Abstract
Emerging data indicate that adults with binge eating may exhibit an attentional bias toward highly palatable foods, which may promote obesogenic eating patterns and excess weight gain. However, it is unknown to what extent youth with loss of control (LOC) eating display a similar bias. We therefore studied 76 youth (14.5±2.3y; 86.8% female; BMI-z 1.7± .73) with (n=47) and without (n=29) reported LOC eating. Following a breakfast to reduce hunger, youth participated in a computerized visual probe task of sustained attention that assessed reaction time to pairs of pictures consisting of high palatable foods, low palatable foods, and neutral household objects. Although sustained attentional bias did not differ by LOC eating presence and was unrelated to body weight, a two-way interaction between BMI-z and LOC eating was observed (p = .01), such that only among youth with LOC eating, attentional bias toward high palatable foods versus neutral objects was positively associated with BMI-z. These findings suggest that LOC eating and body weight interact in their association with attentional bias to highly palatable foods cues, and may partially explain the mixed literature linking attentional bias to food cues with excess body weight.
Keywords: attentional bias, loss of control eating, binge eating, obesity, visual probe task
One factor promoting obesity may be an attentional bias to food cues that leads to excess energy intake. An attentional bias to food cues is a biased processing of food-related stimuli, which may result from a heightened salience of food cues in the environment (Berridge, 2009; Nijs & Franken, 2012). In line with incentive-sensitization theory (Robinson & Berridge, 1993), the repeated exposure of a rewarding stimulus produces an exacerbated reward response in susceptible individuals. As a result, increased salience and strong motivational properties are established for the stimulus (Robinson & Berridge, 1993). As the incentive salience of palatable food cues increases, seeking out and consuming palatable foods becomes an important goal, exceeding homeostatic feeding drives (Berridge, 2009). It has been proposed that an attentional bias to palatable food cues represents a vulnerability to overeat in the current obesogenic food environment, consequently promoting or maintaining obesity (Braet & Crombez, 2003; Castellanos et al., 2009; Nijs & Franken, 2012; Nijs, Muris, Euser, & Franken, 2010).
Some (Werthmann et al., 2011), but not all (Graham, Hoover, Ceballos, & Komogortsev, 2011; Loeber et al., 2012), data suggest that overweight and obese adults demonstrate an approach-avoidance pattern to palatable food cues, indicated by increased automatic orientation to palatable food cues followed by decreased sustained attention. This pattern may reflect the competing influences of enhanced salience of food stimuli and attempts to control behavioral responses through avoidance (Nijs & Franken, 2012). Inconsistent results in overweight samples may be due to the heterogeneous etiology of obesity, highlighting the importance of identifying specific phenotypes within individuals prone to obesity (A. E. Field, Camargo, & Ogino, 2013). Indeed, data in adults suggest that attentional bias to food cues is associated with state characteristics such as hunger level and negative affect (Hepworth, Mogg, Brignell, & Bradley, 2010; Loeber, Grosshans, Herpertz, Kiefer, & Herpertz, 2013; Nijs et al., 2010; Tapper, Pothos, & Lawrence, 2010), as well as stable traits such as an increased tendency toward external eating and food-specific cravings, impulsivity, and reward drive (Brignell, Griffiths, Bradley, & Mogg, 2009; Hou et al., 2011; Newman, O’Connor, & Conner, 2008; Tapper et al., 2010; Werthmann, Roefs, Nederkoorn, & Jansen, 2013). Notably, many of these characteristics are reported by adults with binge eating disorder (BED; Filbey, Myers, & Dewitt, 2012; Pinaquy, Chabrol, Simon, Louvet, & Barbe, 2002; Schag, Schönleber, Teufel, Zipfel, & Giel, 2013; Schag, Teufel, et al., 2013), which is robustly associated with obesity (Blomquist et al., 2011; Yanovski, 2003a).
Several studies have examined the cognitive processing of food cues in adults with BED (Balodis et al., 2013; Chamberlain et al., 2012; Mobbs, Iglesias, Golay, & Van der Linden, 2011; Schmitz, Naumann, Trentowska, & Svaldi, 2014; Svaldi et al., 2014; Svaldi, Tuschen-Caffier, Peyk, & Blechert, 2010). One study directly examined attentional bias to food cues using electroencephalography (EEG) recordings in overweight individuals with BED compared to healthy controls (Svaldi et al., 2010). Differences emerged when viewing high palatable food only; women with BED demonstrated an EEG response pattern which suggested that palatable food stimuli consumed greater attentional resources (Svaldi et al., 2010). However, these findings were potentially confounded because BMI was not accounted for in the analyses (Svaldi et al., 2010). A second study examined attentional bias to food cues using two cognitive tasks (a clarification task and a spatial cueing paradigm) in adults with BED compared to a weight-matched control group (Schmitz et al., 2014). Results suggested that individuals with BED demonstrated an increased automatic orientation bias towards food cues compared to the control group; however, both groups demonstrated a bias in sustained attention toward food cues (Schmitz et al., 2014). These two studies provide preliminary evidence that adults with BED have differential attentional bias to food cues compared to adults without the disorder.
There are limited data on attentional biases and obesity in youth. One study in adolescents found that attentional bias to food cues, as measured by neural activity in reward and attentional processing regions during an fMRI scan, was not only positively correlated with BMI cross-sectionally, but also predicted increased BMI percentile gain one year later (Yokum, Ng, & Stice, 2011). However, no known study has directly examined the relationship of attentional bias to food cues and obesity in youth using a visual probe task. While a variety of attention bias paradigms exist, many are indirect measures, such as the modified Stroop task. Instead, the visual probe task is advantageous because varying the stimulus duration allows for the differentiation between automatic orientation and sustained attention (M. Field & Cox, 2008). Additionally, we opted for this paradigm because many attention bias modification interventions are adaptations of the visual probe task (e.g. Boutelle, Kuckertz, Carlson, & Amir, 2014; Eldar et al., 2012).
While children typically do not meet full criteria for BED, reports of episodes of loss of control (LOC) eating are common, particularly among those prone to excess weight (Shomaker, Tanofsky-Kraff, & Yanovski, 2011; Tanofsky-Kraff, Marcus, Yanovski, & Yanovski, 2008). For youth, the subjective experience of LOC may be a more salient indicator than episode size when describing aberrant eating (Goldschmidt et al., 2008; Shomaker et al., 2010). The presence of LOC eating places youth at high risk for excessive weight and fat gain (Tanofsky-Kraff et al., 2006; Tanofsky-Kraff, Yanovski, et al., 2009) and the development of partial or full-syndrome BED (Hilbert, Hartmann, Czaja, & Schoebi, 2013; Sonneville et al., 2013; Tanofsky-Kraff et al., 2011). Notably, youth with LOC eating appear to have an increased preference for highly palatable foods. Data collected from self-report measures (Theim et al., 2007) and in the laboratory (Tanofsky-Kraff, McDuffie, et al., 2009), indicate that youth with reported LOC eating consume more snack- and dessert-type foods, as well as more energy from carbohydrates and less from protein compared to youth without LOC eating (Tanofsky-Kraff, McDuffie, et al., 2009; Theim et al., 2007). These data support the notion that youth with LOC eating may be particularly susceptible to attentional bias toward palatable food cues, which may promote the obesogenic eating patterns that distinguish the LOC phenotype. Examining attention biases among youth with LOC eating may provide novel information about the role of attention biases in developmental risk models for eating disorders.
