Abstract
Obstructive sleep apnea (OSA) is a common disorder with major neurocognitive and cardiovascular sequelae. It is estimated that more than one quarter of the population is at risk for OSA, with increased prevalence noted in populations with hypertension, coronary artery disease, stroke, and atrial fibrillation. A number of epidemiologic and mechanistic studies have recently generated interest in the role of OSA in the pathophysiology of cardiovascular disease, a link that continues to require extensive investigation. This chapter reviews these epidemiologic studies, the current understanding of the mechanisms by which OSA may contribute to the progression of cardiovascular diseases, and the effects of OSA treatment on cardiovascular disease outcomes.
Keywords: Lung, Heart, Sleep apnea, Cardiovascular, Hypoxemia, Vascular
Introduction
Obstructive sleep apnea (OSA) is a common disorder and is highly prevalent in patients with hypertension, coronary artery disease, stroke, and atrial fibrillation [1, 2]. Interest in the cardiovascular consequences of sleep disorders developed with recognition of the hypoxemia that could occur with OSA. Early studies by Rosthchild et al. [3] and Barach et al. [4] established a link between systemic hypoxemia and electrocardiographic ST segment abnormalities. Other authors subsequently proposed that hypoxemic stresses caused by OSA could contribute to the pathogenesis of cardiac arrhythmias and conduction disturbances. The role of OSA in the pathophysiology of cardiovascular disease has generated considerable recent interest.
Cardiovascular Effects of Normal Sleep
Many studies indicate that normal sleep is a period of relative cardiovascular quiescence. Normally, blood pressure drops by about 5%–10% in sleep stages 1 and 2, and 10%–15% in deeper stages (N3). Heart rate and cardiac output often decline by 5%–10% during these non-rapid eye movement (NREM) stages as well. In REM sleep, blood pressure, heart rate, and cardiac output are typically higher, although more variable, than in NREM sleep [5, 6].
Studies have also demonstrated that sinus arrhythmia often develops during sleep. In one early study of sleep in 50 male medical students, nearly one third developed sinus pauses and three students developed Mobitz type I heart block. In addition, a study of young adults without organic heart disease documented episodes of sinus arrest lasting up to 9 s during REM sleep. Ventricular ectopy has also been found to occur in more than 50% of healthy subjects during normal sleep, despite an overall reduction compared to wakefulness [7, 8]. In summary, normal sleep is a period of relative cardiovascular quiescence and infrequent arrhythmias. Any sleep disorder that disrupts normal sleep may therefore increase the frequency of cardiovascular stress by reducing this period of quiescence and potentiating arrhythmogenesis.
OSA: Epidemiology, Pathophysiology and Diagnosis
Epidemiology
In the United States, the prevalence of OSA with daytime sleepiness is approximately 3%–7% in adult white men and 2%–5% for adult white women [9, 10••]. Disease prevalence is higher in older individuals, racial minorities, males, and overweight individuals [1]. Studies with large sample sizes have provided similar prevalence estimates for OSA in Australia, Europe, and Asia [11, 12].
Pathophysiology
OSA is characterized by obstructive apneas and hypopneas that are caused by repetitive collapse of the upper airway. These recurrent airflow reductions can lead to acute derangements in gas exchange and arousals from sleep. Patients who experience symptoms such as snoring, witnessed apneas, insomnia with frequent awakenings, or excessive daytime sleepiness should undergo diagnostic evaluation, especially if additional risk factors are present.
Diagnosis
OSA in adults is diagnosed based on the frequency of obstructive respiratory events—apneas, hypopneas, and respiratory effort-related arousals (RERAs)—during sleep as measured by overnight polysomnography [1]. OSA is defined as more than 15 of these obstructive respiratory events per hour of sleep (apnea-hypopnea index [AHI] >15/h) in an asymptomatic patient or more than five events per hour of sleep (AHI >5/h) in a patient with excessive daytime sleepiness. However, AHI is only a modest predictor of sleep apnea consequences, emphasizing the need for improved metrics of disease severity and predictors of its complications [13].
Acute Cardiovascular Consequences of OSA
Airway occlusion causing cycles of hypoxemia and carbon dioxide retention with catecholamine surges during respiratory efforts in OSA has multiple important acute cardiovascular consequences (Fig. 1).
Fig. 1.
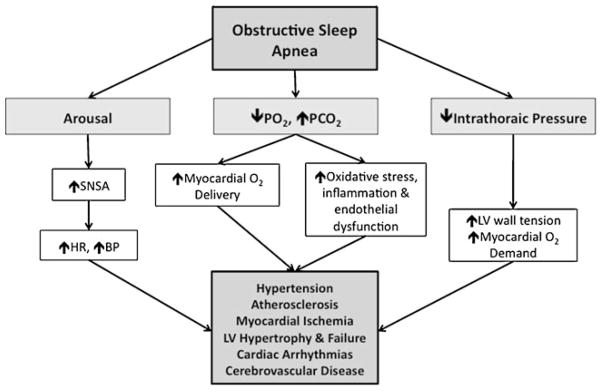
Effects of obstructive sleep apnea on the cardiovascular system. BP—blood pressure; HR—heart rate; PCO2—partial pressure of carbon dioxide; PO2—partial pressure of oxygen; SNSA—sympathetic nervous system activity
Intrathoracic Pressure Changes
The generation of higher-than-normal negative pleural and intrathoracic pressures when inspiring against a closed upper airway leads to a decrease in right atrial pressure and, therefore, an increase in venous return to the right side of the heart [14]. By ventricular interdependence, the increased right ventricular end-diastolic volume leads to a reduction in left ventricular stroke volume [15, 16]. Furthermore, the larger reductions in intrathoracic pressure increase ventricular transmural pressure, and therefore ventricular afterload [17].
