Abstract
Functional and anatomical relationships between working and declarative memory were investigated by contrasting regional cerebral blood flow (rCBF) change during standard working (Wisconsin Card Sorting Test, WCST) and declarative memory (Paired Associate Recognition Test, PART) tasks using identical stimulus–response modalities. The tasks and a resting baseline were administered to 30 participants (16 men, 14 women) during successive 10-min positron emission tomography 15O-water measures of rCBF. For both tasks, rCBF increased over baseline in inferior frontal and occipitotemporal regions, with more consistent dorsolateral prefrontal activation for WCST than PART. Additional orbitofrontal increases and dorsomedial decreases were seen for the PART. Activation patterns diverged when performance was considered. For the WCST, high performers activated dorsolateral and inferior frontal regions, whereas top PART performers activated only the occipitotemporal region. These results suggest operation of a frontotemporal network subserving both types of memory function that becomes more focal as performance increases.
Working memory, defined as a temporary store of limited capacity that holds information “on-line,” is managed by a central executive that allocates attention and visuospatial sketch pad slave systems responsible for maintaining information over brief delays (Baddeley, 1992). Declarative (or explicit) memory refers to memory for previously experienced facts or events (Squire, 1992). Declarative memory subsumes all stages of information processing but is commonly conceptualized as storage and long-term retrieval of information after it has left the immediate working memory span. Focal lesion studies traditionally attributed executive functions including working memory primarily to the prefrontal cortex (e.g., Baddeley, Logic, Bressi, Delia Sala, & Spinnler, 1986; Benton, 1968; Milner, 1963) and attributed long-term declarative memory primarily to mesial temporal regions including temporal cortex (e.g., Milner, 1966; Milner, Corkin, & Teuber, 1968), the hippocampus and amygdala (e.g., Squire, 1992), and the rhinal cortex (e.g., Mishkin & Murray, 1994).
These two memory processes have been functionally dissociated based on findings that amnestic patients such as H. M., who had experienced bilateral temporal lobe damage, could still rehearse and remember short lists of items over brief time spans. Although this functional distinction remains, recent lesion studies suggest that frontal and temporal brain regions may be involved in both types of memory processes. For example, hippocampal and temporal lobe pathology has been associated with deficits on the Wisconsin Card Sorting Test (WCST; Corcoran & Upton, 1993; Weinberger, Berman, Suddath, & Torrey, 1992), and frontal lobe lesions have been found to disrupt declarative memory performance (Wheeler, Stuss, & Tulving, 1995). The first goal of this study is to investigate this frontotemporal network by contrasting cognitive tasks designed to probe primarily working versus declarative memory during functional neuroimaging.
The WCST (Heaton, 1981) was chosen as a measure of working memory. The WCST requires participants to utilize examiner feedback to establish rules for correctly sorting a deck of cards to one of four key cards and then to inhibit previous rules and generate new strategies when examiner feedback changes. The WCST has been classified as a working memory task (e.g., Berman et al., 1995), because participants must maintain previous response information over brief delays to guide their next response. In this respect, WCST performance involves aspects of both spatial and object working memory (Wilson, O’Scalaidhe, & Goldman-Rakic, 1993). Although classified primarily as a working memory task, the WCST also involves other cognitive components. Factor analytic studies (Sullivan et al., 1993) yield at least three factors that measure different aspects of intradimensional (i.e., application of the previous response rule to the next card sort) versus extradimensional (i.e., switching to a new rule) set shifting demands that are differentially sensitive to frontal versus temporal lobe damage (Owen, Roberts, Polkey, Sahakian, & Robbins, 1991; Eslinger & Grattan, 1993).
The Paired Associate Recognition Test (PART; Ragland, Gur, Deutsch, Censits, & Gur, 1995) was chosen as a measure of declarative memory. The PART was developed and validated using WCST stimuli and response requirements to ensure compatibility of nonspecific aspects of sensory and motor activation between tasks. Rather than matching target cards with key cards based on a sorting principle as in the WCST, the PART requires participants to learn and to retrieve paired associates (PAs) composed of one key card and one target card. On each trial, participants are presented with four PAs (one for each key card) and, following a 2-min delay, are asked to indicate which key card was paired with each of the target cards. The PART is a reliable and valid measure of declarative memory that correlates with standard Wechsler Memory Scale visual recall (Ragland et al., 1995). Although the PART utilizes WCST cards, performance on the task is not correlated with the WCST, suggesting minimal overlap in cognitive demands between the two tasks.
Functional imaging studies of the WCST commonly demonstrate selective activation of the dorsolateral region of the prefrontal cortex (e.g., Catafau et al., 1994; Kawasaki et al., 1993;Parellada et al., 1994; Weinberger, Berman,& Zec, 1986). However, as with lesion studies (see Mountain & Snow, 1993), it appears that WCST performance is not dependent solely on the prefrontal cortex, because other investigators have either failed to demonstrate prefrontal activation (Graae, Warkentin, Franzen, & Risberg, 1991), found decreases in frontal lobe areas (Devous, 1989), or found additional activation in temporal, parietal, and cerebellar regions (Berman et al., 1995; Marenco, Coppola, Daniel, Zigun, & Weinberger, 1993).
Imaging studies of declarative memory have likewise found that task performance is not localized to only one region of the brain. Although a few studies find hippocampal activation (e.g., Squire et al., 1992), a larger number of studies have found memory-related activation primarily in prefrontal regions. For example, in a series of studies investigating encoding and retrieval of either words or sentences (Kapur et al., 1994; Tulving et al., 1994b), investigators concluded that left prefrontal regions are involved in the encoding of episodic memory and retrieval of semantic memory, whereas right prefrontal regions are more involved in retrieval of episodic memory (Tulving, Kapur, Craik, Moscovitch, & Houle, 1994a). Grady et al. (1995) found evidence of both prefrontal and hippocampal activation when they demonstrated in young adults that encoding of faces was associated with regional cerebral blood flow (rCBF) change in hippocampal and prefrontal regions, whereas recognition of faces was associated with prefrontal and parietal change.
Converging evidence of temporal lobe involvement in the WCST and prefrontal involvement in declarative memory may partially reflect the difficulty of developing a memory task that does not include components of both memory processes. However, this evidence may also reflect operation of a network of reciprocal pathways linking prefrontal cortex with mesial temporal regions and temporal and parietal association cortices (Goldman-Rakic, 1988; Goldman-Rakic, Selemon, & Schwartz, 1984). Thus, either working or declarative memory may engage a common network of frontotemporal regions.
The second goal of this study is to relate topography of activation with task performance. Gur and Reivich (1980) used a verbal analogies and spatial gestalt completion task in a l33Xe clearance study of CBF. Although CBF increase for the spatial task was not significantly lateralized to the right hemisphere for the whole sample of 36 healthy volunteers, better performance was associated with greater right hemisphere asymmetry. Correlations between specificity of task-related CBF change with performance were also obtained in subsequent CBF studies using both the same tasks (Gur, Ragland, et al., 1994) and word and face-recognition memory probes (Gur et al., 1993). In the latter study, greater right hemispheric CBF laterality in the mid-temporal region was correlated with improved face recognition, and better word recognition correlated with more left-lateralized activation of frontal pole and somatosensory regions. There is, thus, a growing body of evidence that the topography of task-related CBF activation may relate to participant performance, such that better performance is associated with more specific and task-appropriate patterns of hemispheric and regional CBF change.