Individual differences in behavioral phenotypes promoting obesity (A. E. Field et al., 2013) may account for the heterogeneity of findings regarding attentional biases and weight status. Indeed, it is possible that the relationship between attentional biases to food cues and weight status may vary as a function of LOC eating or BED presence. Individuals with LOC eating or BED, who have been shown to be more impulsive and sensitive to the rewarding properties of palatable foods relative to weight-matched controls (Filbey et al., 2012; Schag, Schönleber, et al., 2013), may experience greater difficulties with diverting attention from foods regardless of their goals. Such sustained attention towards palatable foods may be associated with exacerbated cravings (Kemps & Tiggemann, 2009) that trigger LOC episodes and frequent overconsumption, which may promote excessive weight gain and obesity. By contrast, it is possible that overweight youth without LOC eating exhibit the approach-avoidance bias pattern, as they may possess improved attentional control capacity in the face of palatable food cues relative to those with LOC eating. However, these hypotheses require empirical evaluation.
Therefore, we examined biases in sustained attention toward high palatable foods using a visual probe task in youth with and without reported LOC eating. We hypothesized that children with LOC eating would display a greater sustained attentional bias toward palatable foods compared to youth without LOC eating. Additionally, given the prior literature in overweight adults (e.g. Castellanos et al., 2009; Werthmann et al., 2011), we explored whether BMI-z and LOC eating interacted in their relationship to attentional bias to highly palatable foods. In order to determine whether findings were specific to high palatable foods, we also examined biases in sustained attention toward low palatable foods; however, we hypothesized that there would be relevant differences between youth with and without LOC eating on high palatable foods only.
Materials and Methods
Participants and Recruitment
Participants were a convenience sample of children and adolescents, aged 8–17 years, recruited through multiple methods: advertisements in local newspapers, referrals from physicians’ offices, mailings to local area parents, flyers posted at the National Institutes of Health (NIH) and the Uniformed Services University of the Health Sciences (USUHS) in Bethesda, Maryland, and local public facilities, with permission. Flyers were also distributed through local elementary, middle, and high school parent listservs. Participants were drawn from three separate studies. Two were non-intervention studies. The first, carried out at the NIH, examined eating behaviors in adolescent boys and girls (13–17 years) with and without reported LOC eating of all weight strata (ClinicalTrials.gov ID: NCT00631644). The second non-intervention study took place at USUHS and recruited overweight (BMI ≥ 85th percentile) adolescent (12–17 years) girls with reported LOC eating for a study of mood and eating behaviors. The third protocol was a prevention trial, carried out at the NIH, that included overweight and obese (BMI ≥ 85th percentile) boys and girls with LOC eating (ClinicalTrials.gov ID: NCT00263536), all of whom were studied prior to receiving any intervention. All studies excluded individuals with major medical or psychiatric disorders (other than BED), as well as individuals taking prescription medications that could affect eating and/or weight. The Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH and USUHS institutional review board approvals were obtained for each study at the respective sites. Parents and participants provided written consent and assent, respectively, for study participation.
Procedure
Across the three studies, all data were collected at participants’ screening visits following an overnight fast. Height and weight were collected, and then participants consumed a breakfast meal (a breakfast shake, granola bars, or a muffin) to ensure satiety. Approximately 5 to 10 minutes after eating breakfast, youth completed a questionnaire to assess hunger and, immediately following, completed a visual probe task. For the non-intervention studies, the EDE was completed in the afternoon following the visual probe task. For the prevention study, the baseline assessments took place over two days, and the EDE was completed on a separate day from the visual probe task.
Measures
Body mass index (BMI)
Height was measured in triplicate by stadiometer and weight was measured by calibrated scale to the nearest 0.1 kg. BMI (kg/m2) was calculated using height, averaged across the three measurements, and weight. Age and sex were included to produce a BMI-z score based on the Center for Disease Control and Prevention growth standards (Centers for Disease Control and Prevention, 2000).
Loss of control (LOC) eating
The Eating Disorder Examination (EDE) is a semi-structured interview that was used to assess LOC eating. Children were administered either the EDE Version 12.0D (Fairburn & Cooper, 1993) with updates from versions 14 and 15, or the child version (Bryant-Waugh, Cooper, Taylor, & Lask, 1996). Both the adult and child versions measure the same constructs and have been successfully combined in previous studies (e.g. Glasofer et al., 2007; Tanofsky-Kraff, McDuffie, et al., 2009) and have shown excellent inter-rater reliability (Glasofer et al., 2007; Tanofsky-Kraff et al., 2004). LOC eating was deemed present if youth endorsed at least one objective binge episode (defined as consuming an objectively large amount of food while experiencing a lack of control over eating) or subjective binge episode (defined as consuming an ambiguously large amount of food while experiencing a lack of control over eating) within the past 28 days. The number of LOC eating episodes over the past 28 days was collected.
Hunger ratings
Following breakfast, all participants rated their level of hunger on a visual analog scale that ranged from “not at all” to “extremely” (on a scale of 0 to 100) immediately prior to participating in the visual probe task. Previous studies indicate that the visual analog scale is valid, reliable, and positively correlated with food intake (Parker et al., 2004; Stubbs et al., 2000).
Visual probe task
The visual probe task to measure bias in sustained attention consisted of 180 trials in which pairs of color photographs were presented on a HP laptop screen. The visual probe task was coded using E-Prime 2.0. The task used 90 photos from one of three categories: 30 high palatable (HP) foods (e.g. pizza, donuts), 30 low palatable (LP) foods (e.g. pineapples, mushrooms), and 30 neutral non-food (NF) control stimuli, which consisted of emotionally neutral images of household items (e.g. paper shredder, paintbrush). Each photo was shown a total of four times. All of the food stimuli and the majority of the neutral stimuli were drawn from a previously validated database. Additional neutral items were drawn from the International Affective Pictures System (Lang, Bradley, & Cuthbert, 1999). The majority of pictures from the database (94.3%) have been used in previous studies (e.g. Simmons et al., 2013) and have been validated in a sample of older adolescents and young adults by providing ratings of typicality (indicators of how typical each picture was of its respective food category) and palatability. This sample provided typicality ratings (how typical each picture is within its respective food category) and appetizing scores, both rated 1 to 7, with 7 representing the most typical or appetizing. Typicality was acceptable for low palatable foods (M = 5.28, SD = 0.71), high palatable foods (M = 5.68, SD = 0.46), and neutral stimuli (M = 5.38, SD = 0.76). As expected, appetizing scores were significantly higher for high palatable foods (M = 5.33, SD = 0.72) than for low palatable foods (M = 4.52, SD = 1.11; t(39.71) = 3.12, p = .003). The remaining pictures from the database were not validated.
Across the task, trials were divided into three pairing categories (60 of each): HP-LP in which a high palatable and low palatable image were paired; HP-NF in which a high palatable and neutral non-food item were paired; and LP-NF in which a low palatable and neutral non-food item were paired. Although the images varied considerably in visual features such as color and complexity, each pairing was matched to make images within pairs as homogeneous as possible (e.g. matching on shape to pair a pizza pie with a clock), and specific pairings were maintained across all participants in the study. Fifteen such pairings were created for each category, and each pair was presented four times with the location of stimuli and probe counterbalanced as described below. The order of stimulus presentation was randomized.