Cardiovascular Variability
The cyclic hypoxemia caused by OSA results in pulmonary vasoconstriction, bradycardia, and decreased cardiac output, with regional cerebral and myocardial vasodilation to preserve oxygen delivery to these critical organs. Furthermore, cardiac contractility and diastolic relaxation may be directly impaired by cyclic hypoxemia in OSA [18, 19].
Sympathetic Activation
The cycles of hypoxemia and hypercapnia have effects on cardiac parasympathetic and sympathetic nervous activity. At apnea termination, asphyxia triggers a sudden arousal from sleep that increases sympathetic activity, which then causes surges in blood pressure and heart rate [20, 21]. These autonomic effects have been shown to be sustained after awakening [22].
Oxidative Stress, Inflammation, and Endothelial Dysfunction
Intermittent hypoxemia can induce free radical production and impair vascular endothelial function [23•, 24]. Endothelial dysfunction in OSA is the result of complex processes, including oxidation of lipoproteins, increased expression of adhesion molecules, increased monocyte adherence to endothelial cells, vascular smooth muscle proliferation, and increased platelet activation and aggregation [25].
Insulin Resistance
Evidence shows that undiagnosed OSA is highly prevalent in people with type 2 diabetes mellitus [26]. Furthermore, multiple linear regressions have found diabetes to be a significant independent predictor of OSA. Both increased sympathetic activity and sleep disruption, which are common in OSA, are also associated with insulin resistance and contribute to cardiovascular disease [27]. Various studies have shown an association between insulin resistance and OSA, independent of obesity [27]. More recently, a study by Aronsohn et al. [28••] found that in patients with type 2 diabetes, increasing severity of OSA is associated with worsening glucose control, even after controlling for the degree of adiposity and the number of diabetes medications. In this study, the adjusted mean glycated hemoglobin (HbA1C) was increased by 3.69% in patients with severe OSA.
Chronic Cardiovascular Effects of OSA and the Effect of Positive Airway Pressure Therapy
Hypertension
Prevalence and Pathophysiology
About 50% of OSA patients are hypertensive, and about 30% of hypertensive patients also have OSA [29]. Several epidemiologic studies, including the Wisconsin Sleep Cohort Study, have found that even after adjustment for confounding variables, systemic hypertension is more common among those with OSA than among those without (Table 1) [30]. Notably, some recent studies have failed to demonstrate a major association [31]; the reason for the conflicting results is unclear but may be due to a difference in baseline patient characteristics.
Table 1.
Summary of community-based epidemiologic studies that used polysomnography to investigate potential links between obstructive sleep apnea and cardiovascular disease
| Cross-sectional (prevalence)
|
Prospective (incidence)
|
|||
|---|---|---|---|---|
| Unadjusteda | Adjustedb | Unadjusteda | Adjustedb | |
| Hypertension | Yes [30] | Yes [30] | Yes [30]/No [31] | Yes [30]/No [31] |
| Dysglycemia | Yes [27] | Yes [27] | Yes | No |
| Coronary artery disease | Yes [42] | Yes [42] | Yes/No [63] | Yes/No [63] |
| Heart failure | Yes [42] | Yes [42] | NA | NA |
| Bradyarrythmias | No [52] | No [52] | NA | NA |
| Atrial fibrillation | Yes [52] | Yes [52] | NA | NA |
| Ventricular ectopy | Yes [52] | Yes [52] | NA | NA |
| Cerebrovascular disease | Yes [56] | Yes [56] | Yes [56] | Yes [64]/No [56] |
Findings of univariable analysis or multivariate analyses with partial adjustment
Findings of multivariate analyses in which adjustments have been made for all known confounding factors
NA no data available; No—available data do not support a significant association; Yes—available data support a significant association
(Adapted from Bradley TD, Floras JS: Obstructive sleep apnea and its cardiovascular consequences. Lancet 2009, 373:82–93; with permission from Elsevier. [65])
Logan et al. [32] noted that the prevalence of OSA in patients with drug-resistant hypertension was nearly 83% and speculated that resistant hypertension in OSA patients was likely due to mechanisms related to increased aldosterone production [32]. Similarly, patients with a blunted decline in nocturnal blood pressure have been found to be more likely to have co-existing OSA and an increase in all-cause mortality [33].