Method
Participants
The sample included 30 healthy participants (16 men, 14 women; 23 Caucasian, 6 African American, and 1 Asian) classified as right-handed based on a standard behavioral and self-report inventory (Raczkowski, Kalat, & Nebes, 1974). They were (M ± SD) 26.30 ± 6.25 years old (range, 18.3–43.0 years), with 15.33 ± 1.97 years of education. Participants were from a sample of 36 consecutive accruals to the Mental Health Clinical Research Center (MHCRC) who responded to newspaper and community ads. Data from 6 participants were not processed due to either head movement (n = 1), lack of arterial samples (n = 1), participant withdrawal before all scans were completed (n = 1), or discovery of psychiatric history, substance abuse, or other medical problem during follow-up after the study was completed (n = 3). Participants underwent a comprehensive evaluation (Shtasel et al., 1991) that included medical, neurologic, and structured psychiatric examinations (Spitzer, Williams, Gibbon, & First, 1989) and laboratory testing. The laboratory screening tests included urine toxicology examinations. Participants were free of any present or past disorder or injury that might affect brain function, including substance abuse. Informed consent was obtained before participation in the study.
Neurobehavioral Probes
Images were acquired during a standard resting baseline condition (eyes open, ears unoccluded; Gur et al., 1982), a control baseline number-matching task (Weinberger, Berman, & Illowsky, 1988), and PART and WCST tasks. It was decided before data analysis not to utilize data from the number-matching control task, because of theoretical uncertainty about what extraneous cognitive components may have been engaged by number matching, thereby complicating interpretation of the activation data if the task was subtracted from PART and WCST conditions. Values from the resting baseline condition were used to measure activation effects (see Data Analysis subsection). Tasks began with the start of infusion (see PET Procedures subsection and Figure 1) and continued for either 18 min (if Scan 1) or 16 min (Scans 2–4). CBF measurements were recorded the last 10 min of each task period. For both tasks, verbal instructions and feedback following each response (“correct” or “incorrect” for WCST, “OK” for PART) were given during task and scan time.
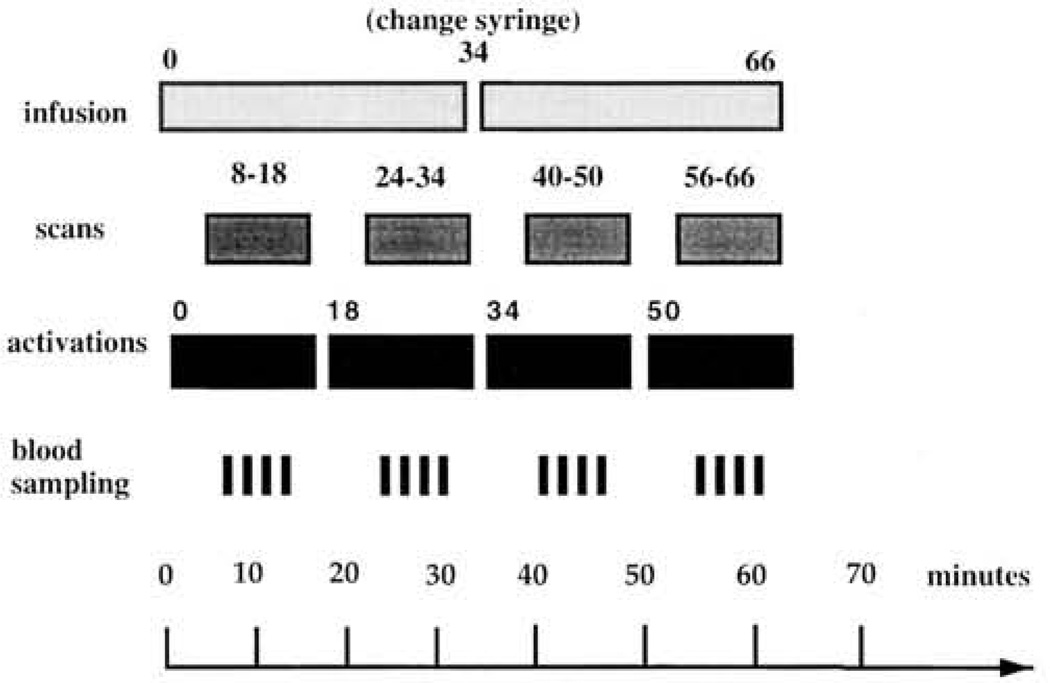
Figure 1.
Schematic showing time line (in minutes) for positron emission tomography (PET) protocol. First line shows timing of 15oxygen infusion. Second line shows timing of 10-min PET scans. Third line shows timing of task administration. Fourth line shows occurrence of arterial sampling.
WCST
Participants took the WCST following standard administration and scoring procedures (Heaton, 1981), with the exception that, because of positron emission tomography (PET) time constraints, the test was not discontinued after six categories and varied from two to three decks long. Because the WCST is self-paced, not all participants received an equal number of stimuli. Although self-paced administration complicates interpretation of activation effects because better performers generally receive more stimuli, it was decided to remain with standard self-paced administration so that results could be compared to existing WCST behavioral and functional imaging data that relies on self-paced administration. Because all but 1 participant received two complete decks, performance data were calculated for the 29 participants based on combined Deck 1 and Deck 2 data. Although this approach excludes additional data from the 15 participants who also completed Deck 3, it allows better comparison with more standard two-deck WCST data and eliminates redundant performance information (participants who completed three decks did so because they had mastered the task).
PART
The PART was designed to measure declarative memory using stimuli and response procedures that mirror the WCST so that the primary difference is the cognitive demand. Task construction, psychometric evaluation, and normative data on the PART were previously described (Ragland et al., 1995).
Briefly, the PART contains six trials of four items each, in which participants are first required to learn and then to correctly recognize PAs composed of WCST stimuli following a delay. It, therefore, encompasses acquisition, storage, and retrieval stages of information processing. PA stimuli consist of 1 of 64 WCST target cards arranged below 1 of 4 WCST key cards. Recognition probes consist of 1 previously administered WCST under the array of 4 key cards. The test begins with three practice trials during which the participant is presented with a PA for 5 s and asked to remember it. After removing the PA, participants are immediately presented with the recognition probe and asked to indicate which of the 4 key cards had been paired with the target card. If the participant responds incorrectly, the practice PA is presented a second time, and participants are retested following the same procedure. The test is discontinued if the participant is unable to perform correctly the second time on any of the three practice trials. Practice trials occur at the start of the 6- or 8-min equilibration period before the 10-min scan begins (see Figure 1). After completing the practice session, participants are presented with the first trial of four PAs at a rate of 5 s each. Participants are instructed to “try to remember which cards were shown together,” and then after a 2-min delay they are presented with four recognition probes in the same order and asked to indicate which key card was paired with each target card. During this recognition period, responses are self-paced. The five remaining trials are administered in the same fashion, and participants are told at the beginning of each successive trial that “none of the targets I show you now will be the same as the ones you saw before.” Trials (consisting of four targets, a 2-min delay, followed by four recognition probes) are presented sequentially until the end of the measurement epoch.