For each trial, a fixation cross appeared. After the fixation cross disappeared, stimuli were presented side-by-side (2000 ms), after which both images disappeared and a probe appeared in a location previously occupied by one of the two pictures (1000 ms). The probe consisted of either a left or a right arrow, and participants were instructed to respond, as quickly and accurately as possible, with the left arrow button if pointing left, and the right arrow button if pointing right. In order to minimize automaticity, the inter-trial interval randomly fluctuated across three durations of 500ms, 1000ms, or 1500 ms. The total visual probe task duration was approximately 10 minutes (Figure 1 illustrates a visual depiction of the task).
Figure 1.
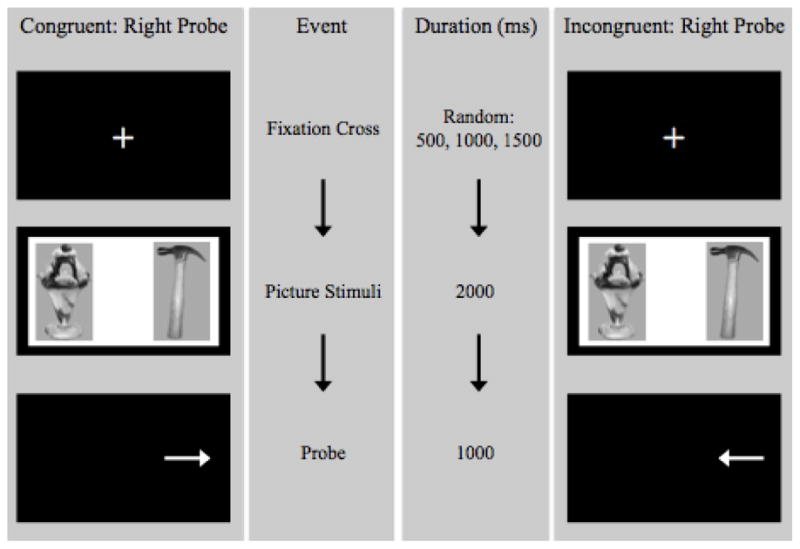
Visual depiction of the visual probe task.
Visual probe tasks are frequently built upon a probe position paradigm, during which participants respond based on the probe position (i.e. participants respond using a key that corresponds to whether the probe appeared on the left or the right). A potential disadvantage of this approach is that participants may adopt a biased monitoring strategy that favors either the left or the right region of the task (Mogg & Bradley, 1999). To encourage equal monitoring of the left and right regions of the task, for the present study, the visual probe task was built upon a probe classification task as in previous studies (Bradley, Mogg, Falla, & Hamilton, 1998; Mogg & Bradley, 1999; O’Toole & Dennis, 2012; Rogers, Appleton, et al., 2008; Rogers, Smith, Heatherley, & Pleydell-Pearce, 2008). Specifically, the participant was instructed to respond based upon the type of probe (i.e., left or right arrow) as opposed to the position of the probe. Prior to initiation of the visual probe task, participants completed a practice task (Mogg, Field, & Bradley, 2005; Nijs et al., 2010; Werthmann, Roefs, Nederkoorn, Mogg, et al., 2013) that consisted of 20 trials.
Data Analysis
All analyses were conducted using SPSS Version 19.0. Data were examined for outliers and screened for normality. Trials were excluded from analysis if participants failed to respond within the 1000 ms probe display window, or if the response was incorrect. One extreme outlier was identified across all visual probe task variables and was therefore recoded to 1.5 times the interquartile range above the 75th percentile to minimize its influence on the data analyses (Behrens, 1997). Additionally, the number of LOC episodes in the past 28 days was log transformed for normality.
An attentional bias score for sustained attention was calculated for each participant on each of the three pair types based on reaction times. The reaction time to the more salient of the paired images was subtracted from the reaction time to the less salient image in the pair, resulting in bias scores in which higher scores represent greater bias. Faster reaction times are suggestive of a greater attentional bias towards the cue; therefore, negative attentional bias scores indicate a bias away from the salient cues, whereas positive attentional bias scores indicate a bias toward the salient cues. The attentional bias score for HP-LP pairs was generated by subtracting reaction times for high palatable food cues from reaction times for low palatable food cues; therefore, positive attentional bias scores for HP-LP pairs indicate a bias towards the HP cues. The attentional bias score for HP-NF pairs was generated by subtracting reaction times for high palatable food cues reaction times from reaction times for neutral non-food cues; therefore, positive attentional bias scores for HP-NF pairs indicate a bias towards the HP cues. Lastly, for LP-NF pairs, reaction times for low palatable food cues were subtracted from reaction times for neutral non-food cues. Therefore, positive attentional bias scores for LP-HF pairs indicate a bias towards the LP cues.
A 2 × 3 mixed model analysis of covariance (ANCOVA) was conducted to examine attentional bias across all trials, with two independent variables: pair type (HP-LP bias; HP-NF bias; LP-NF bias), and LOC eating status (presence versus absence). Due to the varying inclusion criteria across the three studies, differences in demographics and BMI-z score were expected. Therefore, we adjusted for study (breakfast type), age, sex, race, and BMI-z score as well as self-reported state hunger prior to the task in all ANCOVA models. In addition to serving as a covariate, BMI-z was also included as an interaction term with LOC eating status. Follow-up ANCOVAs were conducted within each significant pair type. For significant interactions, the slopes of the interaction (i.e. the slope of the line for each LOC eating status group) were analyzed using a linear regression t-test to determine if each slope significantly differed from zero. A slope of 0 would indicate that the relation between attentional bias and BMI-z score did not differ within each LOC eating group. Additionally, the relationship between LOC episode frequency and attentional bias scores were examined within all participants using bivariate correlations, as well as using partial bivariate correlations controlling for BMI-z. Lastly, the relationship between BMI-z and attentional bias scores were examined continuously using correlations and categorically across weight groups using independent samples t-tests. The assumptions of all analyses were checked, and no assumptions were violated. Differences were considered significant when p values were ≤ 0.05. All tests were two-tailed.
Results
Seventy-seven children and adolescents participated in the visual probe task. One participant was excluded from all analyses, as this child did not properly complete the visual probe task. Therefore, data from 76 children and adolescents were analyzed. Youth ranged in age from 8 to 17 years, with a mean of 14.45 years (SD = 2.30). The majority of participants were female (86.8%) and the sample spanned a wide BMI-z range (M = 1.71, SD = 0.73, range = −0.50–2.75). The ethnic/racial breakdown was 46.1% Non-Hispanic Black, 42.1% Non-Hispanic White, 2.6% Hispanic, and 9.2% other or unknown. An average of 15.9 (8.8%) trials was excluded per participant due to incorrect responses. The mean hunger rating after breakfast was low (M = 26.5 on a scale of 1 to 100, SD = 23.1), as has similarly been reported in prior attentional bias studies (e.g. Kemps, Tiggemann, Orr, & Grear, 2013). Hunger rating did not correlate with HP-NF bias [r(70) = −.11, p = .34], HP-LP bias [r(70) = .18, p = .14), or LP-NF bias [r(70) = −.04, p = .74]. Over half (61.8%) of participants reported the presence of LOC eating in the past month. Participant characteristics by LOC eating status are shown in Table 1. The number of LOC episodes in the past 28 days ranged from 1 to 29 (M=5.60, SD=6.18). There were no differences in the number of incorrect and excluded trials based on LOC eating status [t(71.58) = 1.87, p = .07].
Table 1.
