Abstract
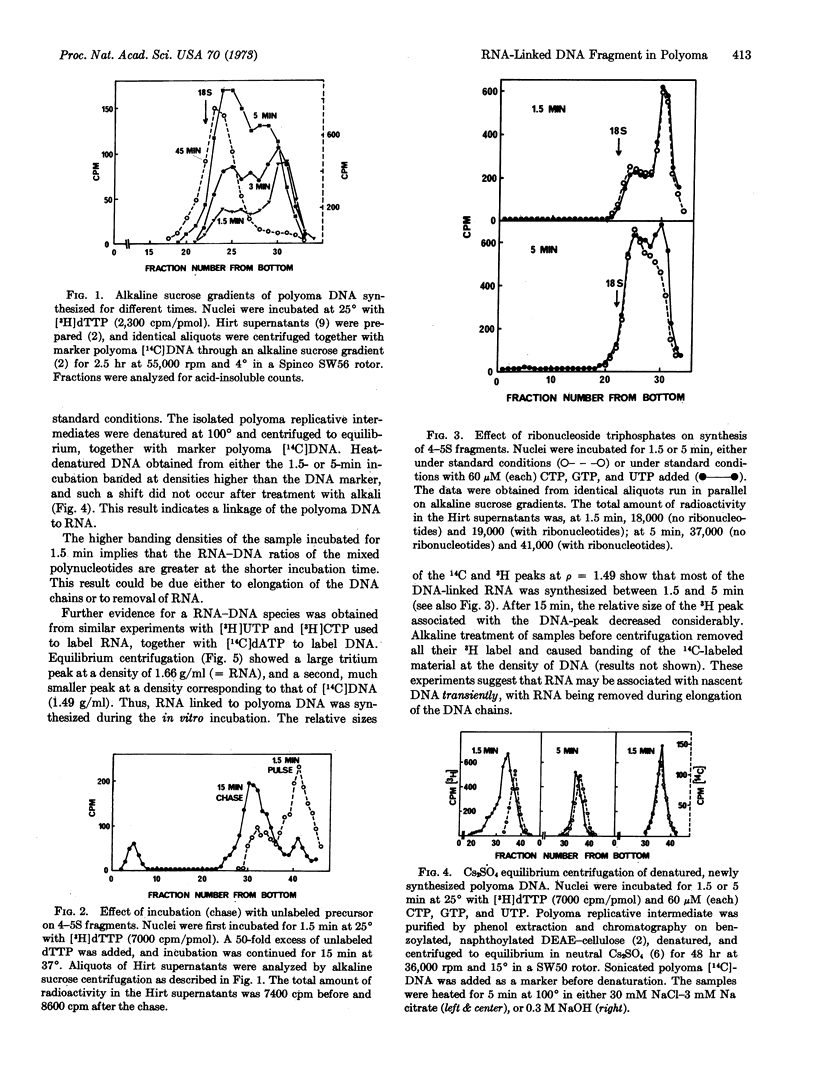
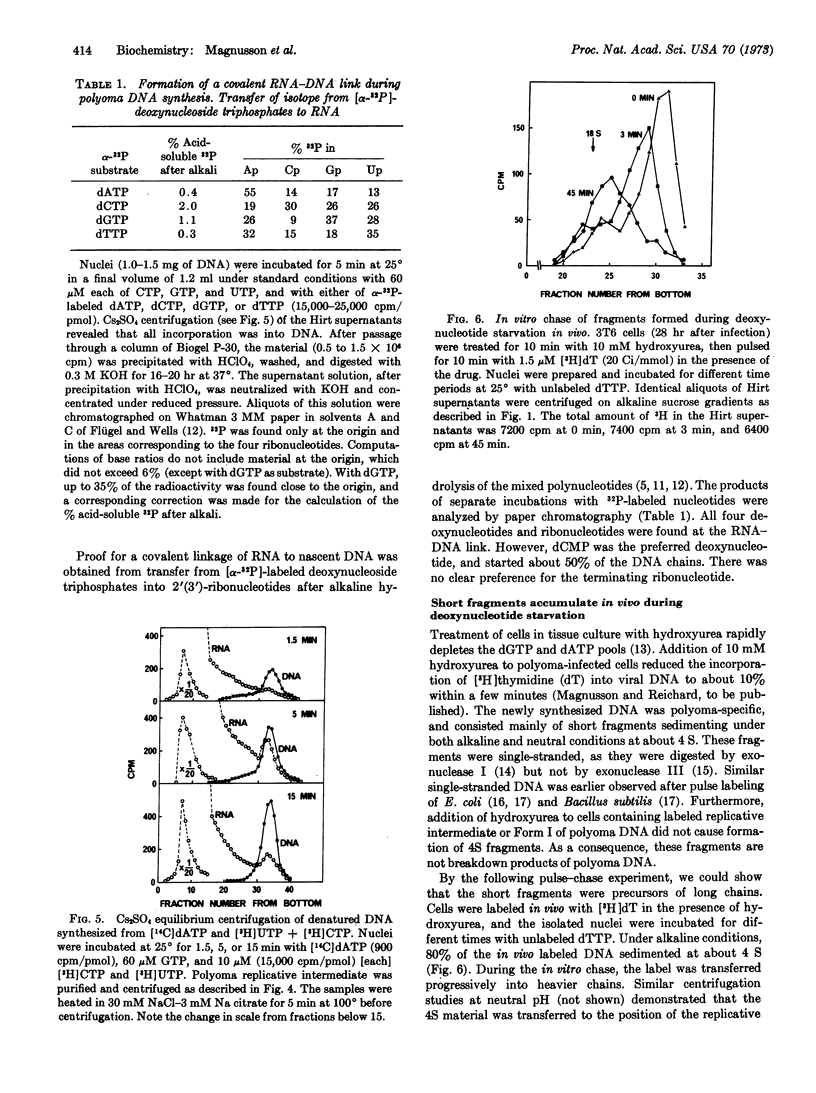
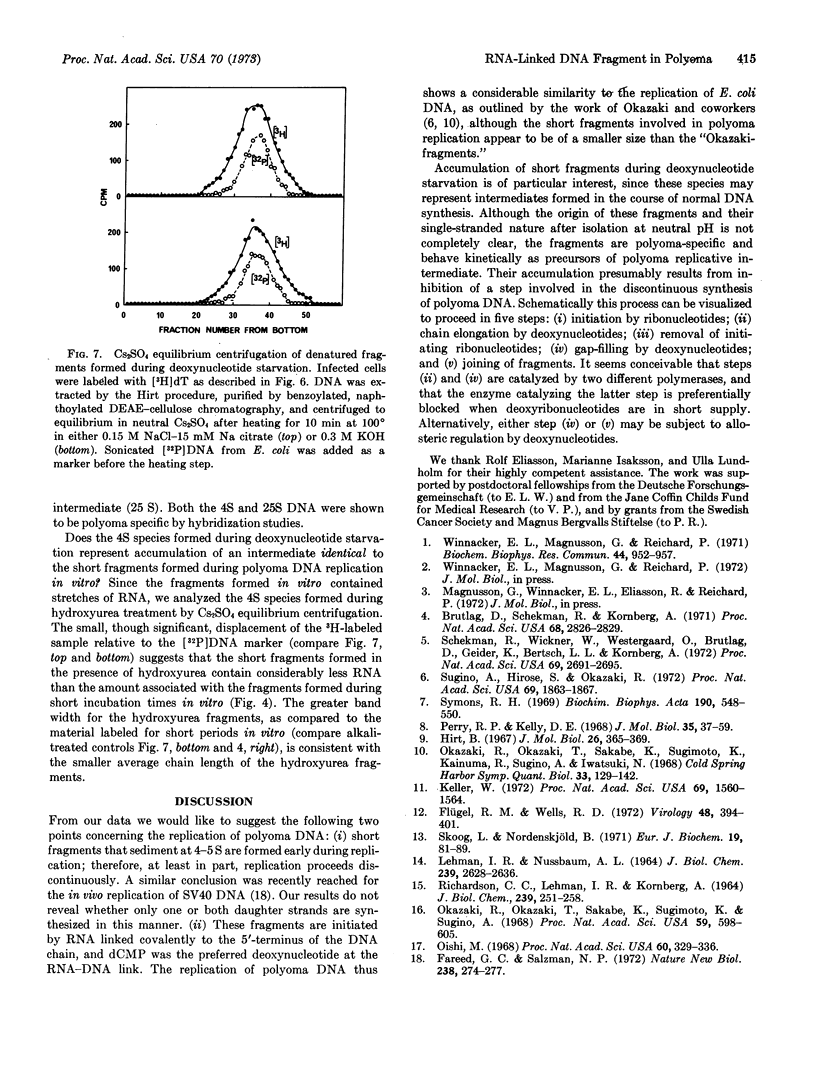
During in vitro incubation, nuclei from polyoma-infected cells elongate the daughter strands of the replicative intermediate of polyoma DNA. This process is now shown to involve the transient formation of short fragments (4-5 S), a process that is stimulated by the addition of ribonucleoside triphosphates. The presence of stretches of RNA at the 5′-end of short DNA chains was determined from Cs2SO4 equilibrium centrifugation and from the finding that isotope from α-32P-labeled deoxynucleoside triphosphates was recovered in 2′(3′)-ribonucleotides after alkaline hydrolysis. Transfer occurred preferentially with [α-32P]dCTP as substrate. Starvation for deoxynucleotides by in vivo treatment with hydroxyurea resulted in the accumulation of short fragments that are deficient in RNA. Our results suggest that a late step during the discontinuous synthesis of polyoma DNA is selectively inhibited when deoxynucleotides are in short supply.
