Abstract
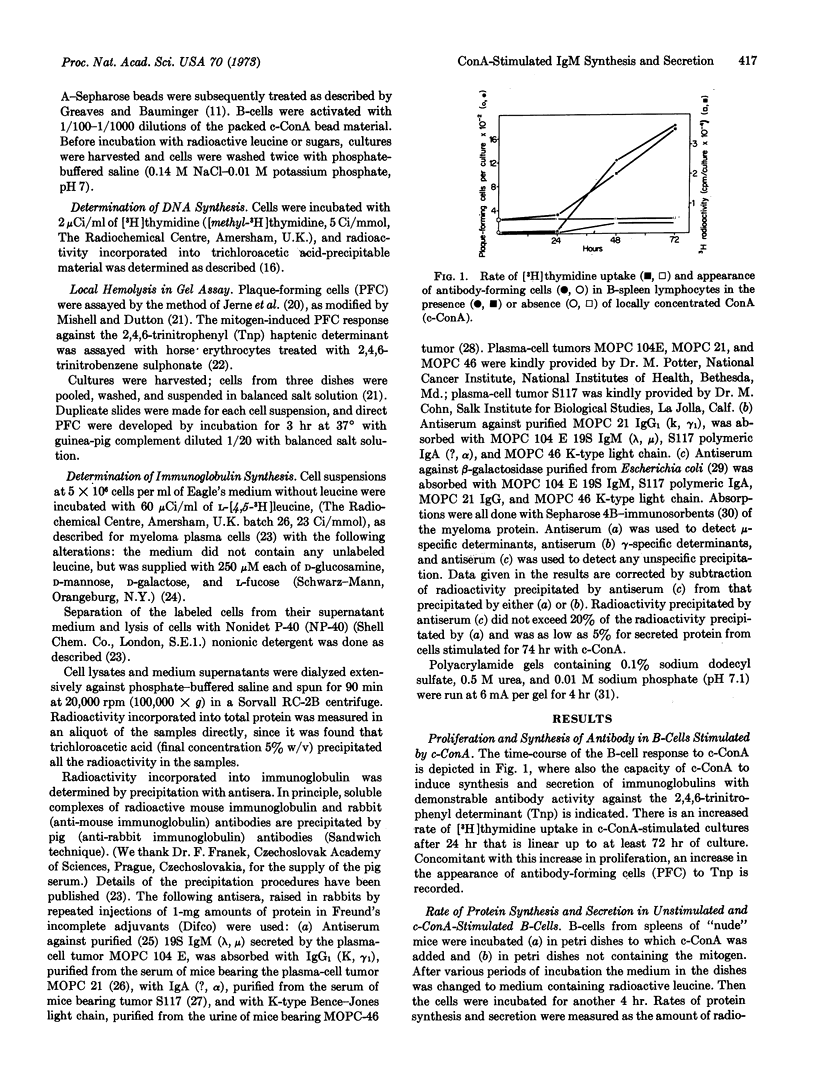
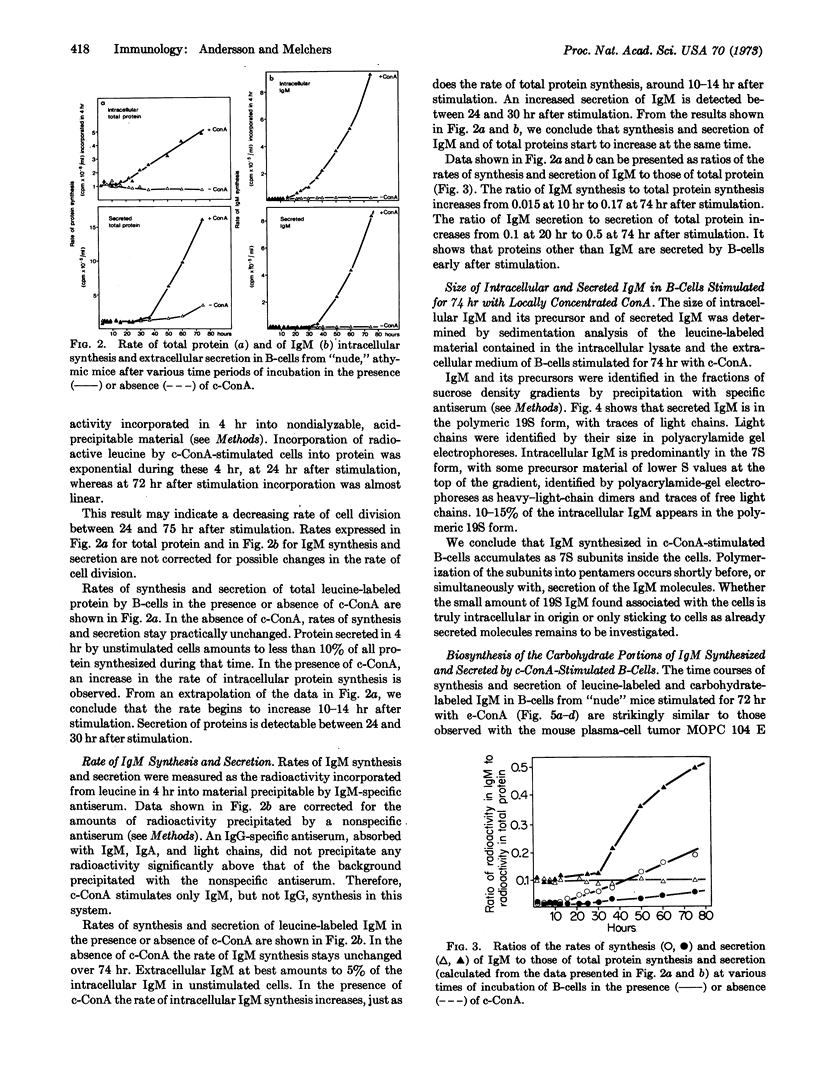
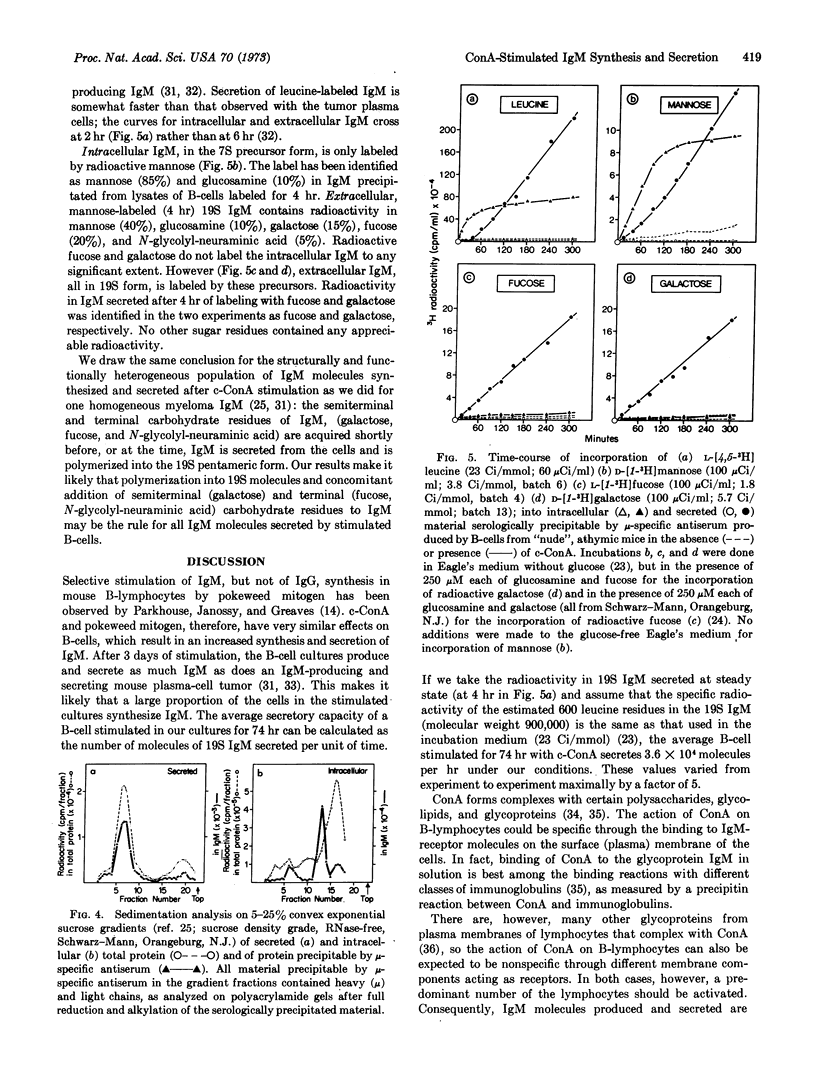
Stimulation of bone-marrow-derived lymphocytes by locally concentrated concanavalin A involves first an increased intracellular synthesis of total protein and of immunoglobulin M at 10-14 hr, followed by the initiation of secretion of protein and of IgM at 24-30 hr after stimulation. Differentiation into immunoglobulin-producing and -secreting cells after stimulation is manifested by (a) a rate of IgM synthesis that increases continuously over total protein synthesis, and (b) an increased rate of IgM secretion with concomitant decrease of secretion of other proteins. Within stimulated bone-marrow-derived cells, IgM molecules were found mainly in their 7S form, together with some light-μ-heavy-chain precursors. Small amounts of polymerized 19S IgM are found associated with the cells. Only fully assembled 19S IgM was found secreted into the extracellular medium. Polymerization into the pentamer therefore takes place shortly before, or simultaneously with, secretion. Intracellular 7S IgM contains only glucosamine and mannose residues, with traces of fucose and galactose residues. Secreted 19S IgM has the full complement of carbohydrates with glucosamine, mannose, fucose, galactose, and N-glycolylneuraminic acid residues. During polymerization and secretion from the cells, IgM molecules therefore acquire all of the semiterminal and terminal residues of their carbohydrate groups.
Keywords: mitogens, differentiation of lymphocytes, lymphocyte stimulation, polymerization of IgM, carbohydrate attachment to IgM