Participant Characteristics
| LOC eating (n = 47)
|
No LOC eating (n = 29)
|
p
|
|
|---|---|---|---|
| Age in years, M (SD) | 13.8 (2.4) | 15.6 (1.6) | .001 |
| Sex, n (%) | .73 | ||
| Male | 7 (14.9%) | 3 (10.3%) | |
| Female | 40 (85.1%) | 26 (89.7%) | |
| Race, n (%) | .006 | ||
| Non-Hispanic White | 14 (29.8%) | 18 (62.1%) | |
| Non-Hispanic Black | 27 (57.4%) | 8 (27.6%) | |
| Hispanic | 2 (4.3%) | 0 (0.0%) | |
| Other/Unknown | 4 (8.5%) | 3 (10.3%) | |
| BMI-z score, M (SD) | 2.03 (0.40) | 1.19 (0.84) | < .001 |
| Weight status, n (%) | |||
| Overweight | 7 (14.9%) | 10 (34.5%) | < .001 |
| Obese | 40 (85.1%) | 8 (27.6%) | |
| Hunger Rating, M (SD) | 25.1 (21.0) | 28.8 (26.4) | .51 |
| HP-LP bias | −5.07 (27.45) | 1.86 (30.89) | .31 |
| HP-NF bias | 12.89 (41.46) | 1.16 (25.34) | .17 |
| LP-NF bias | 1.63 (36.19) | 1.84 (31.34) | .98 |
Note: LOC, loss of control; HP-LP bias, bias for high palatable foods versus low palatable foods; HP-NF bias, bias for high palatable foods versus neutral non-food stimuli; LP-NF bias, bias for low palatable foods versus neutral non-food stimuli
Attentional Bias, LOC Eating, and Body Weight
The 2 × 3 mixed model ANCOVA revealed no main effects for pair type [F(2, 124) = .78, p = .46] or LOC eating status [F(1, 62) = <.001, p = .99]. Additionally, there was no interaction between pair type and LOC eating status, F(2, 124) = .03, p = .98. As shown in Table 1, youth with and without reported LOC eating did not differ significantly on HP-LP bias (LOC: M = −5.07, SD = 27.45, No LOC: M = 1.86, SD = 30.88), HP-NF bias (LOC: M = 12.89, SD = 41.46, No LOC: M = 1.16, SD = 25.34), or LP-NF bias (LOC: M = 1.63, SD = 36.19, No LOC: M = 1.84, SD = 31.34).
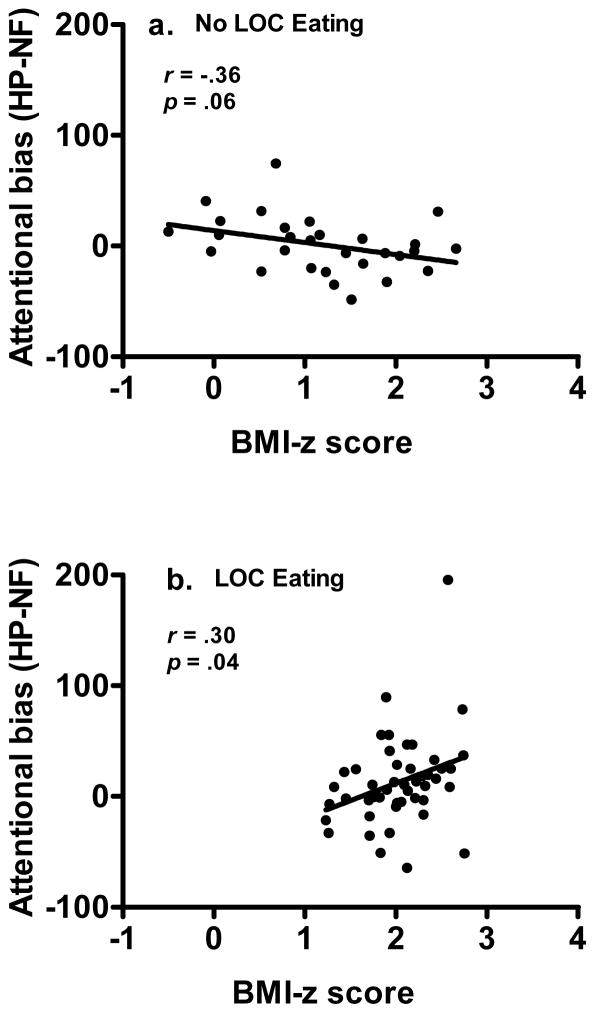
A significant three-way interaction between BMI-z score, LOC eating status, and pair type was observed, F(2, 124) = 3.56, p = .03, η2p = .054. Follow-up analyses (shown in Table 2) showed a two-way interaction between BMI-z score and LOC eating status was not significant for LP-NF bias [F(1, 62) = 2.25, p = .14, η2p = .035] or for HP-LP bias [F(1, 62) = 0.61, p = .44, η2p = .010]. However, there was a significant two-way interaction between BMI-z score and LOC eating status for HP-NF bias only, F(1, 62) = 7.78, p = .007, η2p = .111. The interaction for HP-NF bias revealed a slight negative association between attentional bias score and BMI-z among children without LOC eating (Figure 2a), and a positive association between attentional bias score and BMI-z among participants with LOC (Figure 2b). In participants without LOC, those with higher BMI-z demonstrated a trend toward a greater bias in sustained attention away from highly palatable foods compared to neutral non-food cues (slope of the interaction, F(1,27) = 3.98, p = .06, 95% CI [−21.99, 0.31]). By contrast, in participants with LOC, bias in sustained attention toward highly palatable foods increased as BMI-z increased (slope of the interaction was positive and significantly different from zero, F(1, 45) = 4.60, p = .04, 95% CI [1.88, 60.98]). No other pair type interactions were significant for attentional bias in children either with or without reported LOC eating.
Table 2.
Attentional Bias by Pair Type
|
df
|
F
|
η2p
|
p
|
|
|---|---|---|---|---|
| HP-NF Bias | ||||
| Study Dummy Code 1 | 1 | 2.46 | .04 | .12 |
| Study Dummy Code 2 | 1 | 0.48 | .008 | .49 |
| LOC Status | 1 | 0.00 | .00 | >.99 |
| Sex | 1 | 0.62 | .01 | .44 |
| Race | 1 | 1.61 | .03 | .21 |
| Age | 1 | 0.32 | .005 | .58 |
| BMI-z | 1 | 1.83 | .03 | .18 |
| Hunger Level | 1 | 1.50 | .02 | .23 |
| LOC Status * BMI-z | 1 | 7.78 | .11 | .007* |
| Error | 62 | |||
| HP-LP Bias | ||||
| Study Dummy Code 1 | 1 | 1.92 | .03 | .17 |
| Study Dummy Code 2 | 1 | 1.35 | .02 | .25 |
| LOC Status | 1 | 0.04 | .85 | .85 |
| Sex | 1 | 0.19 | .003 | .67 |
| Race | 1 | 0.10 | .002 | .75 |
| Age | 1 | 0.75 | .01 | .39 |
| BMI-z | 1 | 0.98 | .02 | .33 |
| Hunger Level | 1 | 1.85 | .03 | .18 |
| LOC Status * BMI-z | 1 | 0.61 | .01 | .44 |
| Error | 62 | |||
| LP-NF Bias | ||||
| Study Dummy Code 1 | 1 | 5.26 | .08 | .03* |
| Study Dummy Code 2 | 1 | 2.56 | .04 | .12 |
| LOC Status | 1 | .02 | .00 | .90 |
| Sex | 1 | 0.30 | .005 | .59 |
| Race | 1 | 0.13 | .002 | .72 |
| Age | 1 | 5.92 | .09 | .02* |
| BMI-z | 1 | .00 | .00 | .99 |
| Hunger Level | 1 | 1.13 | .02 | .29 |
| LOC Status * BMI-z | 1 | 2.25 | .04 | .14 |
| Error | 62 | |||
Note:
Significant at p < .05
Figure 2.