There is strong experimental evidence from animal studies that OSA contributes to hypertension through mechanisms such as intermittent hypoxemia, sympathetic activation, and alterations in the renin-angiotensin system [22, 34]. For instance, in an acute setting, OSA simulated in dogs caused an acute increase in blood pressure of nearly 20 mm Hg that persisted for several hours. A longer-term dog model found that mimicking repetitive nocturnal airway occlusion resulted in hypertension, during both sleep and wakefulness, which resolved on reversal of the OSA.
As a result of compelling epidemiologic and experimental evidence, the most recent Joint National Committee on the Detection and Management of Hypertension recognized OSA as an identifiable cause of hypertension [35].
Treatment of OSA: The Effect on Hypertension
Several randomized trials and meta-analyses have demonstrated that effective treatment of OSA with continuous positive airway pressure (CPAP) reduces daytime blood pressure, regardless of whether the patients were hypertensive at baseline [36]. In general, blood pressure reduction was modest in normotensive subjects and more evident in hypertensive patients. The largest of these studies, with 118 normotensive OSA patients, found a 24-h mean blood pressure reduction of 2.5 mm Hg with CPAP therapy compared to sham CPAP. Notably, in the subset of patients already taking antihypertensive medication, CPAP therapy resulted in a 24-h mean blood pressure reduction of more than 5 mm Hg, and a greater reduction was also seen in those with more severe OSA. Two other studies of OSA patients demonstrated that therapeutic CPAP was more effective in reducing both daytime and nocturnal blood pressure than either subtherapeutic CPAP or sham CPAP.
Notably, some randomized trials have not found significant blood pressure reduction with CPAP therapy for OSA. Campos-Rodriguez et al. [37] studied 68 OSA patients with medication-controlled hypertension and found that therapeutic CPAP had no further antihypertensive effect after 1 month. Robinson et al. [38] similarly found that after 1 month, therapeutic CPAP had no effect on blood pressure in 68 hypertensive patients with OSA. More recently, a study of 359 non-sleepy OSA patients with hypertension, demonstrated that after 1 year of treatment, CPAP was associated with a non-significant 1.89-mm Hg decrease in systolic blood pressure (P=0.065) but a significant 2.19-mm Hg decrease in diastolic blood pressure (P=0.0008) [39]. The most significant blood pressure reduction was observed in those patients who used CPAP for more than 5.6 h per night.
Three meta-analyses have been published on the effect of CPAP treatment on blood pressure in OSA patients. Overall, a significant reduction of approximately 2 mm Hg was found in two of the three studies. Notably, both the positive meta-analyses included studies with both normotensive and hypertensive patients.
In all, the preponderance of data suggest that treatment of OSA with CPAP therapy can lower blood pressure modestly, and that patients with uncontrolled hypertension and more severe OSA are likely to have the greatest benefit. In those hypertensive patients who cannot tolerate CPAP, oral appliances may also slightly lower diastolic blood pressure [40]. However, given that the blood pressure improvement with apnea therapy is modest as compared to antihypertensive medications, the motivation for sleep apnea therapy needs to extend beyond blood pressure alone [41].
Heart Failure
Prevalence and Pathophysiology
Cross-sectional data from the Sleep Heart Health Study, which included over 6,000 patients, found a 2.4-times increased likelihood of having OSA with underlying heart failure [42]. Several prospective and case series studies have also found a prevalence of OSA, ranging from 11%–37% in patients with systolic dysfunction [34, 43].
Several mechanisms have been studied regarding the role of OSA in the pathogenesis of heart failure: 1) increased sympathetic drive to the heart and kidney, 2) increased aldosterone secretion, 3) increased left ventricular afterload and wall stress, 4) increased risk of myocardial infarction, and 5) increased incidence of hypoxemia-induced pulmonary hypertension [44, 45].
Treatment of OSA: The Effect on Heart Failure
Kaneko et al. [46], in a study of 24 patients with ejection fraction (EF) <45% and OSA, found that over a period of 1 month CPAP increased mean EF by 9% and lowered morning systolic blood pressure and heart rate (Table 2). In another study of 40 OSA patients with systolic heart failure, Mansfield et al. [47] found that CPAP therapy led to a 5% increase in mean EF as well as improvements in quality-of-life and sleepiness indices. Conversely, a randomized trial of CPAP therapy in OSA patients with decreased systolic function by Smith et al. showed no effect on left ventricular EF; differences in patient characteristics likely explain these discordant findings. Recent observational data also show a trend toward lower mortality in patients with heart failure and OSA treated with CPAP, although randomized trial data examining hard outcomes are clearly required.
Table 2.