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ada G. L. Antigen binding cells in tolerance and immunity. Transplant Rev. 1970;5:105–129. doi: 10.1111/j.1600-065x.1970.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Andersson J., Edelman G. M., Möller G., Sjöberg O. Activation of B lymphocytes by locally concentrated concanavalin A. Eur J Immunol. 1972 Jun;2(3):233–235. doi: 10.1002/eji.1830020307. [DOI] [PubMed] [Google Scholar]
- Andersson J., Möller G., Sjöberg O. Selective induction of DNA synthesis in T and B lymphocytes. Cell Immunol. 1972 Aug;4(4):381–393. doi: 10.1016/0008-8749(72)90040-8. [DOI] [PubMed] [Google Scholar]
- Andersson J., Sjöberg O., Möller G. Induction of immunoglobulin and antibody synthesis in vitro by lipopolysaccharides. Eur J Immunol. 1972 Aug;2(4):349–353. doi: 10.1002/eji.1830020410. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., MERRICK J. M. PROTEIN-CARBOHYDRATE INTERACTION. I. THE INTERACTION OF POLYSACCHARIDES WITH CONCANAVALIN A. Biochim Biophys Acta. 1965 Jan 4;97:68–76. doi: 10.1016/0304-4165(65)90270-9. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Bauminger S. Activation of T and B lymphocytes by insoluble phytomitogens. Nat New Biol. 1972 Jan 19;235(55):67–70. doi: 10.1038/newbio235067a0. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Möller E. Studies on antigen-binding cells. I. The origin of reactive cells. Cell Immunol. 1970 Oct;1(4):372–385. doi: 10.1016/0008-8749(70)90015-8. [DOI] [PubMed] [Google Scholar]
- Knopf P. M., Parkhouse R. M., Lennox E. S. Biosynthetic units of an immunoglobulin heavy chain. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2288–2295. doi: 10.1073/pnas.58.6.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon M. A. Concanavalin A reaction with human normal immunoglobulin G and myeloma immunoglobulin G. Science. 1967 Dec 8;158(3806):1325–1326. doi: 10.1126/science.158.3806.1325. [DOI] [PubMed] [Google Scholar]