Interaction between loss of control eating and BMI-z for attentional bias to high palatable foods versus neutral non-food stimuli (HP-NF bias), p = .01. (A) Youth without loss of control eating have a negative association between bias in sustained attention to high palatable foods and BMI-z score, with bias in sustained attention decreasing as BMI-z increases, r(27) = −.36, p = .06. (B) Youth with loss of control eating have a positive association between bias in sustained attention to high palatable foods and BMI-z score, with bias in sustained attention increasing as BMI-z increases, r(45) = .30, p = .04.
Exploratory Analyses
When examining the data continuously, LOC episode frequency was significantly and positively correlated with attentional bias for HP-NF bias, r(74) = .24, p = .04. After adjusting for BMI-z, the relationship between LOC eating frequency and HP-NF bias was attenuated, r(73) = .22, p = .058. Moreover, BMI-z score did not significantly correlate with HP-LP bias [r(74) = −.14, p = .24], HP-NF bias [r(74) = .10, p = .39], or LP-NF bias [r(74) = −.10, p = .41]. Attention bias was also examined categorically by weight status. Weight status was defined as healthy weight (BMI-z < 1.04), overweight (BMI-z between 1.04 and 1.64), or obese (BMI-z greater than 1.64). When collapsing across LOC eating status and examining attention bias categorically by weight status, no differences were found for high versus low palatable foods [F(2, 73) = .72, p = .49], high palatable foods versus neutral non-food stimuli [F(2, 73) = 2.03, p = .14], or low palatable foods versus neutral non-food stimuli [F(2, 73) = .31, p = .74].
Discussion
Using a visual probe task designed to measure sustained attention, we found that neither BMI-z nor LOC eating status was directly related to attentional bias to highly palatable foods. However, in youth with LOC eating, bias in sustained attention toward highly palatable foods increased as BMI-z increased. The opposite pattern trended towards significance among youth without LOC eating.
For high palatable food, we found no difference in attentional bias by weight status when collapsed across LOC condition. This finding contradicts the only other known study in youth that examined attentional bias to food cues across the weight spectrum in youth, which found significant negative correlations between reaction times to food cues and BMI (Yokum et al., 2011). However, this study primarily examined automatic orientation to food cues and the reallocation of attention to food cues (Yokum et al., 2011), while our study examined biases in sustained attention. Additionally, Yokum and colleagues (2011) used fMRI to examine attentional bias to food cues during an attention network task, and did not use a reaction time difference score across picture types. Lastly, participants in this study fasted for 4–6 hours before the study (Yokum et al., 2011), while our participants were satiated. Similarly, two studies examining cognitive interference due to food cues in obese youth yielded conflicting results. Using food words (e.g. whipped cream, bread, peach), one study found that obese children displayed cognitive interference for food words as measured by a modified Stroop task (Braet & Crombez, 2003), while the second study found no interference for high calorie food words (e.g. pizza, cake) as measured by an imbedded word task (Soetens & Braet, 2007).
Since attentional bias to food cues may be measured through a variety of methods, including visual probe tasks and neuroimaging (Field & Cox, 2008), a potentially complicating factor is that subcomponents of attention allocation may be differentially measured across paradigms (Posner & Peterson, 1990). For example, orienting to sensory events, detecting signals for processing, and maintaining a vigilant state are subsystems of attention. Such differences across subcomponents and methods render generalizability across studies a challenge. However, the findings of this study, in conjunction with future research, may make it possible to identify the specific attentional subcomponents implicated in adolescent LOC eating.
We did not find that sustained attentional bias to highly palatable foods in youth with LOC eating differed from those without LOC eating. However, our data support the approach-avoidance pattern to palatable food cues versus neutral non-food items (Werthmann et al., 2011), but only among youth without LOC eating. Consistent with this pattern, in youth without LOC eating, as BMI-z score increased, bias in sustained attention decreased. Among youth without LOC eating, leaner youth generally had a slight or absent attentional bias toward highly palatable food cues, with attentional bias shifting increasingly away from such food cues among heavier youth. In those with LOC eating, the opposite pattern was observed; leaner youth exhibited a slight attentional bias away from highly palatable food cues, with the attentional bias shifting increasingly toward palatable food cues among heavier youth. Overall, findings indicate that heavier youth without LOC eating may be more likely to demonstrate purposeful avoidance of highly palatable food cues, whereas heavier youth with LOC eating generally may have a sustained approach bias toward highly palatable food cues.
These findings may explain the inconsistent results between obesity and attentional bias to food cues across adult studies (Nijs & Franken, 2012). As there was no main effect of BMI-z on attentional bias, our study lends support to the importance of understanding attentional bias across varying obesity phenotypes (A. E. Field et al., 2013). Indeed, overweight youth with LOC eating may represent a group particularly vulnerable to sustained attentional bias toward highly palatable foods. As a bias in sustained attention toward highly palatable foods represents cognitive difficulty in disengaging attention from these foods, this may explain laboratory and self-report data showing that youth with LOC tend to consume highly palatable foods (Tanofsky-Kraff, McDuffie, et al., 2009; Theim et al., 2007). This possibility is supported by studies showing that have found that experimentally manipulated attention bias to specific types of food cues to can produce changes in food consumption patterns (Kakoschke, Kemps, & Tiggemann, 2014; Kemps, Tiggemann, Orr, & Grear, 2014). As no effects were observed for attentional bias towards low palatable foods, youth with LOC eating may experience difficulty in disengaging solely from high palatable foods. Adults with BED may be physiologically prone to cravings for carbohydrate-rich and palatable foods (Gendall, Joyce, & Abbott, 1999; Yanovski, 2003b), and analogous effects may occur in youth with LOC eating. Therefore, low palatable foods may be less rewarding for youth with LOC eating compared to high palatable foods. Alternatively, both age and study were significant covariates in the analysis comparing low palatable food versus neutral non-food stimuli. Notably, these variables were not significant in other comparisons, suggesting that both age and study source may have particular relevance for attentional biases toward low palatable foods and may explain why we did not observe significant effects for low palatable foods.
Although our data are cross-sectional, the interaction between LOC eating and BMI-z score suggests that a combination of excess body weight and the LOC phenotype could promote, or be the result of, attentional bias to highly palatable foods. According to the incentive-sensitization theory, some individuals may have underlying biological vulnerabilities that render them susceptible to the development of sensitization to a rewarding stimulus (Robinson & Berridge, 1993). Similarly, obese adults with BED display increased food-related impulsivity, which may also represent a biological vulnerability for increased attentional bias to food cues (Schag, Schönleber, et al., 2013). Thus, it is possible that LOC eating and obesity may underlie the development of attentional bias to palatable foods. Alternatively, a particular susceptibility for attentional bias to palatable food cues may promote LOC eating and/or obesity. While past research has shown that LOC eating predicts excess weight gain (Tanofsky-Kraff, Yanovski, et al., 2009), no prospective study has examined whether weight status itself predicts the development of LOC eating. However, within a cross-sectional sample of children with LOC eating, the majority retrospectively reported becoming overweight before LOC eating developed (Tanofsky-Kraff, Faden, Yanovski, Wilfley, & Yanovski, 2005). Prospective data are required to disentangle the relationships among body weight, LOC eating and attentional bias to highly palatable foods so that effective, highly targeted interventions for specific phenotypes may be developed.