Summary of randomized trials of treatment for obstructive sleep apnea on cardiovascular outcomes in patients with heart failurea
| Treatment | Patients enrolled, n | Patients completing trial, n | Treatment period | Treatment outcomes | |
|---|---|---|---|---|---|
| Kaneko et al. [46] | Therapeutic CPAP vs no CPAP | 24 | 24 | 1 month | 9% increase in LVEF; 10-mm Hg decrease in systolic blood pressure; decrease in heart rate of four beats per minute |
| Mansfield et al. [47] | Therapeutic CPAP vs no CPAP | 55 | 40 | 3 months | 5% increase in LVEF; no change in blood pressure; decrease in nocturnal urinary concentration of norepinephrine; improved QOL |
| Usui et al.b | Therapeutic CPAP vs no CPAP | 17 | 17 | 1 month | 17% decrease in muscle SNA; 15-mm Hg decrease in awake systolic blood pressure |
| Gilman et al.b | Therapeutic CPAP vs no CPAP | 19 | 19 | 1 month | Increase in high-frequency HRV |
| Ryan et al.b | Therapeutic CPAP vs no CPAP | 18 | 18 | 1 month | 58% decrease in frequency of VPB during sleep |
| Egea et al.b | Therapeutic vs sham CPAP | 61 | 45 | 2 months | 2%–7% increase in LVEF; no change in blood pressure, QOL, or 6-MWT |
Only trials in which most patients had elevated blood pressure at enrollment are included
Overlap exists with some patients in these trials because these reports represent components of a larger clinical trial that was extended after the findings by Kaneko et al. [46] were reported
6-MWT 6-minute walk test distance; CPAP continuous positive airway pressure; HRV heart rate variability; LVEF left ventricular ejection fraction; QOL quality of life; SNA sympathetic nervous system activity; VPB ventricular premature beats
(Adapted from Bradley TD, Floras JS: Obstructive sleep apnea and its cardiovascular consequences. Lancet 2009, 373:82–93; with permission from Elsevier. [65])
Pulmonary Arterial Hypertension
Prevalence and Pathophysiology
OSA is associated with mild pulmonary arterial hypertension (PAH), most often when there is co-existing chronic lung or heart disease [48]. Two observational studies have illustrated this, demonstrating that among patients with OSA, cor pulmonale occurred predominantly in those who had daytime hypoxemia [48].
OSA likely contributes to the pathogenesis of PAH by cyclic hypoxemia, which reflexively increases pulmonary artery pressures. However, proof that OSA causes PAH has been limited in existing studies by a lack of control for co-morbidities such as obesity and other respiratory conditions. Nonetheless, the most recent clinical classification of PAH still identifies sleep-disordered breathing within the respiratory disorders associated with this disease.
Treatment of OSA: The Effect on PAH
Studies of OSA patients with PAH have demonstrated increased hypoxemia-induced pulmonary vascular reactivity, and CPAP has been shown to decrease this reactivity [48]. Furthermore, Arias et al. demonstrated that 12 weeks of effective CPAP therapy was associated with a significant 5-mm Hg reduction in echocardiographic measurement of pulmonary artery pressure, compared to sham CPAP in OSA patients. However, larger and longer-term randomized studies are still needed to establish the sustained effects of CPAP on PAH, right ventricular function, and mortality.
Acute Cardiovascular Events
Prevalence and Pathophysiology
The prevalence of sleep-disordered breathing in patients with coronary artery disease is about twofold greater than in patients without coronary artery disease. Studies have demonstrated that severe OSA was associated with an increased risk of fatal and non-fatal cardiac events, a finding confirmed by other recent studies [45]. Interestingly, a study by Gami et al. [49] found that more than half of sudden cardiac deaths in patients with OSA occur during sleep. Although OSA appears to increase the propensity for sudden cardiac death during sleep, more studies are needed to determine whether OSA increases the overall risk of sudden cardiac death.
Treatment of OSA: The Effect on Acute Cardiovascular Events
Several observational studies have shown that treatment of OSA with CPAP reduces the incidence of cardiovascular events. A recent prospective cohort study of 450 OSA patients followed over 6 years found that the treatment with CPAP was associated with a reduced likelihood of cardiovascular events [50]. However, randomized trials verifying these apparent benefits of OSA therapy are still lacking but are essential to draw conclusions, given that CPAP therapy may be a marker of a good prognosis [51•].
Cardiac Arrhythmias
Prevalence and Pathophysiology
Cardiac arrhythmias occur frequently in patients with OSA, as nocturnal arrhythmias may occur in up to 50% of OSA patients. An observational study of 566 patients found that the patients with sleep-disordered breathing had a high prevalence of atrial fibrillation, non-sustained ventricular tachycardia, and complex ventricular ectopy [52].
With regard to bradyarrhythmia, data from the European Multicenter Polysomnographic Study found that 60% of patients with pacemakers placed for dilated cardiomyopathy (29%), high-degree atrioventricular block (34%), and sinus node disease (37%) also had OSA [53]. OSA was also evident in 68% of patients with atrioventricular block. However, bradycardia is somewhat controversial because some studies show no important association with OSA [52].
The prevalence of atrial fibrillation in patients with OSA is low, although overall it is higher than in the general population. Recurrent atrial fibrillation and post-operative atrial fibrillation are more likely to occur in patients with OSA than those without.
Premature ventricular contractions are significantly more common in patients with OSA than those without. However, the mechanisms that cause ventricular arrhythmias in these patients are still unclear. In most patients with OSA, ventricular arrhythmias usually occur during the apneic periods, particularly when oxygen saturation falls below 60%. Therefore, hypoxemia and sympathetic activation induced by apneic events likely play important roles in the pathogenesis of ventricular ectopy.