Test Procedures
After placement of arterial and venous catheters (see PET Procedures subsection), participants were positioned in the scanner in a supine position using a foam head holder. Two lasers at right angles to each other were used to align the head along the orbitomeatal line. After positioning, and after starting the 15O-water infusion (see later discussion), we made four 10-min CBF determinations in counterbalanced order (Latin square design): resting baseline, number matching, PART task, and WCST task. Test stimuli were presented, using a 35-mm slide projector, on a screen mounted from the ceiling at an angle perpendicular to the line of sight. Participants responded with a light pointer attached to their feet via a foot pedal apparatus. Responses consisted of making a bilateral foot movement to point the light beam to one of the four WCST key cards mounted in a vertical array at the top of the screen.
PET Procedures
A quantitative equilibrium infusion technique with arterial sampling was used to measure CBF with 15O-labeled water (Jones et al., 1985). 15O water was infused at a rate of about 4 mCi/min for a total study time of 66 min. Activity was measured with a volume imaging camera that has a spatial resolution of 5.5-mm in all directions (UGM Medical Systems, Philadelphia; Karp, Kinahan, & Muehllehner, 1993). Infusion of 15O water and simultaneous start of the first task began at Tune 0 followed by four 10-min scans. The first scan started 8 min after start of infusion, and subsequent scans were separated by 6 min to allow for reequilibration (Jones et al., 1985). Participants performed tasks during the 6- or 8-min reequilibration period, as well as during the 10-min scans. Participants did not know when reequilibration ended and scanning began. The imaging protocol is summarized in Figure 1.
Image Processing
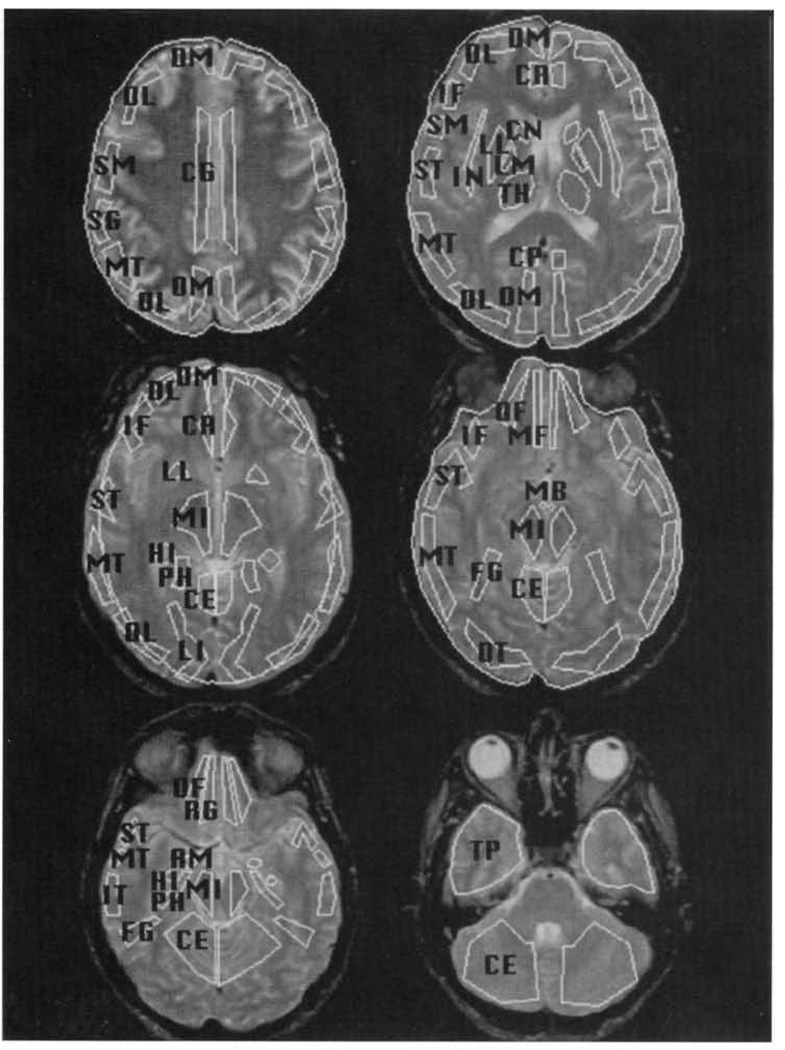
Radioactivity was localized on the reconstructed PET by coregistering the PET scan with contemporaneously acquired MRIs using a standard MHCRC protocol (Turetsky et al., 1995). Both MRI and PET scans were obliquely reformatted on graphics workstations by linear interpolation of the original three-dimensional arrays. Images were resliced in planes along the anterior and posterior commissures (AC–PC line). A set of templates with multiple regions of interest (ROIs) was then custom fit on each MRI slice by operators trained to an interrater reliability criterion of >.85 (Resnick, Karp, Turetsky, & Gur, 1994). Representative ROIs are illustrated in Figure 2. The templates and their regions were based on the Talairach Atlas (Talairach & Tournoux, 1988) as presented in Resnick et al. (1994). These custom-fit ROI templates were transfered to each individual’s PET image by globally adjusting the template to fit whole-brain boundaries on each slice (Gur et al., 1987).
Figure 2.
Placement of representative regions of interest on MRI images. SF = superior frontal; DL = dorsolateral prefrontal; DM = dorsomedial prefrontal; IF = inferior frontal; OT = occipitotemporal; MT = mid-temporal; IT = inferior temporal; TP = temporal pole; PH = parahippocampal gyrus; HI = hippocampus; AM = amygdala; OF = orbital frontal.
Data Quantification
CBF
Absolute CBF values were calculated using the equilibrium infusion technique (Frackowiak, Lenzi, Jones, & Heather, 1980; Lammertsma et al., 1981; Smith, Shao, Freifelder, Karp, & Ragland, 1995). CBF for each region (rCBF) was calculated by volume averaging over all slices in which that region could be identified. Uncertainties in activity concentration measurements in tissue and blood resulted in propagated errors in whole-brain CBF of +25/−18%. Statistical errors in tissue activity concentrations for various regions ranged from 8.6% for a 0.35-cm2 region to 3.4% for a 9-cm2 region. A commonly used method for reducing these propagated errors is to use relative rCBF (Blauenstein, Halsey, Wilson, Wills, & Risberg, 1977; Risberg, 1980) denned here as: relative rCBF = rCBF/wCBF, where wCBF is whole-brain CBF and rCBF is regional CBF. Because the largest source of measurement error is the variability in measured arterial activity concentration, and because this variability is the same for each region as for the whole brain, this error cancels out for relative rCBF. Therefore, we used quantitative data to determine that there were no differences between conditions in whole-brain CBFs, so that relative rCBFs could be used for examining regional specificity of activation.
Task performance
Number of categories (WCSTCAT) and number of perseverative errors (WCSTPE) on the WCST were calculated using standard procedures (Heaton, 1981) with two-deck data.
Participant responses to each recognition probe on the PART were recorded, were scored as either true or false, and were summed to obtain a total score on each trial. Because of individual differences in task order, response speed, and the time required for practice trials, the number of trials administered varied from four to six (i.e., 16–24 target and 16–24 recognition items). To allow comparison across participants, a percent correct score (PART_%COR) was calculated by dividing the total number correct by the total number of responses.
Because the sampling distribution of a proportion does not satisfy the assumptions underlying standard parametric analysis (e.g., analysis of variance), PART_%COR was transformed using the following equation: PART_%COR_T = 2(sin−1)(total correct/total responses)1/2. This transformation stabilizes the variance and normalizes the distribution, facilitating use of parametric statistical methods (Cohen, 1988). To ensure compatibility across tests, we rescaled both WCST and PART variables to standard equivalents (i.e., z score) with M = 0 and SD = 1.