Future research should examine whether attentional bias modification may be an effective intervention in this population. This approach has been shown to be effective in reducing biases to highly palatable foods in primarily healthy young adults (Kakoschke et al., 2014; Kemps et al., 2013). With regard to children, one small pediatric study found that a single laboratory session of computerized attentional bias modification relatively reduced eating in the absence of hunger among obese children compared to the control condition (Boutelle et al., 2014). While this finding was primarily driven by an increase in eating in the absence of hunger by children in the control condition (Boutelle et al., 2014), these data suggest that it may be beneficial to examine the effectiveness of an attentional bias modification intervention in overweight children who experience LOC eating.
Strengths of this study include the recruitment of racially diverse boys and girls across a wide weight stratum and the use of a structured clinical interview to assess LOC eating. Additionally, we controlled for pre-task hunger, which has been found to affect biases to palatable food (Loeber et al., 2013; Nijs et al., 2010; Tapper et al., 2010). In addition, the visual probe task used in this study was a probe classification task, which encourages equal monitoring of the left and right stimuli regions to produce a more accurate measurement of attentional bias. Limitations include that the visual probe task relied on reaction time only, which only captures an individual’s attention allocation for at the time immediately before the probe appears. Eye tracking was not used in this study, which would have allowed for greater understanding of attentional allocation throughout the entire stimuli duration. Additionally, the sample was one of convenience and combined children across multiple studies. Each study involved a different standardized breakfast and varying recruitment strategies. While we adjusted for study in all analyses and hunger levels were comparable across protocols, it is possible that other unknown aspects (e.g. social desirability) may have impacted our results. Lastly, BMI-z was unequal between groups, as all participants who reported LOC eating were overweight or obese. While a limitation, we did account for BMI-z in all analyses. Future studies should utilize weight-matched groups (Tanofsky-Kraff et al., 2004).
There are several additional considerations for interpreting the study results. First, we measured height and fasting weight before the visual probe task. While no known study has examined whether collecting height and weight before a visual probe task could influence attentional bias, future studies should consider measuring height and weight after the task. Second, the range of LOC episodes reported by youth varied broadly. Stronger effects may have been found with a higher frequency threshold (e.g. once weekly for the past month) to determine whether attentional biases differ between youth without LOC eating compared to those with recurrent episodes. However, a cut-off of at least one episode of LOC eating in the past month has been used in previous studies (e.g. Shomaker et al., 2010; Tanofsky-Kraff, McDuffie, et al., 2009), provides predictive validity (Tanofsky-Kraff et al., 2011; Tanofsky-Kraff, Yanovski, et al., 2009), and few children endorse full-syndrome binge eating disorder (Shomaker et al., 2011). Moreover, a benefit of using subthreshold criteria is the ability to better understand precursors to adverse outcomes. Third, the task stimuli were not systematically matched on features that could affect attention such as color, luminance, and contrast (Knudsen, 2007). While pictures were matched on features such as shape and color, it is possible that systematic differences existed within picture pairs on features such as color, luminance, and contrast. However an important point is that the pairings were the same across all subjects and while this may have introduced non-semantic biases within stimulus pairs across subjects this would not differentially impact groups. Therefore, we have greater confidence that any such biases are unlikely to impact our primary results. Lastly, study participants represented a broad age range, which may have influenced results and increased the variability of attentional bias scores. Reaction times tend to differ as a function of age, but the use of a difference score to measure attentional bias likely mitigates these concerns. Additionally, there may be differences in attention bias to food cues across the age range. Future research should focus upon developmental differences in attentional bias to food cues.
In conclusion, heavier youth with LOC eating may have a greater sustained attentional bias to highly palatable foods. It warrants testing to what extent modifying such biases is an effective approach to reducing obesity and exacerbated disordered eating in these vulnerable youth.
Acknowledgments
Research support: Intramural Research Program, NIH, grant 1ZIAHD000641 from the NICHD with supplemental funding from NIMHD, the Bench to Bedside Program and the Office of Behavioral and Social Sciences Research (OBSSR) of the NIH, NIDDK 1R01DK080906; Intramural Research Program, NIMH, and NIMH F31MH095348.
References
- Balodis IM, Molina ND, Kober H, Worhunsky PD, White MA, Rajita S, Potenza MN. Divergent neural substrates of inhibitory control in binge eating disorder relative to other manifestations of obesity. Obesity (Silver Spring) 2013;21(2):367–377. doi: 10.1002/oby.20068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens JT. Principles and procedures of exploratory data analysis. Psychological Methods. 1997;2(2):131–160. [Google Scholar]
- Berridge KC. ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiol Behav. 2009;97(5):537–550. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomquist KK, Barnes RD, White MA, Masheb RM, Morgan PT, Grilo CM. Exploring weight gain in year before treatment for binge eating disorder: a different context for interpreting limited weight losses in treatment studies. Int J Eat Disord. 2011;44(5):435–439. doi: 10.1002/eat.20836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutelle KN, Kuckertz JM, Carlson J, Amir N. A pilot study evaluating a one-session attention modification training to decrease overeating in obese children. Appetite. 2014;76:180–185. doi: 10.1016/j.appet.2014.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, Falla SJ, Hamilton LR. Attentional bias for threatening facial expressions in anxiety: Manipulation of stimulus duration. Cognition and Emotion. 1998;12(6):737–753. [Google Scholar]
- Braet C, Crombez G. Cognitive interference due to food cues in childhood obesity. J Clin Child Adolesc Psychol. 2003;32(1):32–39. doi: 10.1207/S15374424JCCP3201_04. [DOI] [PubMed] [Google Scholar]