Treatment of OSA: The Effect on Arrhythmias
CPAP therapy reduces the incidence of nocturnal ventricular asystole and bradycardia in OSA patients. In a study by Simantirakis et al., 23 patients with moderate to severe OSA had implantable loop recorders placed and followed after instituting CPAP therapy. Nocturnal ventricular asystole and bradycardia were noted in 47% of the patients prior to initiation of CPAP, both of which decreased by 8 weeks of therapy. Furthermore, there were no nocturnal arrhythmias detected during the last 6 months of treatment. Nasal CPAP has been associated with reduced recurrence risk of atrial fibrillation after cardioversion as compared to untreated OSA, although randomized trials are now ongoing [54, 55].
Stroke
Although there are substantial data implicating OSA in cardiovascular disease and hypertension, there is as of yet no definitive evidence that OSA is an independent risk factor for stroke [56]. A study by Bassetti and Aldrich found an AHI >10 in 62% of patients with a transient ischemic attack (TIA) compared with 12% of control subjects. However, these data were not confirmed in a subsequent case control study of TIA patients that found the likelihood of OSA in TIA and control subjects was similar. Several prospective observational studies have shown an increased incidence of stroke among OSA patients, although effects of apnea therapy were not assessed. Therefore, the relationship between stroke and OSA is still unclear. Studies have shown that stroke patients with OSA have decreased survival and limited rehabilitation success compared with those without OSA. Emerging data support the treatment of OSA following stroke, at least for functional outcomes.
Treatment Options in OSA
Positive Airway Pressure Therapy
For many years, the only treatment available to patients with OSA was surgical bypass of the obstructed airway (i.e., tracheostomy). This situation changed with the development of positive airway pressure therapy, which prevents pharyngeal collapse by maintaining increased airway pressure [57]. Although CPAP is not tolerated by all individuals, it remains the treatment of choice for OSA.
Oral Appliances
Customized dental appliances are designed to increase airway size and to facilitate airflow by advancing the mandible or tongue or lifting the soft palate. Although success with oral appliances often is difficult to predict, these devices may offer a viable treatment option in non-obese patients with micrognathia or retrognathia who have snoring or mild-to-moderate OSA, particularly those who do not tolerate CPAP therapy [40].
Surgical Treatment
Surgical options include procedures designed to increase upper airway size, procedures designed to bypass the upper airway, and procedures that promote weight loss. The latter two procedures have traditionally been reserved for the more severely affected patients. Even in the best situations, however, surgery is seldom as efficacious as treatment with CPAP. Most studies have shown that uvulopalatopharyngoplasty (UPPP) and tonsillectomy have a 50%–60% success rate for OSA, compared with over 95% with effective CPAP therapy. However, because adherence to CPAP therapy remains an issue for many patients, some have argued that even an incomplete response to UPPP may provide a better outcome compared to CPAP therapy among patients with poor or variable adherence [58, 59].
Behavior Modification
Lifestyle changes can be the most difficult changes to accomplish, but they can make a major impact on OSA severity. Weight loss is effective in reducing OSA severity in selected patients [60]. In a randomized study of obese patients with type II diabetes, weight loss resulted in significant improvements in OSA. Participants with a weight loss of 10 kg or more had the greatest reductions in OSA severity. Body repositioning to avoid sleeping in the supine position (the position with the greatest risk for upper airway collapse during sleep) can also be helpful in some cases [61].
Conclusions
Convincing data have revealed the deleterious effects of sleep-disordered breathing on cardiovascular health. There are still, however, gaps in scientific and clinical knowledge in this field. Randomized trials are needed to determine how best to reduce the cardiovascular risk attributed to OSA. To date, there have been no such trials evaluating the impact of treating OSA, either by CPAP or other modalities, on hospitalization rates and mortality. A better understanding of the mechanisms underlying cardiovascular complications of OSA will also contribute to better recognition and treatment of these cardiovascular issues. In addition, increased public awareness of OSA and its health impact will be as essential as increased awareness among health care providers. Moving forward, a multidisciplinary effort will be crucial to the recognition, treatment, and prevention of sleep-disordered breathing and its cardiovascular consequences.
