
- 67.Frame PT, Watanakunakorn C, Mclaurin RL, et al. Penetration of Nafcillin, Methicillin, and Cefazolin into Human-Brain Tissue. Neurosurgery. 1983;12:142–147. doi: 10.1227/00006123-198302000-00002. [DOI] [PubMed] [Google Scholar]
- 68.Bedford H, de Louvois J, Halket S, et al. Meningitis in infancy in England and Wales: follow up at age 5 years. Bmj. 2001;323:533–536. doi: 10.1136/bmj.323.7312.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bassler D, Stoll BJ, Schmidt B, et al. Using a count of neonatal morbidities to predict poor outcome in extremely low birth weight infants: added role of neonatal infection. Pediatrics. 2009;123:313–318. doi: 10.1542/peds.2008-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klinger G, Chin CN, Beyene J, et al. Predicting the outcome of neonatal bacterial meningitis. Pediatrics. 2000;106:477–482. doi: 10.1542/peds.106.3.477. [DOI] [PubMed] [Google Scholar]
- 71.Klinger G, Chin CN, Otsubo H, et al. Prognostic value of EEG in neonatal bacterial meningitis. Pediatr Neurol. 2001;24:28–31. doi: 10.1016/s0887-8994(00)00221-6. [DOI] [PubMed] [Google Scholar]
- 72.Schrag SJ, Verani JR. Intrapartum antibiotic prophylaxis for the prevention of perinatal group B streptococcal disease: experience in the United States and implications for a potential group B streptococcal vaccine. Vaccine. 2013;31(Suppl 4):D20–D26. doi: 10.1016/j.vaccine.2012.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Puopolo KM, Eichenwald EC. No change in the incidence of ampicillin-resistant, neonatal, early-onset sepsis over 18 years. Pediatrics. 2010;125:e1031–e1038. doi: 10.1542/peds.2009-1573. [DOI] [PubMed] [Google Scholar]
- 74.Alarcon A, Pena P, Salas S, et al. Neonatal early onset Escherichia coli sepsis: trends in incidence and antimicrobial resistance in the era of intrapartum antimicrobial prophylaxis. Pediatr Infect Dis J. 2004;23:295–299. doi: 10.1097/00006454-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 75.Castor ML, Whitney CG, Como-Sabetti K, et al. Antibiotic resistance patterns in invasive group B streptococcal isolates. Infect Dis Obstet Gynecol. 2008;2008:727505. doi: 10.1155/2008/727505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oster G, Edelsberg J, Hennegan K, et al. Prevention of group B streptococcal disease in the first 3 months of life: Would routine maternal immunization during pregnancy be costeffective? Vaccine. 2014 doi: 10.1016/j.vaccine.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 77.Novartis Vaccines. A Phase I, Randomized, Single-blind, Controlled, Single Center Study to Evaluate the Safety and Immunogenicity of a Dose Range of Glycoconjugate Antigen Vaccine of Group B Streptococcus in Healthy Women 18–40 Years of Age. [Accessed July 28, 2014]; ClinicalTrials.gov [Internet]. 2000 Available at: https://clinicaltrials.gov/ct2/show/NCT00645346.
- 78.Novartis Vaccines. Safety and Immunogenicity of a Group B Streptococcus Vaccine in Non Pregnant and Pregnant Women 18–40 Years of Age. [Accessed July 28, 2014]; ClinicalTrials.gov [Internet]. 2000 Available at: https://clinicaltrials.gov/ct2/show/NCT01193920.
- 79.Novartis Vaccines. Immune Response Induced by a Vaccine Against Group B Streptococcus and Safety in Pregnant Women and Their Offsprings. [Accessed July 28, 2014]; ClinicalTrials.gov [Internet]. 2000 Available at: https://clinicaltrials.gov/ct2/show/NCT01446289.
- 80.Novartis Vaccines. Safety and Immunogenicity of a Trivalent Group B Streptococcus Vaccine in Healthy Pregnant Women. [Accessed July 28, 2014]; ClinicalTrials.gov [Internet]. 2000 Available at: https://clinicaltrials.gov/ct2/show/NCT02046148.