- Loor F., Forni L., Pernis B. The dynamic state of the lymphocyte membrane. Factors affecting the distribution and turnover of surface immunoglobulins. Eur J Immunol. 1972 Jun;2(3):203–212. doi: 10.1002/eji.1830020304. [DOI] [PubMed] [Google Scholar]
- Melchers F. Biosynthesis of the carbohydrate portion of immunoglobulins. Incorporation of radioactive fucose into immunoglobulin G1 synthesized and secreted by mouse plasma-cell tumour MOPC 21. Biochem J. 1971 Nov;125(1):241–247. doi: 10.1042/bj1250241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers F. Biosynthesis of the carbohydrate portion of immunoglobulins. Kinetics of synthesis and secretion of [3H] leucine-, [3H] galactose- and [3H] mannose-labelled myeloma protein by two plasma-cell tumours. Biochem J. 1970 Oct;119(4):765–772. doi: 10.1042/bj1190765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers F. Difference in carbohydrate composition and a possible conformational difference between intracellular and extracellular immunoglobulin M. Biochemistry. 1972 May 23;11(11):2204–2208. doi: 10.1021/bi00761a031. [DOI] [PubMed] [Google Scholar]
- Melchers F., Lennox E. S., Facon M. A carbohydrate-containing mouse light chain-protein. Biochem Biophys Res Commun. 1966 Jul 20;24(2):244–251. doi: 10.1016/0006-291x(66)90727-3. [DOI] [PubMed] [Google Scholar]
- Melchers F., Sela M. Search for antibodies against the carbohydrate portion of two mouse myeloma proteins. Immunochemistry. 1970 Sep;7(9):749–754. doi: 10.1016/0019-2791(70)90217-x. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Thymus and antigen-reactive cells. Transplant Rev. 1969;1:3–42. doi: 10.1111/j.1600-065x.1969.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller G. Immunocyte triggering. Cell Immunol. 1970 Dec;1(6):573–582. doi: 10.1016/0008-8749(70)90023-7. [DOI] [PubMed] [Google Scholar]