Footnotes
Disclosure Puja Kohli reports no potential conflict of interest relevant to this article. Jay S. Balachandran has received a grant from the National Institutes of Health. Atul Malhotra has received grants from the National Institutes of Health, the American Heart Association, Sepracor, Cephalon, and Philips. He has also received consulting fees from Philips, Pfizer, Merck, SGS, SHC, Ethicon, Medtronic, Apnex, Apnicure, and Itamar.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360(9328):237–245. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 2.Caples SM, Gami AS, Somers VK. Obstructive sleep apnea. Annals of internal medicine. 2005;142(3):187–197. doi: 10.7326/0003-4819-142-3-200502010-00010. [DOI] [PubMed] [Google Scholar]
- 3.Rosthchild, et al. Production of the anginal syndrome by induced general anoxemia. American heart journal. 1933;8:729–744. [Google Scholar]
- 4.Barach, et al. The physiologic action of oxygen and carbon dioxide on the coronary circulation as shown by the blood gas and electrocardiogram studies. American heart journal. 1941;22:13–34. [Google Scholar]
- 5.Khatri IM, Freis ED. Hemodynamic changes during sleep. J Appl Physiol. 1967;22(5):867–873. doi: 10.1152/jappl.1967.22.5.867. [DOI] [PubMed] [Google Scholar]
- 6.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. The New England journal of medicine. 1993;328(5):303–307. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- 7.Guilleminault C, Pool P, Motta J, Gillis AM. Sinus arrest during REM sleep in young adults. The New England journal of medicine. 1984;311(16):1006–1010. doi: 10.1056/NEJM198410183111602. [DOI] [PubMed] [Google Scholar]
- 8.Pickering TG, Johnston J, Honour AJ. Comparison of the effects of sleep, exercise and autonomic drugs on ventricular extrasystoles, using ambulatory monitoring of electrocardiogram and electroencephalogram. The American journal of medicine. 1978;65(4):575–583. doi: 10.1016/0002-9343(78)90844-6. [DOI] [PubMed] [Google Scholar]
- 9.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proceedings of the American Thoracic Society. 2008;5(2):136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10••.Punjabi NM, Newman AB, Young TB, Resnick HE, Sanders MH. Sleep-disordered breathing and cardiovascular disease: an outcome-based definition of hypopneas. Am J Respir Crit Care Med. 2008;177(10):1150–1155. doi: 10.1164/rccm.200712-1884OC. This study investigated the criteria used to define breathing abnormalities during sleep. Many prior recommendations had been based on expert opinion rather than outcome data. These data provided evidence for the current definitions and initiated a discussion regarding the predictive value of various OSA definitions for predicting different outcome measures. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young T, Peppard P, Gottlieb D. The epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 12.Sharma SK, Ahluwalia G. Epidemiology of adult obstructive sleep apnoea syndrome in India. The Indian journal of medical research. 2010;131:171–175. [PubMed] [Google Scholar]
- 13.Gottlieb DJ, et al. Relation of sleepiness to respiratory disturbance index: the Sleep Heart Health Study. Am J Respir Crit Care Med. 1999;159(2):502–507. doi: 10.1164/ajrccm.159.2.9804051. [DOI] [PubMed] [Google Scholar]
- 14.Jellinek H, Krenn H, Oczenski W, Veit F, Schwarz S, Fitzgerald R. Influence of positive airway pressure on the pressure gradient for venous return in humans. J Appl Physiol. 2000;88:926–932. doi: 10.1152/jappl.2000.88.3.926. [DOI] [PubMed] [Google Scholar]
- 15.Fessler HE. Heart-lung interactions: applications in the critically ill. Eur Respir J. 1997;10(1):226–237. doi: 10.1183/09031936.97.10010226. [DOI] [PubMed] [Google Scholar]
- 16.Malhotra A, Muse V, Mark E. Clinical Pathological Case. New Engl J Med. 2002 in press. [Google Scholar]
- 17.Marrone O, Bellia V, Ferrara G, Milone F, Romano L, Salvaggio A, Stallone A, Bonsignore G. Transmural pressure measurements. Importance in the assessment of pulmonary hypertension in obstructive sleep apneas. Chest. 1989;95(2):338–342. doi: 10.1378/chest.95.2.338. [DOI] [PubMed] [Google Scholar]
- 18.Gomez A, Mink S. Interaction between effects of hypoxia and hypercapnia on altering left ventricular relaxation and chamber stiffness in dogs. Am Rev Respir Dis. 1992;146(2):313–320. doi: 10.1164/ajrccm/146.2.313. [DOI] [PubMed] [Google Scholar]
- 19.Kusuoka H, Weisfeldt ML, Zweier JL, Jacobus WE, Marban E. Mechanism of early contractile failure during hypoxia in intact ferret heart: evidence for modulation of maximal Ca2+-activated force by inorganic phosphate. Circulation research. 1986;59(3):270–282. doi: 10.1161/01.res.59.3.270. [DOI] [PubMed] [Google Scholar]
- 20.Horner RL, Brooks D, Kozar LF, Gan K, Phillipson EA. Respiratory-related heart rate variability persists during central apnea in dogs: mechanisms and implications. J Appl Physiol. 1995;78(6):2003–2013. doi: 10.1152/jappl.1995.78.6.2003. [DOI] [PubMed] [Google Scholar]
- 21.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. The Journal of clinical investigation. 1995;96(4):1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brooks D, Horner RL, Kozar LF, Render-Teixeira CL, Phillipson EA. Obstructive sleep apnea as a cause of systemic hypertension. Evidence from a canine model. Journal of Clinical Investigation. 1997;99(1):106–109. doi: 10.1172/JCI119120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Lavie L. Oxidative stress—a unifying paradigm in obstructive sleep apnea and comorbidities. Progress in cardiovascular diseases. 2009;51(4):303–312. doi: 10.1016/j.pcad.2008.08.003. This is an excellent review regarding the basic science underlying oxidative stress and how sleep apnea mechanisms, including hypoxemia with re-oxygenation, may contribute to oxidative stress. [DOI] [PubMed] [Google Scholar]