Data Analysis
Of the 36 available ROIs, four regions each were chosen from frontal, temporal, and limbic areas. By reducing the number of ROIs analyzed, we reduced the chance of Type I error and contained the problem of multiple comparisons. These ROIs were chosen a priori based on the literature.
To address the first goal of examining the network of regions activated by each task, a repeated measures multivariate analysis of variance (MANOVA; Proc GLM [general linear model procedure]; SAS, 1987) was performed. This MANOVA entered the relative rCBF values listed in Table 1 into a 3 (task conditions) × 2 (hemispheres) × 12 (brain regions) design, with repeated measures for all three factors. Any significant task interactions were decomposed using separate post hoc contrasts. Each contrast used the same repeated measures MANOVA design and compared either WCST versus baseline, PART versus baseline, or WCST versus PART. Evidence of a significant Task × Region interaction was required as a prerequisite for subsequent paired t-test analysis of rCBF change scores (rCBFΔ). Results from the overall MANOVA were also used to determine whether hemispheric or bilateral change indices were examined. A Task × Hemisphere interaction was a prerequisite to examining rCBF laterality indices, otherwise bilateral values would be examined. Following these MANOVA analyses, rCBFΔ values were calculated by subtracting relative CBF during baseline from relative CBF during PART or WCST tasks for each of the 12 ROIs. This was done both within each hemisphere, producing 12 left and 12 right hemispheric rCBFΔ values, and for the regional average of both hemispheres, producing 12 bilateral rCBFΔ scores for each task. These hemispheric or bilateral rCBFΔ values were entered as dependent measures into standard paired t tests for the whole sample, with H0: t = 0 and H1: t ≠ 0
Table 1.
Left and Right Hemisphere rCBF (reg/wb) for Baseline, Paired Associate Recognition (PART), and Wisconsin Card Sorting (WCST) Tasks
| Resting baseline | PART | WCST | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | |||||||
| Region | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD |
| SF | 0.96 | 0.14 | 0.83 | 0.14 | 0.97 | 0.13 | 0.82 | 0.13 | 0.94 | 0.15 | 0.81 | 0.14 |
| DL | 1.07 | 0.12 | 0.86 | 0.10 | 1.11 | 0.12 | 0.87 | 0.10 | 1.10 | 0.12 | 0.88 | 0.11 |
| DM | 1.01 | 0.14 | 0.90 | 0.10 | 0.98 | 0.14 | 0.88 | 0.11 | 0.99 | 0.15 | 0.89 | 0.11 |
| IF | 1.13 | 0.13 | 0.90 | 0.11 | 1.18 | 0.11 | 0.93 | 0.11 | 1.18 | 0.13 | 0.93 | 0.12 |
| OT | 0.99 | 0.18 | 0.94 | 0.18 | 1.03 | 0.20 | 0.99 | 0.19 | 1.03 | 0.21 | 0.99 | 0.20 |
| MT | 0.97 | 0.08 | 0.84 | 0.08 | 0.98 | 0.08 | 0.83 | 0.09 | 0.97 | 0.08 | 0.84 | 0.08 |
| IT | 1.03 | 0.18 | 0.83 | 0.11 | 1.02 | 0.18 | 0.84 | 0.11 | 1.03 | 0.19 | 0.83 | 0.11 |
| TP | 0.89 | 0.15 | 0.82 | 0.13 | 0.90 | 0.15 | 0.84 | 0.12 | 0.90 | 0.149 | 0.84 | 0.13 |
| PH | 1.29 | 0.18 | 1.25 | 0.17 | 1.30 | 0.14 | 1.22 | 0.15 | 1.31 | 0.16 | 1.22 | 0.17 |
| HI | 1.32 | 0.19 | 1.21 | 0.15 | 1.28 | 0.16 | 1.22 | 0.13 | 1.27 | 0.15 | 1.21 | 0.14 |
| AM | 1.49 | 0.32 | 1.47 | 0.28 | 1.47 | 0.31 | 1.50 | 0.27 | 1.48 | 0.33 | 1.48 | 0.26 |
| OF | 1.23 | 0.26 | 1.00 | 0.16 | 1.25 | 0.26 | 1.05 | 0.16 | 1.22 | 0.26 | 1.05 | 0.17 |
Note. Regional cerebral blood flow change scores may be calculated by subtracting the average of left and right regional values for baseline from respective left and right regional averages for task conditions. rCBF = regional cerebral blood flow; reg/wb = region to whole brain; SF = superior frontal; DL = dorsolateral prefrontal; DM = dorsomedial prefrontal; IF = inferior frontal; OT = occipitotemporal; MT = mid-temporal; IT = inferior temporal; TP = temporal pole; PH = parahippocampal gyrus; HI = hippocampus; AM = amygdala; OF = orbital frontal brain regions.
To address the second goal of relating patterns of rCBFΔ with task performance, participants were divided into the top, middle, and bottom third performance range on each task. Hemispheric or bilateral rCBFΔ values were then examined for each performance group using paired t tests, with H0: t = 0 and H1: t ≠ 0. The significance criterion for all MANOVA and paired t-test analyses was set at an alpha level of .05, two-tailed.
Results
Task Performance
All participants correctly performed PART practice trials and were given the complete task. Because participants varied in the time they took to complete practice items, because the PART was administered in a counterbalanced order between Scan 1 (18 min) and Scan 2–4 (16 min), and because reaction times varied, each participant did not receive exactly the same number of stimuli (from 16–24 target and 16–24 response cards, M = 36.2 ± 4.6 of each). Participants’ mean (±SD) performance on the PART was 70.9 ± 20.2% correct (range, 18.7–100%), which is comparable to the average of 71.2 ± 19.3% correct obtained in the original normative sample (Ragland et al., 1995). On the WCST, participants sorted a varied number of cards due to differences in reaction time (from 91–246, M = 186 ± 30.9 cards). Taking into account that two-deck data (128 cards) were analyzed, participants also performed within the standard range (Heaton, 1981), obtaining an average of 7.8 ± 2.9 categories (range, 0–10), and making a total of 16.3 ± 17.0 perseverative errors (range, 7–96). The large range of perseverative errors was due to 1 outlier who never comprehended the task and continued to sort cards by color.
Replicating previous results, PART_%COR was not correlated with either WCSTCAT (r = −.19, p = .30) or WCSTPE (r = .22, p = .23). As expected, WCSTCAT and WCSTPE showed a strong negative correlation (r = −.72, p = .0001), indicating that higher category attainment was associated with lower rates of perseverative responding.
Whole-Brain CBF
Because sex differences in CBF have been consistently reported (e.g., Gur et al., 1982, 1988; Rodrigues, Warkentin, Risberg, & Rosadini, 1988; Shaw et al., 1984), whole-brain CBF was examined for sex effects using a 2 (men, women) × 3 (tasks) MANOVA, with repeated measures for the second factor. Replicating previous findings, there was a main effect for sex, F(1, 28) = 5.24, p = .029, with men having lower blood flow across task conditions. No other main effects or interactions were significant, and subsequent analyses were performed across sex groups. Absolute whole-brain CBF values (M ± SD) for baseline, PART, and WCST were 31.94 ± 5.3, 32.6 ± 4.7, and 33.1 ± 4.6 ml/min/100g, respectively. Lack of significant difference between whole-brain values supported the use of relative (or region-whole brain) rCBF to investigate activation effects.