- Brignell C, Griffiths T, Bradley BP, Mogg K. Attentional and approach biases for pictorial food cues. Influence of external eating. Appetite. 2009;52(2):299–306. doi: 10.1016/j.appet.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Bryant-Waugh R, Cooper PJ, Taylor CL, Lask B. The use of the eating disorder examination with children: A pilot study. Int J Eat Disord. 1996;19(4):391–397. doi: 10.1002/(SICI)1098-108X(199605)19:4<391::AID-EAT6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Castellanos EH, Charboneau E, Dietrich MS, Park S, Bradley BP, Mogg K, Cowan RL. Obese adults have visual attention bias for food cue images: evidence for altered reward system function. Int J Obes (Lond) 2009;33(9):1063–1073. doi: 10.1038/ijo.2009.138. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2000 CDC growth charts for the United States: Methods and Development. Vital and Health Statistics. 2000;11(246) [PubMed] [Google Scholar]
- Chamberlain SR, Mogg K, Bradley BP, Koch A, Dodds CM, Tao WX, Nathan PJ. Effects of mu opioid receptor antagonism on cognition in obese binge-eating individuals. Psychopharmacology (Berl) 2012;224(4):501–509. doi: 10.1007/s00213-012-2778-x. [DOI] [PubMed] [Google Scholar]
- Eldar S, Apter A, Lotan D, Perez-Edgar K, Naim R, Fox NA, Bar-Haim Y. Attention bias modification treatment for pediatric anxiety disorders: A randomized controlled trial. Am J Psychiatry. 2012;169:213–220. doi: 10.1176/appi.ajp.2011.11060886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairburn CG, Cooper Z. The eating disorder examination. In: Fairburn CG, Wilson GT, editors. Binge eating: Nature, assessment, and treatment. 12. New York: Guilford Press; 1993. pp. 317–360. [Google Scholar]
- Field AE, Camargo CA, Ogino S. The merits of subtyping obesity: One size does not fit all. JAMA. 2013;310(20):2147–2148. doi: 10.1001/jama.2013.28150110.1038/onc.2013.244. [DOI] [PubMed] [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97(1–2):1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Myers US, Dewitt S. Reward circuit function in high BMI individuals with compulsive overeating: similarities with addiction. Neuroimage. 2012;63(4):1800–1806. doi: 10.1016/j.neuroimage.2012.08.073. [DOI] [PubMed] [Google Scholar]
- Gendall KA, Joyce PR, Abbott RM. The effects of meal composition on subsequent craving and binge eating. Addictive Behaviors. 1999;24(3):305–315. doi: 10.1016/s0306-4603(98)00046-x. [DOI] [PubMed] [Google Scholar]
- Glasofer DR, Tanofsky-Kraff M, Eddy KT, Yanovski SZ, Theim KR, Mirch MC, Yanovski JA. Binge eating in overweight treatment-seeking adolescents. J Pediatr Psychol. 2007;32(1):95–105. doi: 10.1093/jpepsy/jsl012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt AB, Jones M, Manwaring JL, Luce KH, Osborne MI, Cunning D, Taylor CB. The clinical significance of loss of control over eating in overweight adolescents. Int J Eat Disord. 2008;41(2):153–158. doi: 10.1002/eat.20481. [DOI] [PubMed] [Google Scholar]
- Graham R, Hoover A, Ceballos NA, Komogortsev O. Body mass index moderates gaze orienting biases and pupil diameter to high and low calorie food images. Appetite. 2011;56(3):577–586. doi: 10.1016/j.appet.2011.01.029. [DOI] [PubMed] [Google Scholar]
- Hepworth R, Mogg K, Brignell C, Bradley BP. Negative mood increases selective attention to food cues and subjective appetite. Appetite. 2010;54(1):134–142. doi: 10.1016/j.appet.2009.09.019. [DOI] [PubMed] [Google Scholar]
- Hilbert A, Hartmann AS, Czaja J, Schoebi D. Natural course of preadolescent loss of control eating. J Abnorm Psychol. 2013;122(3):684–693. doi: 10.1037/a0033330. [DOI] [PubMed] [Google Scholar]
- Hou R, Mogg K, Bradley BP, Moss-Morris R, Peveler R, Roefs A. External eating, impulsivity and attentional bias to food cues. Appetite. 2011;56(2):424–427. doi: 10.1016/j.appet.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Kakoschke N, Kemps E, Tiggemann M. Attentional bias modification encourages healthy eating. Eat Behav. 2014;15(1):120–124. doi: 10.1016/j.eatbeh.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Kemps E, Tiggemann M. Attentional bias for craving-related (chocolate) food cues. Exp Clin Psychopharmacol. 2009;17(6):425–433. doi: 10.1037/a0017796. [DOI] [PubMed] [Google Scholar]
- Kemps E, Tiggemann M, Orr J, Grear J. Attentional retraining can reduce chocolate consumption. Journal of Experimental Psychology: Applied. 2013 doi: 10.1037/xap0000005. [DOI] [PubMed] [Google Scholar]
- Kemps E, Tiggemann M, Orr J, Grear J. Attentional retraining can reduce chocolate consumption. J Exp Psychol Appl. 2014;20(1):94–102. doi: 10.1037/xap0000005. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Fundamental components of attention. Annu Rev Neurosci. 2007;30:57–78. doi: 10.1146/annurev.neuro.30.051606.094256. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Technical manual and affective ratings. Gainesville: University of Florida, Center for Research in Psychophysiology; 1999. [Google Scholar]
- Loeber S, Grosshans M, Herpertz S, Kiefer F, Herpertz SC. Hunger modulates behavioral disinhibition and attention allocation to food-associated cues in normal-weight controls. Appetite. 2013;71:32–39. doi: 10.1016/j.appet.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Loeber S, Grosshans M, Korucuoglu O, Vollmert C, Vollstadt-Klein S, Schneider S, Kiefer F. Impairment of inhibitory control in response to food-associated cues and attentional bias of obese participants and normal-weight controls. Int J Obes (Lond) 2012;36(10):1334–1339. doi: 10.1038/ijo.2011.184. [DOI] [PubMed] [Google Scholar]
- Mobbs O, Iglesias K, Golay A, Van der Linden M. Cognitive deficits in obese persons with and without binge eating disorder. Investigation using a mental flexibility task. Appetite. 2011;57(1):263–271. doi: 10.1016/j.appet.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Some methodological issues in assessing attentional biases for threatening faces in anxiety: A replication study using a modified version of the probe detection task. Behav Res Ther. 1999;37:595–604. doi: 10.1016/s0005-7967(98)00158-2. [DOI] [PubMed] [Google Scholar]
- Mogg K, Field M, Bradley BP. Attentional and approach biases for smoking cues in smokers: an investigation of competing theoretical views of addiction. Psychopharmacology (Berl) 2005;180(2):333–341. doi: 10.1007/s00213-005-2158-x. [DOI] [PubMed] [Google Scholar]
- Newman E, O’Connor DB, Conner M. Attentional biases for food stimuli in external eaters: possible mechanism for stress-induced eating? Appetite. 2008;51(2):339–342. doi: 10.1016/j.appet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Nijs IM, Franken IH. Attentional Processing of Food Cues in Overweight and Obese Individuals. Curr Obes Rep. 2012;1(2):106–113. doi: 10.1007/s13679-012-0011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijs IM, Muris P, Euser AS, Franken IH. Differences in attention to food and food intake between overweight/obese and normal-weight females under conditions of hunger and satiety. Appetite. 2010;54(2):243–254. doi: 10.1016/j.appet.2009.11.004. [DOI] [PubMed] [Google Scholar]
- O’Toole L, Dennis TA. Attention training and the threat bias: an ERP study. Brain Cogn. 2012;78(1):63–73. doi: 10.1016/j.bandc.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker BA, Sturm K, MacIntosh CG, Feinle C, Horowitz M, Chapman IM. Relation between food intake and visual analogue scale ratings of appetite and other sensations in healthy older and young subjects. Eur J Clin Nutr. 2004;58(2):212–218. doi: 10.1038/sj.ejcn.1601768. [DOI] [PubMed] [Google Scholar]