- Oudin J., Michel M. Idiotypy of rabbit antibodies. II. Comparison of idiotypy of various kinds of antibodies formed in the same rabbits against Salmonella typhi. J Exp Med. 1969 Sep 1;130(3):619–642. doi: 10.1084/jem.130.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelouris E. M. Absence of thymus in a mouse mutant. Nature. 1968 Jan 27;217(5126):370–371. doi: 10.1038/217370a0. [DOI] [PubMed] [Google Scholar]
- Parkhouse R. M., Askonas B. A. Immunoglobulin M biosynthesis. Intracellular accumulation of 7S subunits. Biochem J. 1969 Nov;115(2):163–169. doi: 10.1042/bj1150163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhouse R. M. Immunoglobulin M biosynthesis. Production of intermediates and excess of light-chain in mouse myeloma MOPC 104E. Biochem J. 1971 Jul;123(4):635–641. doi: 10.1042/bj1230635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhouse R. M., Janossy G., Greaves M. F. Selective stimulation of IgM synthesis in mouse B lymphocytes by pokeweed mitogen. Nat New Biol. 1972 Jan 5;235(53):21–23. doi: 10.1038/newbio235021a0. [DOI] [PubMed] [Google Scholar]
- Parkhouse R. M., Melchers F. Biosynthesis of the carbohydrate portions of immunoglobulin M. Biochem J. 1971 Nov;125(1):235–240. doi: 10.1042/bj1250235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernis B., Forni L., Amante L. Immunoglobulins as cell receptors. Ann N Y Acad Sci. 1971 Dec 31;190:420–431. doi: 10.1111/j.1749-6632.1971.tb13552.x. [DOI] [PubMed] [Google Scholar]
- Porath J., Axen R., Ernback S. Chemical coupling of proteins to agarose. Nature. 1967 Sep 30;215(5109):1491–1492. doi: 10.1038/2151491a0. [DOI] [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Vicari G., Sher A., Cohn M., Kabat E. A. Immunochemical studies on a mouse myeloma protein with specificity for certain beta-linked terminal residues of N-acetyl-D-glucosamine. Immunochemistry. 1970 Oct;7(10):829–838. doi: 10.1016/0019-2791(70)90059-5. [DOI] [PubMed] [Google Scholar]


