Abstract
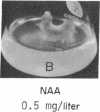
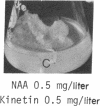
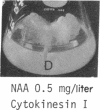
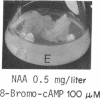
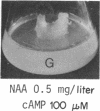
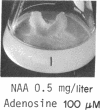
8-Bromoadenosine 3′:5′-cyclic monophosphate, when used in association with an auxin, can completely replace the cell-division-promoting activity of either a cytokinesin or a 6-substituted adenylyl cytokinin in excised tobacco pith parenchyma tissue. The 8-bromo derivative of adenosine 3′:5′-cyclic monophosphate was found to be far more resistant to degradation by plant adenosine 3′:5′-cyclic monophosphate phosphodiesterases than was adenosine 3′:5′-cyclic monophosphate. These findings appear to provide further support for the suggestion made earlier that the cytokinesins, which are potent inhibitors of both plant and animal adenosine 3′:5′-cyclic monophosphate phosphodiesterases, exert their cell-division-promoting effects as regulators of adenosine 3′:5′-cyclic monophosphate.
Keywords: auxins, phosphodiesterase, cytokinesins, cytokinins
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAUN A. C., NAF U. A non-auxinic growth-promoting factor present in crown gall tumor tissue. Proc Soc Exp Biol Med. 1954 Jun;86(2):212–214. doi: 10.3181/00379727-86-21052. [DOI] [PubMed] [Google Scholar]
- BRAUN A. C. The activation of two growth-substance systems accompanying the conversion of normal to tumor cells in crown gall. Cancer Res. 1956 Jan;16(1):53–56. [PubMed] [Google Scholar]
- DAS N. K., PATAU K., SKOOG F. Autoradiographic and microspectrophotometric studies of DNA synthesis in excised tobacco pith tissue. Chromosoma. 1958;9(7):606–617. doi: 10.1007/BF02568095. [DOI] [PubMed] [Google Scholar]
- Ikehara M., Uesugi S. Studies on nucleosides and nucleotides. 38. Synthesis of 8-bromoadenosine nucleotides. Chem Pharm Bull (Tokyo) 1969 Feb;17(2):348–354. doi: 10.1248/cpb.17.348. [DOI] [PubMed] [Google Scholar]
- Muneyama K., Bauer R. J., Shuman D. A., Robins R. K., Simon L. N. Chemical synthesis and biological activity of 8-substituted adenosine 3',5'-cyclic monophosphate derivatives. Biochemistry. 1971 Jun 8;10(12):2390–2395. doi: 10.1021/bi00788a033. [DOI] [PubMed] [Google Scholar]
- NEWCOMB E. H. Effect of auxin on ascorbic oxidase activity in tobacco pith cells. Proc Soc Exp Biol Med. 1951 Mar;76(3):504–509. doi: 10.3181/00379727-76-18538. [DOI] [PubMed] [Google Scholar]
- Shodell M. Environmental stimuli in the progression of BHK-21 cells through the cell cycle. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1455–1459. doi: 10.1073/pnas.69.6.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A. L., Kipnis D. M., Utiger R., Parker C. Radioimmunoassay for the measurement of adenosine 3',5'-cyclic phosphate. Proc Natl Acad Sci U S A. 1969 Sep;64(1):367–373. doi: 10.1073/pnas.64.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood H. N., Lin M. C., Braun A. C. The inhibition of plant and animal adenosine 3':5'-cyclic monophosphate phosphodiesterases by a cell-division-promoting substance from tissues of higher plant species. Proc Natl Acad Sci U S A. 1972 Feb;69(2):403–406. doi: 10.1073/pnas.69.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood H. N. Revised identification of the chromophore of a cell division factor from crown gall tumor cells of Vinca rosea L. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1283–1287. doi: 10.1073/pnas.67.3.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]