Regional CBF
The relative hemispheric rCBF values for the four frontal, four temporal, and four limbic ROIs are presented in Table 1 for each condition.
When these values were entered into a 3 (task condition) × 2 (hemisphere) × 12 (ROIs) MANOVA, main effects of hemisphere, F(1, 27) = 117.13, p <.0001, and of region were found, F(11, 17) = 21.16, p < .0001. The hemispheric effect reflected higher left than right CBF across task conditions. The main effect of region demonstrated that CBF values differed between regions across tasks. A significant interaction was also observed between the three task conditions and brain region, F(16, 12) = 2.55, p <.05, indicating that the regional topography of blood flow across hemispheres was different between task conditions. Brain region also interacted with hemisphere, F(11, 17) = 15.44, p <.0001, illustrating that not all regions were similarly lateralized. No other main effects or interactions were found. Post hoc MANOVAs revealed that the overall Task × Region interaction was also present for WCST versus baseline, F(11, 17) = 2.74, p < .05, and PART versus baseline contrasts, F(11, 17) = 3.83, p <.01. However, no Task × Region effect was found for the WCST versus PART contrast. Bilateral rCBFΔ indices were therefore calculated for task-baseline but not task-task subtractions for the subsequent exploration of activation effects.
Task-related activation
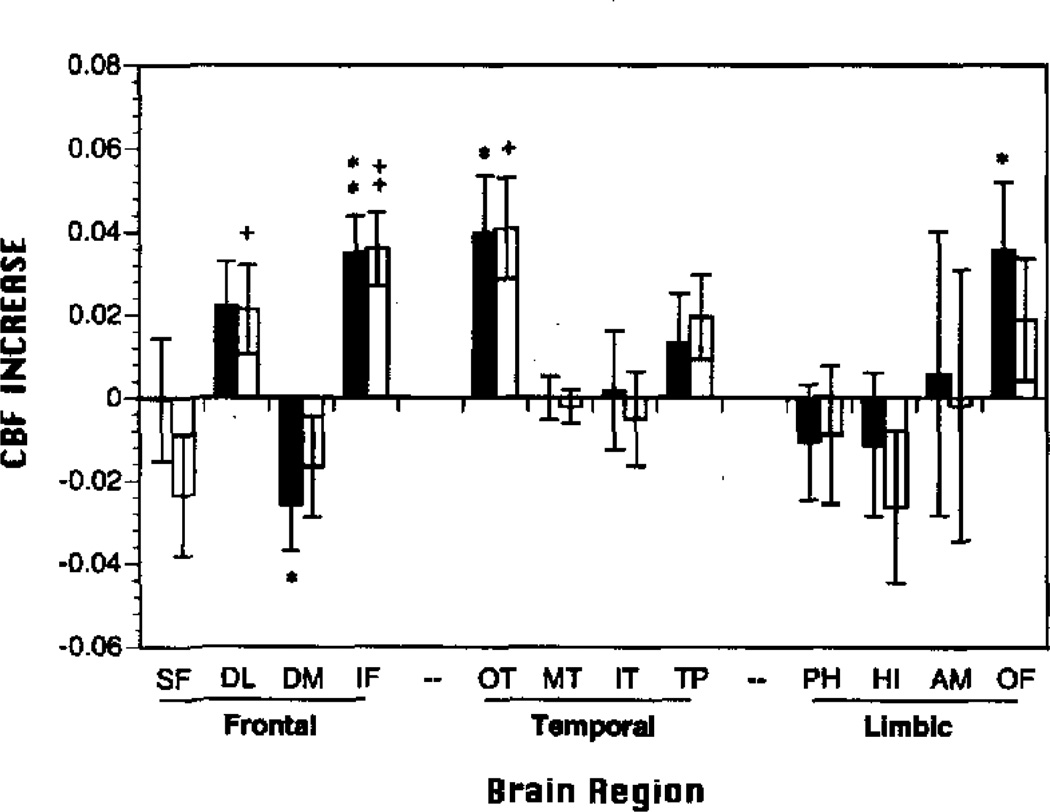
As can be seen in Figure 3, all except one rCBFΔ change value was positive, and the pattern of activation was similar for both tasks. Both the WCST and PART activated inferior frontal (IF) and occipitotemporal regions (OT). The WCST produced additional CBF increase in the dorsolateral prefrontal (DL) cortex. This DL region also showed a somewhat smaller increase over baseline for the PART, which occurred at a trend level (p = .06). The PART produced additional activation in the orbital-frontal (OF) region and a decrease in the dorsomedial prefrontal cortex (DM), which was not seen for the WCST.
Figure 3.
Mean (±SEM) regional cerebral blood flow change scores (rCBFΔ; resting baseline subtacted) in frontal, temporal, and limbic regions of interest for Paired Associates Recognition Task (PART black bars) and Wisconsin Card Sorting Task (WCST open bars). SF = superior frontal; DL = dorsolateral prefrontal; DM = dorsomedial prefrontal; IF = inferior frontal; OT = occipitotemporal; MT = mid-temporal; IT = inferior temporal; TP = temporal pole; PH = parahippocampal gyrus; HI = hippocampus; AM = amygdala; OF = orbital frontal; *p < .05, **p < .005 PART; +p <.05, †p < .005 WCST; all two-tailed.
Relationship with task performance
Figures 4 and 5 present the pattern of regional activation for top, middle, and bottom performers on WCST and PART tasks, respectively. When performance level was taken into account, the pattern of activation diverged.
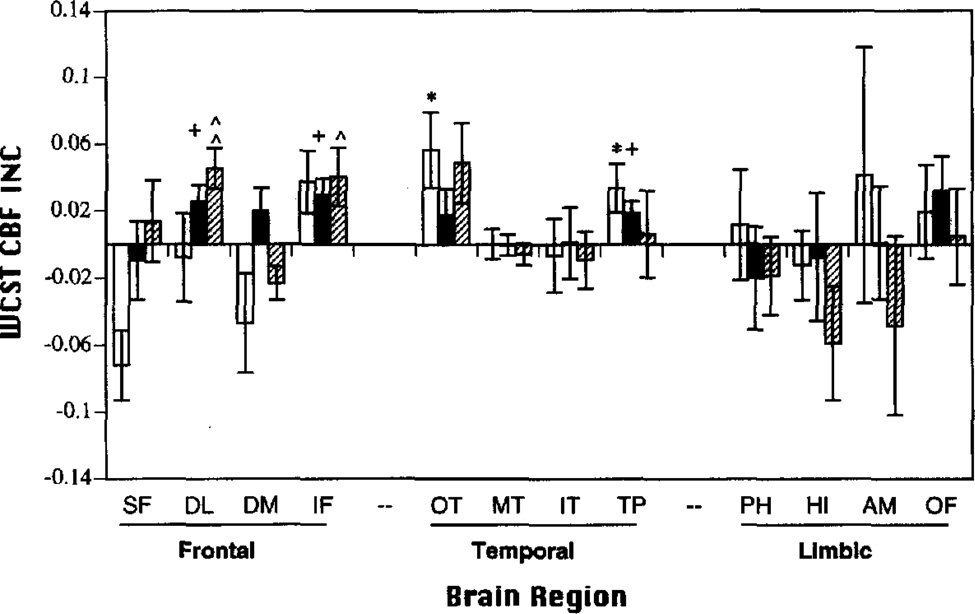
Figure 4.