- 24.Atkeson A, Yeh SY, Malhotra A, Jelic S. Endothelial function in obstructive sleep apnea. Progress in cardiovascular diseases. 2009;51(5):351–362. doi: 10.1016/j.pcad.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahangdale S, Yim YehS, Novack V, Stevenson K, Barnard M, Furman M, Malhotra A. The influence of intermittent hypoxemia on platelet activation in obese obstructive sleep apnea patients. Journal of Clinical Sleep Medicine. in press. [PMC free article] [PubMed] [Google Scholar]
- 26.Foster GD, Sanders MH, Millman R, Zammit G, Borradaile KE, Newman AB, Wadden TA, Kelley D, Wing RR, Sunyer FX, Darcey V, Kuna ST. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes care. 2009;32(6):1017–1019. doi: 10.2337/dc08-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Punjabi N, Sorkin J, Katzel L, Goldberg A, Schwartz A, Smith P. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir and Crit Care Med. 2002;165:677–682. doi: 10.1164/ajrccm.165.5.2104087. [DOI] [PubMed] [Google Scholar]
- 28••.Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med. 2010;181(5):507–513. doi: 10.1164/rccm.200909-1423OC. This study shows the impact of sleep apnea on glycemic control in patients with diabetes. The data show worsening glycemic control with increasing sleep apnea severity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pimenta E, Calhoun DA, Oparil S. Sleep apnea, aldosterone, and resistant hypertension. Progress in cardiovascular diseases. 2009;51(5):371–380. doi: 10.1016/j.pcad.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Peppard P, Young T, Palta M, Skatrud J. Prospective study of the association between sleep disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 31.O’Connor GT, Caffo B, Newman AB, Quan SF, Rapoport DM, Redline S, Resnick HE, Samet J, Shahar E. Prospective study of sleep-disordered breathing and hypertension: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2009;179(12):1159–1164. doi: 10.1164/rccm.200712-1809OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Logan A, Tkacova R, Perlikowski S, Leung R, Tisler A, Floras J, Bradley T. Refractory hypertension and sleep apnoea: effect of CPAP on blood pressure and baroreflex. Europ Respir J. 2003;21:241–247. doi: 10.1183/09031936.03.00035402. [DOI] [PubMed] [Google Scholar]
- 33.Wright J, Johns R, Watt I, Melville A, Sheldon T. Health effects of obstructive sleep apnoea and the effectiveness of. Bmj. 1997;314 (7084):851–860. doi: 10.1136/bmj.314.7084.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leung RS, Bradley TD. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med. 2001;164(12):2147–2165. doi: 10.1164/ajrccm.164.12.2107045. [DOI] [PubMed] [Google Scholar]
- 35.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. Jama. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 36.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, Mullins R, Jenkinson C, Stradling JR, Davies RJ. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet. 2002;359(9302):204–210. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- 37.Campos-Rodriguez F, Grilo-Reina A, Perez-Ronchel J, Merino-Sanchez M, Gonzalez-Benitez MA, Beltran-Robles M, Almeida-Gonzalez C. Effect of continuous positive airway pressure on ambulatory BP in patients with sleep apnea and hypertension: a placebo-controlled trial. Chest. 2006;129(6):1459–1467. doi: 10.1378/chest.129.6.1459. [DOI] [PubMed] [Google Scholar]
- 38.Robinson GV, Smith DM, Langford BA, Davies RJ, Stradling JR. Continuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patients. Eur Respir J. 2006;27(6):1229–1235. doi: 10.1183/09031936.06.00062805. [DOI] [PubMed] [Google Scholar]
- 39.Barbe F, Duran-Cantolla J, Capote F, de la Pena M, Chiner E, Masa JF, Gonzalez M, Marin JM, Garcia-Rio F, de Atauri JD, Teran J, Mayos M, Monasterio C, del Campo F, Gomez S, de la Torre MS, Martinez M, Montserrat JM. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181(7):718–726. doi: 10.1164/rccm.200901-0050OC. [DOI] [PubMed] [Google Scholar]
- 40.Gotsopoulos H, Kelly JJ, Cistulli PA. Oral appliance therapy reduces blood pressure in obstructive sleep apnea: a randomized, controlled trial. Sleep. 2004;27(5):934–941. doi: 10.1093/sleep/27.5.934. [DOI] [PubMed] [Google Scholar]
- 41.Pepin JL, Tamisier R, Barone-Rochette G, Launois SH, Levy P, Baguet JP. Comparison of continuous positive airway pressure and valsartan in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;182(7):954–960. doi: 10.1164/rccm.200912-1803OC. [DOI] [PubMed] [Google Scholar]
- 42.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier Nieto F, O’Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163(1):19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 43.MacDonald M, Fang J, Pittman SD, White DP, Malhotra A. The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J Clin Sleep Med. 2008;4(1):38–42. [PMC free article] [PubMed] [Google Scholar]
- 44.Sajkov D, Cowie RJ, Thornton AT, Espinoza HA, McEvoy RD. Pulmonary hypertension and hypoxemia in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 1994;149(2 Pt 1):416–422. doi: 10.1164/ajrccm.149.2.8306039. [DOI] [PubMed] [Google Scholar]