Keywords: isolated nuclei, discontinuous synthesis, deoxynucleotide starvation, hydroxyurea
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brutlag D., Schekman R., Kornberg A. A possible role for RNA polymerase in the initiation of M13 DNA synthesis. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2826–2829. doi: 10.1073/pnas.68.11.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed G. C., Salzman N. P. Intermediate in SV40 DNA chain growth. Nat New Biol. 1972 Aug 30;238(87):274–277. doi: 10.1038/newbio238274a0. [DOI] [PubMed] [Google Scholar]
- Flügel R. M., Wells R. D. Nucleotides at the RNA-DNA covalent bonds formed in the endogenous reaction by the avian myeloblastosis virus DNA polymerase. Virology. 1972 May;48(2):394–401. doi: 10.1016/0042-6822(72)90050-5. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Keller W. RNA-primed DNA synthesis in vitro. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1560–1564. doi: 10.1073/pnas.69.6.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEHMAN I. R., NUSSBAUM A. L. THE DEOXYRIBONUCLEASES OF ESCHERICHIA COLI. V. ON THE SPECIFICITY OF EXONUCLEASE I (PHOSPHODIESTERASE). J Biol Chem. 1964 Aug;239:2628–2636. [PubMed] [Google Scholar]
- Oishi M. Studies of DNA replication in vivo. I. Isolation of the first intermediate of DNA replication in bacteria as single-stranded DNA. Proc Natl Acad Sci U S A. 1968 May;60(1):329–336. doi: 10.1073/pnas.60.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki R., Okazaki T., Sakabe K., Sugimoto K., Sugino A. Mechanism of DNA chain growth. I. Possible discontinuity and unusual secondary structure of newly synthesized chains. Proc Natl Acad Sci U S A. 1968 Feb;59(2):598–605. doi: 10.1073/pnas.59.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Messenger RNA-protein complexes and newly synthesized ribosomal subunits: analysis of free particles and components of polyribosomes. J Mol Biol. 1968 Jul 14;35(1):37–59. doi: 10.1016/s0022-2836(68)80035-x. [DOI] [PubMed] [Google Scholar]
- RICHARDSON C. C., LEHMAN I. R., KORNBERG A. A DEOXYRIBONUCLEIC ACID PHOSPHATASE-EXONUCLEASE FROM ESCHERICHIA COLI. II. CHARACTERIZATION OF THE EXONUCLEASE ACTIVITY. J Biol Chem. 1964 Jan;239:251–258. [PubMed] [Google Scholar]
- Schekman R., Wickner W., Westergaard O., Brutlag D., Geider K., Bertsch L. L., Kornberg A. Initiation of DNA synthesis: synthesis of phiX174 replicative form requires RNA synthesis resistant to rifampicin. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2691–2695. doi: 10.1073/pnas.69.9.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog L., Nordenskjöld B. Effects of hydroxyurea and 1-beta-D-arabinofuranosyl-cytosine on deoxyribonucleotide pools in mouse embryo cells. Eur J Biochem. 1971 Mar 1;19(1):81–89. doi: 10.1111/j.1432-1033.1971.tb01290.x. [DOI] [PubMed] [Google Scholar]
- Sugino A., Hirose S., Okazaki R. RNA-linked nascent DNA fragments in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1863–1867. doi: 10.1073/pnas.69.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons R. H. Preparation of [alpha-32P]nucleoside and deoxynucleoside 5'-triphosphates from 32Pi and protected and unprotected nucleosides. Biochim Biophys Acta. 1969 Oct 22;190(2):548–550. doi: 10.1016/0005-2787(69)90105-1. [DOI] [PubMed] [Google Scholar]
- Winnacker E. L., Magnusson G., Reichard P. Synthesis of polyoma DNA by isolated nuclei. Biochem Biophys Res Commun. 1971 Aug 20;44(4):952–957. doi: 10.1016/0006-291x(71)90804-7. [DOI] [PubMed] [Google Scholar]