Mean (±SEM) Wisconsin Card Sorting Task (WCST) regional cerebral blood flow change scores (rCBFΔ; baseline subtracted) in frontal, temporal, and limbic regions of interest for top (hatched bar), middle (black bar), and bottom (open bar) performers. SF = superior frontal; DL = dorsolateral prefrontal; DM = dorsomedial prefrontal; IF = inferior frontal; OT = occipitotemporal; MT = mid-temporal; IT = inferior temporal; TP = temporal pole; PH = parahippocampal gyrus; HI = hippocampus; AM = amygdala; OF = orbital frontal; INC = increase. *p < .05 bottom; +p < .05 middle; ^p < .05, ^^p < .005 top; all two-tailed.
Figure 5.
Mean (±SEM) Paired Associates Recognition Task (PART) regional cerebral blood flow change scores (rCBFΔ; baseline subtracted) in frontal, temporal, and limbic regions of interest for top (hatched bar), middle (black bar), and bottom (open bar) performers. SF = superior frontal; DL = dorsolateral prefrontal; DM = dorsomedial prefrontal; IF = inferior frontal; OT = occipitotemporal; MT = mid-temporal; IT = inferior temporal; TP = temporal pole; PH = parahippocampal gyrus; HI = hippocampus; AM = amygdala; OF = orbital frontal; INC = increase. **p < .005 bottom; +p < .05 middle; ^^p < .005 top; all two-tailed.
For the WCST (see Figure 4), top performers (9–10 categories) showed significant rCBF increases in DL and IF regions. In contrast, top PART performers (87.5–100% correct) activated only the OT region. Thus, top performance was associated with nonoverlapping and more selective patterns of regional activation on each task. Activation patterns did overlap for middle and bottom performers. The IF region was activated by middle WCST (8–9 categories) and middle and bottom PART performers (60–81.25% correct and 18.75–60% correct, respectively). Middle WCST performers also activated DL and TP (temporal pole) regions. Bottom WCST performers (0–8 categories) showed additional activation of OT and TP regions of interest. There were no regional decreases observed.
Discussion
The study provides evidence that a common anatomical network may mediate aspects of both working and declarative memory. The WCST and PART produced similar bilateral CBF increases in IF and OT brain regions, with a more consistent DL activation for WCST than PART conditions. The PART produced additional increases in OF and decreases in DM regions. This topographic similarity of task-related CBF change was found even though performance on the two tasks was uncorrelated. When level of task performance was taken into account, a divergence in the topography of activation effects was observed. Top performers on the PART activated only OT regions, whereas top WCST performers activated only DL and IF regions.
The brain regions activated by both tasks comprise anterior and posterior portions of a visual processing system that has been shown in primates to mediate perceptual and mnemonic processing of object identity and object location. DL and IF regions comprise the anterior portion of this network. A series of primate delayed-recognition memory studies using either focal lesion or neural recording techniques have shown that DL regions maintain spatial information on-line over brief delays, whereas IF regions are involved in working memory for objects and faces (see Wilson et al., 1993, for review). These frontal lobe regions also share pathways with OT areas in the primate extrastriate visual cortex (Goldman-Rakic, 1987). The extrastriate cortex includes primary visual areas in the occipital lobe that project to either parietal or OT and inferior temporal regions (Baizer, Ungerleider, & Desimone, 1991). Primate lesion studies show that the occipitoparietal pathway is necessary for correctly perceiving the spatial relations between objects, and the OT pathway for identifying objects and performing pattern discrimination tasks (Mishkin & Ungerleider, 1982; Desimone & Ungerleider, 1990). A recent PET CBF study using face-discrimination and dot-localization tasks provides some confirmation of this animal model in humans by demonstrating selective activation of OT regions during face discrimination and superior parietal regions during dot localization (Haxby et al., 1991).
The overlap of frontal and temporal activation effects for the PART and WCST is interpreted as reflecting the reciprocal nature of corticocortical pathways connecting prefrontal cortex and temporal association areas (Goldman-Rakic, 1988). However, it is also possible that the topography of activation effects was similar because either the PART and WCST measured the same cognitive functions or the PET procedures were not sufficiently sensitive to detect subtle task differences. There are several lines of evidence that counter these alternative explanations.
Because the PART utilizes WCST stimuli and response requirements to control nonspecific activation effects, a central concern during task development was that the PART might engage the same cognitive processing strategies as the WCST. To limit the working memory component of the PART and to discourage participants from matching cards as they would during the WCST, PAs were created in which targets did not match key cards on any dimension (e.g., one red triangle was paired with three blue circles). Likewise, a short-term or working memory component was reduced by requiring participants to store information on multiple PAs, thereby discouraging rehearsal, and by introducing a 2-min delay between targets and recognition probes to allow decay of the immediate memory trace.
These design features appear to have been successful, because performance on the PART was highly correlated with standard measures of declarative memory in the normative sample (Ragland et al., 1995) and was uncorrelated with WCST performance in both the normative sample and the current study. However, it is likely that the PART also involved some aspects of working memory (e.g., temporal order) and the WCST involved some aspects of declarative memory (e.g., remembering task instructions). Similarity in activation effects may, therefore, also partially reflect some task overlap of these two memory processes. Because we did not attempt to prevent subvocal mediation of the tasks, it is also possible that operation of an articulatory loop during each task accounted for some of the regional similarities (e.g., inferior frontal activation).
The ability to correlate CBF data with individual differences, such as gender and task performance, supports the sensitivity of our CBF method. Lower rates of whole-brain CBF in men, which has been demonstrated using the 133Xe clearance method (e.g., Gur et al., 1988; Rodrigues et al., 1988), was replicated for PET in the current study. Systematic differences in the topography of task-related rCBF change between good and poor performers also support the sensitivity of the method. Although the performance difference between the top, middle, and bottom third of the distribution on both tasks was relatively small, activation patterns appeared most specific and task appropriate for top performers and more diffuse for the bottom and middle thirds of the distribution. This finding of better performance associated with fewer and more specific activation effects builds on previous work with healthy volunteers (Gur & Reivich, 1980) and patients with schizophrenia (Gur, Jaggi, Shtasel, Ragland, & Gur, 1994) and suggests that although activation of widely distributed brain regions may be sufficient to perform a cognitive task, more selective activation of only those brain regions specific to the task is required for better performance (either within healthy individuals or between patient and nonpatient groups).
Acknowledgments
This research was supported by National Institutes of Health Grants MH-19112, MH-00586, MH-48539, and MH-43880. We thank Sean Riggins, Kevin Kilroy, and Debbie Meehan of the PET Center for assistance in data collection; Teong Thew for programming; and Diane Sandefur for coordination of participant recruitment.
Contributor Information
J. Daniel Ragland, Department of Psychiatry, University of Pennsylvania Health Systems.
David C. Glahn, Department of Psychiatry, University of Pennsylvania Health Systems
Ruben C. Gur, Department of Psychiatry, University of Pennsylvania Health Systems
David M. Censits, Department of Psychiatry, University of Pennsylvania Health Systems
Robin J. Smith, Department of Radiology, University of Pennsylvania Health Systems
P. David Mozley, Department of Radiology, University of Pennsylvania Health Systems.
Abass Alavi, Department of Radiology, University of Pennsylvania Health Systems.