- 45.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365 (9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 46.Kaneko Y, Floras J, Usui K, Plante J, Tkacova R, Kubo T, Ando S, Bradley T. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. New Engl J Med. 2003;348:1233–1241. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 47.Mansfield D, Gollogly N, Kaye D, Richardson M, Bergin P, Naughton M. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med. 2004;169:361–366. doi: 10.1164/rccm.200306-752OC. [DOI] [PubMed] [Google Scholar]
- 48.Sajkov D, McEvoy RD. Obstructive sleep apnea and pulmonary hypertension. Progress in cardiovascular diseases. 2009;51 (5):363–370. doi: 10.1016/j.pcad.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 49.Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. The New England journal of medicine. 2005;352(12):1206–1214. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 50.Buchner NJ, Sanner BM, Borgel J, Rump LC. Continuous positive airway pressure treatment of mild to moderate obstructive sleep apnea reduces cardiovascular risk. Am J Respir Crit Care Med. 2007;176(12):1274–1280. doi: 10.1164/rccm.200611-1588OC. [DOI] [PubMed] [Google Scholar]
- 51•.Platt AB, Kuna ST, Field SH, Chen Z, Gupta R, Roche DF, Christie JD, Asch DA. Adherence to sleep apnea therapy and use of lipid-lowering drugs: a study of the healthy-user effect. Chest. 2010;137(1):102–108. doi: 10.1378/chest.09-0842. This study shows that the use of statins is higher in patients adherent with CPAP as compared to patients non-adherent with CPAP. The findings provide reason for pause in the interpretation of observational studies in which CPAP adherence is associated with improved outcome and further they emphasize the need for randomized trials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mehra R, Benjamin EJ, Shahar E, Gottlieb DJ, Nawabit R, Kirchner HL, Sahadevan J, Redline S. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173(8):910–916. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garrigue S, Pepin JL, Defaye P, Murgatroyd F, Poezevara Y, Clementy J, Levy P. High prevalence of sleep apnea syndrome in patients with long-term pacing: the European Multicenter Polysomnographic Study. Circulation. 2007;115(13):1703–1709. doi: 10.1161/CIRCULATIONAHA.106.659706. [DOI] [PubMed] [Google Scholar]
- 54.Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T, Somers VK. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. Journal of the American College of Cardiology. 2007;49(5):565–571. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 55.Gami AS, Pressman G, Caples SM, Kanagala R, Gard JJ, Davison DE, Malouf JF, Ammash NM, Friedman PA, Somers VK. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110(4):364–367. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 56.Djonlagic I, Malhotra A. Risk of stroke from sleep apnea in men and women. Expert review of neurotherapeutics. 2010;10 (8):1267–1271. doi: 10.1586/ern.10.102. [DOI] [PubMed] [Google Scholar]
- 57.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1(8225):862–865. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 58.Kezirian EJ, Malhotra A, Goldberg AN, White DP. Changes in obstructive sleep apnea severity, biomarkers, and quality of life after multilevel surgery. Laryngoscope. 2010;120(7):1481–1488. doi: 10.1002/lary.20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weaver EM, Maynard C, Yueh B. Survival of veterans with sleep apnea: continuous positive airway pressure versus surgery. Otolaryngol Head Neck Surg. 2004;130(6):659–665. doi: 10.1016/j.otohns.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 60.Foster GD, Borradaile KE, Sanders MH, Millman R, Zammit G, Newman AB, Wadden TA, Kelley D, Wing RR, Pi-Sunyer FX, Reboussin D, Kuna ST. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med. 2009;169(17):1619–1626. doi: 10.1001/archinternmed.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oksenberg A, Silverberg DS, Arons E, Radwan H. Positional vs nonpositional obstructive sleep apnea patients: anthropomorphic, nocturnal polysomnographic, and multiple sleep latency test data. Chest. 1997;112(3):629–639. doi: 10.1378/chest.112.3.629. [DOI] [PubMed] [Google Scholar]
- 62.Yaggi H Klar, MD, MPH, Concato John, MD, MPH, Kernan Walter N, MD, Lichtman Judith H, PhD, MPH, Brass Lawrence M, MD, Mohsenin Vahid., MD Obstructive Sleep Apnea as a Risk Factor for Stroke and Death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 63.Gottlieb DJ, Yenokyan G, Newman AB, O’Connor GT, Punjabi NM, Quan SF, Redline S, Resnick HE, Tong EK, Diener-West M, Shahar E. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122(4):352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Redline Susan, Yenokyan Gayane, Gottlieb Daniel J, Shahar Eyal, O’Connor George T, Resnick Helaine E, Diener-West Marie, Sanders Mark H, Wolf Philip A, Geraghty Estella M, Ali Tauqeer, Lebowitz Michael, Naresh M. Obstructive Sleep Apnea–Hypopnea and Incident Stroke: The Sleep Heart Health Study Punjabi. American Journal of Respiratory and Critical Care Medicine. 2010;182:269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bradley TD, Floras JS. Obstructive sleep apnea and its cardiovascular consequences. Lancet. 2009;373:82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]