- Pinaquy S, Chabrol H, Simon C, Louvet JP, Barbe P. Emotional eating, alexithymia, and binge-eating disorder in obese women. Obesity Research. 2002;11(2):195–201. doi: 10.1038/oby.2003.31. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rogers PJ, Appleton KM, Kessler D, Peters TJ, Gunnell D, Hayward RC, Ness AR. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br J Nutr. 2008;99(2):421–431. doi: 10.1017/S0007114507801097. [DOI] [PubMed] [Google Scholar]
- Rogers PJ, Smith JE, Heatherley SV, Pleydell-Pearce CW. Time for tea: mood, blood pressure and cognitive performance effects of caffeine and theanine administered alone and together. Psychopharmacology (Berl) 2008;195(4):569–577. doi: 10.1007/s00213-007-0938-1. [DOI] [PubMed] [Google Scholar]
- Schag K, Schönleber J, Teufel M, Zipfel S, Giel KE. Food-related impulsivity in obesity and binge eating disorder--a systematic review. Obes Rev. 2013;14(6):477–495. doi: 10.1111/obr.12017. [DOI] [PubMed] [Google Scholar]
- Schag K, Teufel M, Junne F, Preissl H, Hautzinger M, Zipfel S, Giel KE. Impulsivity in binge eating disorder: food cues elicit increased reward responses and disinhibition. PLoS One. 2013;8(10):e76542. doi: 10.1371/journal.pone.0076542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz F, Naumann E, Trentowska M, Svaldi J. Attentional bias for food cues in binge eating disorder. Appetite. 2014;80:70–80. doi: 10.1016/j.appet.2014.04.023. [DOI] [PubMed] [Google Scholar]
- Shomaker LB, Tanofsky-Kraff M, Elliott C, Wolkoff LE, Columbo KM, Ranzenhofer LM, Yanovski JA. Salience of loss of control for pediatric binge episodes: does size really matter? Int J Eat Disord. 2010;43(8):707–716. doi: 10.1002/eat.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomaker LB, Tanofsky-Kraff M, Yanovski JA. Disinhibited eating and body weight in youth. In: Preedy VR, Watson RR, Watson CR, editors. Handbook of Behavior, Food and Nutrition. Springer Publishers; 2011. pp. 2183–2200. [Google Scholar]
- Simmons WK, Rapuano KM, Kallman SJ, Ingeholm JE, Miller B, Gotts SJ, Martin A. Category-specific integration of homeostatic signals in caudal but not rostral human insula. Nat Neurosci. 2013;16(11):1551–1552. doi: 10.1038/nn.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soetens B, Braet C. Information processing of food cues in overweight and normal weight adolescents. Br J Health Psychol. 2007;12(Pt 2):285–304. doi: 10.1348/135910706X107604. [DOI] [PubMed] [Google Scholar]
- Sonneville KR, Horton NJ, Micali N, Crosby RD, Swanson SA, Solmi F, Field AE. Longitudinal associations between binge eating and overeating and adverse outcomes among adolescents and young adults: does loss of control matter? JAMA Pediatr. 2013;167(2):149–155. doi: 10.1001/2013.jamapediatrics.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs RJ, Hughes DA, Johnstone AM, Rowley E, Reid C, Elia M, Blundell J. The use of visual analogue scales to assess motivation to eat in human subjects: A review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. British Journal of Nutrition. 2000;84:405–415. doi: 10.1017/s0007114500001719. [DOI] [PubMed] [Google Scholar]
- Svaldi J, Schmitz F, Trentowska M, Tuschen-Caffier B, Berking M, Naumann E. Cognitive interference and a food-related memory bias in binge eating disorder. Appetite. 2014;72:28–36. doi: 10.1016/j.appet.2013.09.014. [DOI] [PubMed] [Google Scholar]
- Svaldi J, Tuschen-Caffier B, Peyk P, Blechert J. Information processing of food pictures in binge eating disorder. Appetite. 2010;55(3):685–694. doi: 10.1016/j.appet.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Cohen ML, Yanovski SZ, Cox C, Theim KR, Keil M, Yanovski JA. A prospective study of psychological predictors of body fat gain among children at high risk for adult obesity. Pediatrics. 2006;117(4):1203–1209. doi: 10.1542/peds.2005-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Faden D, Yanovski SZ, Wilfley DE, Yanovski JA. The perceived onset of dieting and loss of control eating behaviors in overweight children. Int J Eat Disord. 2005;38(2):112–122. doi: 10.1002/eat.20158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Marcus MD, Yanovski SZ, Yanovski JA. Loss of control eating disorder in children age 12 years and younger: proposed research criteria. Eat Behav. 2008;9(3):360–365. doi: 10.1016/j.eatbeh.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, McDuffie JR, Yanovski SZ, Kozlosky M, Schvey NA, Shomaker LB, Yanovski JA. Laboratory assessment of the food intake of children and adolescents with loss of control eating. Am J Clin Nutr. 2009;89(3):738–745. doi: 10.3945/ajcn.2008.26886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Shomaker LB, Olsen C, Roza CA, Wolkoff LE, Columbo KM, Yanovski JA. A prospective study of pediatric loss of control eating and psychological outcomes. J Abnorm Psychol. 2011;120(1):108–118. doi: 10.1037/a0021406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Yanovski SZ, Schvey NA, Olsen CH, Gustafson J, Yanovski JA. A prospective study of loss of control eating for body weight gain in children at high risk for adult obesity. Int J Eat Disord. 2009;42(1):26–30. doi: 10.1002/eat.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Yanovski SZ, Wilfley DE, Marmarosh C, Morgan CM, Yanovski JA. Eating-disordered behaviors, body fat, and psychopathology in overweight and normal-weight children. J Consult Clin Psychol. 2004;72(1):53–61. doi: 10.1037/0022-006X.72.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper K, Pothos EM, Lawrence AD. Feast your eyes: hunger and trait reward drive predict attentional bias for food cues. Emotion. 2010;10(6):949–954. doi: 10.1037/a0020305. [DOI] [PubMed] [Google Scholar]
- Theim KR, Tanofsky-Kraff M, Salaita CG, Haynos AF, Mirch MC, Ranzenhofer LM, Yanovski JA. Children’s descriptions of the foods consumed during loss of control eating episodes. Eat Behav. 2007;8(2):258–265. doi: 10.1016/j.eatbeh.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werthmann J, Roefs A, Nederkoorn C, Jansen A. Desire lies in the eyes: attention bias for chocolate is related to craving and self-endorsed eating permission. Appetite. 2013;70:81–89. doi: 10.1016/j.appet.2013.06.087. [DOI] [PubMed] [Google Scholar]
- Werthmann J, Roefs A, Nederkoorn C, Mogg K, Bradley BP, Jansen A. Can(not) take my eyes off it: attention bias for food in overweight participants. Health Psychol. 2011;30(5):561–569. doi: 10.1037/a0024291. [DOI] [PubMed] [Google Scholar]
- Werthmann J, Roefs A, Nederkoorn C, Mogg K, Bradley BP, Jansen A. Attention bias for food is independent of restraint in healthy weight individuals-an eye tracking study. Eat Behav. 2013;14(3):397–400. doi: 10.1016/j.eatbeh.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Yanovski S. Binge eating disorder and obesity in 2003: could treating an eating disorder have a positive effect on the obesity epidemic? Int J Eat Disord. 2003a;34(Suppl):S117–120. doi: 10.1002/eat.10211. [DOI] [PubMed] [Google Scholar]
- Yanovski S. Sugar and fat: Cravings and aversions. The Journal of Nutrition. 2003b;133:835S–837S. doi: 10.1093/jn/133.3.835S. [DOI] [PubMed] [Google Scholar]
- Yokum S, Ng J, Stice E. Attentional bias to food images associated with elevated weight and future weight gain: an fMRI study. Obesity (Silver Spring) 2011;19(9):1775–1783. doi: 10.1038/oby.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]