Raquel E. Gur, Department of Psychiatry, University of Pennsylvania Health Systems
References
- Baddeley A. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Logic R, Bressi S, Della Sala S, Spinnler H. Dementia and working memory. The Quarterly Journal of Experimental Psychology. 1986;38A:603–618. doi: 10.1080/14640748608401616. [DOI] [PubMed] [Google Scholar]
- Baizer JS, Ungerleider LG, Desimone R. Organization of visual inputs to the inferior temporal and posterior parietal cortex in macaques. Journal of Neuroscience. 1991;11:168–190. doi: 10.1523/JNEUROSCI.11-01-00168.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton AL. Differential behavioral effects of frontal lobe disease. Neuropsychologia. 1968;6:53–60. [Google Scholar]
- Berman KF, Ostrem JL, Randolph C, Gold J, Goldberg TE, Coppola R, Carson RE, Herscovitch P, Weinberger DR. Physiological activation of a cortical network during performance of the Wisconsin Card Sorting Test: A positron emission tomography study. Neuropsychologia. 1995;33:1027–1046. doi: 10.1016/0028-3932(95)00035-2. [DOI] [PubMed] [Google Scholar]
- Blauenstein UW, Halsey JH, Wilson EM, Wills EL, Risberg J. 133-Xenon inhalation method, analysis of reproducibility: Some of its physiological implications. Stroke. 1977;8:92–102. doi: 10.1161/01.str.8.1.92. [DOI] [PubMed] [Google Scholar]
- Catafau AM, Parellada E, Lomena FJ, Bernardo M, Pavia J, Ros D, Setoain J, Gonzalez-Monclus E. Prefrontal and temporal blood flow in schizophrenia: Resting and activation technetium-99m-HMPAO SPECT patterns in young neuroleptic-naive patients with acute disease. Journal of Nuclear Medicine. 1994;35:935–941. [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Corcoran R, Upton D. Role for the hippocampus in card sorting? Cortex. 1993;29:293–304. doi: 10.1016/s0010-9452(13)80182-7. [DOI] [PubMed] [Google Scholar]
- Desimone R, Ungerleider LG. In: Handbook of neuropsychology. Boller F, Grafman J, editors. Amsterdam: Elsevier; 1990. pp. 267–299. [Google Scholar]
- Devous MD. Imaging brain function by single-photon emission computer tomography. In: Andreasen NC, editor. Brain imaging: Applications in psychiatry. Washington, DC: American Psychiatric Press; 1989. pp. 147–234. [Google Scholar]
- Eslinger PJ, Grattan LM. Frontal lobe and frontal-striatal substrates for different forms of cognitive flexibility. Neuropsychologia. 1993;31:17–28. doi: 10.1016/0028-3932(93)90077-d. [DOI] [PubMed] [Google Scholar]
- Frackowiak RSJ, Lenzi GL, Jones T, Heather JD. Quantitative measurement of regional cerebral blood flow and oxygen metabolism in man using 15O and positron emission tomography: Theory, procedure and normal values. Journal of Computer Assisted Tomography. 1980;4:727–736. doi: 10.1097/00004728-198012000-00001. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Higher functions of the brain, Part 1. In: Plum IF, editor. Handbook of physiology, Section 1: The nervous system. Vol. 5. New York: Oxford University Press; 1987. p. 373. [Google Scholar]
- Goldman-Rakic PS. Topography of cognition: Parallel distributed networks in primate association cortex. Annual Review of Neuroscience. 1988;11:137–156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12:719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Graae E, Warkentin S, Franzen G, Risberg J. Frontal lobe challenge: A comparison of rCBF during activation procedures. Journal of Cerebral Blood Flow and Metabolism. 1991;11:S372. [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, Pietrini P, Schapiro MB, Haxby JV. Age-related reductions in human recognition memory due to impaired encoding. Science. 1995;269:218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- Gur RC, Gur RE, Obrist WD, Hungerbuhler JP, Yoynkin D, Rosen AD, Skolnick BE, Reivich M. Sex and handedness differences in cerebral blood flow during rest and cognitive activity. Science. 1982;217:659–661. doi: 10.1126/science.7089587. [DOI] [PubMed] [Google Scholar]
- Gur RC, Gur RE, Skolnick BE, Resnick SM, Silver FL, Chawluk JB, Muenz L, Obrist WD, Reivich M. Effects of task difficulty on regional cerebral blood flow: Relationships with anxiety and performance. Psychophysiology. 1988;25:392–399. doi: 10.1111/j.1469-8986.1988.tb01874.x. [DOI] [PubMed] [Google Scholar]
- Gur RC, Jaggi JL, Ragland JD, Resnick SM, Shtasel D, Muenz L, Gur RE. Effects of memory processing on regional brain activation: Cerebral blood flow in normal subjects. International Journal of Neuroscience. 1993;72:31–44. doi: 10.3109/00207459308991621. [DOI] [PubMed] [Google Scholar]
- Gur RE, Jaggi JL, Shtasel D, Ragland JD, Gur RC. Cerebral blood flow in schizophrenia: Effects of memory processing on regional activation. Biological Psychiatry. 1994;35:3–15. doi: 10.1016/0006-3223(94)91160-6. [DOI] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Resnick SM, Skolnick BE, Jaggi J, Muenz L, Gur RE. Lateralized increases in cerebral blood flow during performance of verbal and spatial tasks: Relationship with performance level. Brain and Cognition. 1994;24:244–258. doi: 10.1006/brcg.1994.1013. [DOI] [PubMed] [Google Scholar]
- Gur RC, Reivich M. Cognitive task effects on hemispheric blood flow in humans: Evidence for individual differences in hemispheric activation. Brain and Language. 1980;9:78–92. doi: 10.1016/0093-934x(80)90073-5. [DOI] [PubMed] [Google Scholar]
- Gur RE, Resnick SM, Alavi A, Gur RC, Caroff S, Dann R, Silver F, Saykin AJ, Chawluk JB, Kushner M, Reivich M. Regional brain function in schizophrenia: I. A positron emission tomography study. Archives of General Psychiatry. 1987;44:119–125. doi: 10.1001/archpsyc.1987.01800140021003. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Grady CL, Horwitz B, Ungerleider LG, Mishkin M, Carson RE, Herscovitch P, Schapiro MB, Rapoport SI. Dissociation of object and spatial visual processing pathways in human extrastriate cortex. Proceedings of the National Academy of Sciences, USA. 1991;88:1621–1625. doi: 10.1073/pnas.88.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK. The Wisconsin Card Sorting Test manual. Odessa, FL: Psychological Assessment Resources; 1981. [Google Scholar]
- Jones SC, Greenberg JH, Dann R, Robinson GD, Jr, Kushner M, Alavi A, Reivich M. Cerebral blood flow with the continuous infusion of oxygen-15-labeled water. Journal of Cerebral Blood Flow and Metabolism. 1985;5:566–575. doi: 10.1038/jcbfm.1985.85. [DOI] [PubMed] [Google Scholar]
- Kapur S, Craik FI, Tulving E, Wilson AA, Houle S, Brown GM. Neuroanatomical correlates of encoding in episodic memory: Levels of processing effect. Proceedings of the National Academy of Sciences, USA. 1994;91:2008–2011. doi: 10.1073/pnas.91.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp JS, Kinahan PE, Muehllehner G. Effect of increased axial field of view on the performance of a volume PET scanner. IEEE Transactions on Medical Imaging. 1993;12:299–306. doi: 10.1109/42.232259. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Maeda Y, Suzuki M, Katsumi U, Higashima M, Kiba K, Yamaguchi N, Matsuda M, Hisada K. SPECT analysis of regional cerebral blood flow changes in patients with schizophrenia during the Wisconsin Card Sorting Test. Schizophrenia Research. 1993;10:109–116. doi: 10.1016/0920-9964(93)90045-k. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Frackowiak RSJ, Lenzi GL, Heather JD, Pozzilli C, Jones T. Accuracy of the oxygen-15 steady state technique for measuring rCBF and rCMR(O2): Tracer modeling, statistics and spatial sampling. Journal of Cerebral Blood Flow Metabolism. 1981;1:S3–S4. [Google Scholar]
- Marenco S, Coppola R, Daniel DG, Zigun JR, Weinberger DR. Regional cerebral blood flow during the Wisconsin Card Sorting Test in normal subjects studied by Xenon-133 dynamic SPECT: Comparison of absolute values, percent distribution values, and covariance analysis. Psychiatry Research: Neuroimaging. 1993;50:177–192. doi: 10.1016/0925-4927(93)90029-h. [DOI] [PubMed] [Google Scholar]
- Milner B. Effects of different brain lesions on card sorting. Archives of Neurology. 1963;9:90–100. [Google Scholar]
- Milner B. Amnesia following operation on the temporal lobe. In: Whitty CWM, Zangwil OL, editors. Amnesia. London: Butterworth; 1966. pp. 109–133. [Google Scholar]
- Milner B, Corkin S, Teuber HL. Further analyses of the hippocampal amnesic syndrome: Fourteen-year follow-up study of H. M. Neuropsychologia. 1968;6:215–234. [Google Scholar]
- Mishkin M, Murray EA. Stimulus recognition. Current Opinion in Neurobiology. 1994;4:200–206. doi: 10.1016/0959-4388(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Ungerleider LG. Contribution of striate inputs to the visuospatial functions of parieto-preoccipital cortex in monkeys. Behavioral Brain Research. 1982;6:57–77. doi: 10.1016/0166-4328(82)90081-x. [DOI] [PubMed] [Google Scholar]
- Mountain MA, Snow WG. Wisconsin Card Sorting Test as a measure of frontal pathology: A review. The Clinical Neuropsychologist. 1993;7:108–118. [Google Scholar]
- Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1991;29:993–1006. doi: 10.1016/0028-3932(91)90063-e. [DOI] [PubMed] [Google Scholar]
- Parellada E, Catafau AM, Bernado M, Lomena F, Gonzalez-Monclus E, Setoain J. Prefrontal dysfunction in young acute neuroleptic-naive schizophrenic patients: A resting and activation SPECT study. Psychiatry Research. 1994;55:131–139. doi: 10.1016/0925-4927(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Raczkowski D, Kalat J, Nebes R. Reliability and validity of some handedness questionnaire items. Neuropsychologia. 1974;12:43–48. doi: 10.1016/0028-3932(74)90025-6. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Deutsch G, Censits DM, Gur RE. Reliability and construct validity of the Paired-Associate Recognition Memory Test: A test of declarative memory using Wisconsin Card Sorting stimuli. Psychological Assessment. 1995;7:25–32. [Google Scholar]
- Resnick SM, Karp JS, Turetsky B, Gur RE. Comparison of anatomically-defined versus physiologically-based regional localization: Effects on PET-FDG quantitation. Journal of Nuclear Medicine. 1994;34:2201–2207. [PubMed] [Google Scholar]
- Risberg J. Regional cerebral blood flow measurements by 133-Xe inhalation: Methodology and clinical applications in neuropsychology and psychiatry. Brain and Language. 1980;9:9–34. doi: 10.1016/0093-934x(80)90069-3. [DOI] [PubMed] [Google Scholar]
- Rodrigues G, Warkentin S, Risberg J, Rosadini G. Sex differences in regional cerebral blood flow. Journal of Cerebral Blood Flow and Metabolism. 1988;8:783–789. doi: 10.1038/jcbfm.1988.133. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS/STAT Software (Version 6.03) Cary, NC: Author; 1987. [Google Scholar]
- Shaw TG, Mortel KF, Meyer JS, Roger RL, Hardenberg J, Cutaia MM. Cerebral blood flow changes in benign aging and cerebrovascular disease. Neurology. 1984;34:855–862. doi: 10.1212/wnl.34.7.855. [DOI] [PubMed] [Google Scholar]
- Shtasel DL, Gur RE, Mozley PD, Richards J, Taleff MM, Heimberg C, Gallacher F, Gur RC. Volunteers for biomedical research: Recruitment and screening of normal controls. Archives of General Psychiatry. 1991;48:1022–1025. doi: 10.1001/archpsyc.1991.01810350062010. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Shao L, Freifelder R, Karp JS, Ragland JD. Quantitative measurement of cerebral blood flow in volume imaging PET scanners. IEEE Transactions on Nuclear Science. 1995;42:1018–1023. [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSM–III–R—Non-Patient version (SCID-NP, 9/1/89) New York: New York State Psychiatric Institute; 1989. [Google Scholar]
- Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire LR, Ojemann JG, Miezin FM, Petersen SE, Videen TO, Raichle ME. Activation of the hippocampus in normal humans: A functional anatomical study of memory. Proceedings of the National Academy of Sciences, USA. 1992;89:1837–1841. doi: 10.1073/pnas.89.5.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Mathalon DH, Zipursky RB, Kersteen-Tucker Z, Knight RT, Pfefferbaum A. Factors of the Wisconsin Card Sorting Test as measures of frontal-lobe function in schizophrenia and in chronic alcoholism. Psychiatry Research. 1993;46:175–199. doi: 10.1016/0165-1781(93)90019-d. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: An approach to cerebral imaging. New York: Thieme Medical Publishers, Inc.; 1988. [Google Scholar]
- Tulving E, Kapur S, Craik FIM, Moscovitch M, Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: Positron emission tomography findings. Proceedings of the National Academy of Sciences, USA. 1994a;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E, Kapur S, Markowitsch HJ, Craik FI, Habib R, Houle S. Neuroanatomical correlates of retrieval in episodic memory: Auditory sentence recognition. Proceedings of the National Academy of Sciences, USA. 1994b;91:2012–2015. doi: 10.1073/pnas.91.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Cowell PE, Gur RC, Grossman RI, Shtasel DL, Gur RE. Frontal and temporal lobe brain volumes in schizophrenia: Relationship to symptomatology and clinical subtype. Archives of General Psychiatry. 1995;52:1061–1070. doi: 10.1001/archpsyc.1995.03950240079013. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Illowsky BP. Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia. III. A new cohort and evidence for a monoaminergic mechanism. Archives of General Psychiatry. 1988;45:609–615. doi: 10.1001/archpsyc.1988.01800310013001. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Suddath R, Torrey EF. Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: A magnetic resonance imaging and rCBF flow study of discordant monozygotic twins. American Journal of Psychiatry. 1992;7:890–897. doi: 10.1176/ajp.149.7.890. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia: I. Regional cerebral blood flow evidence. Archives of General Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Wheeler MA, Stuss DT, Tulving E. Frontal lobe damage produces episodic memory impairment. Journal of the International Neuropsychological Society. 1995;1:525–536. doi: 10.1017/s1355617700000655. [DOI] [PubMed] [Google Scholar]
- Wilson FAW, O’Scalaidhe SP, Goldman-Rakic PS. Dissociation of object and spatial processing domains in primate prefrontal cortex. Science. 1993;260:1955–1958. doi: 10.1126/science.8316836. [DOI] [PubMed] [Google Scholar]