Abstract
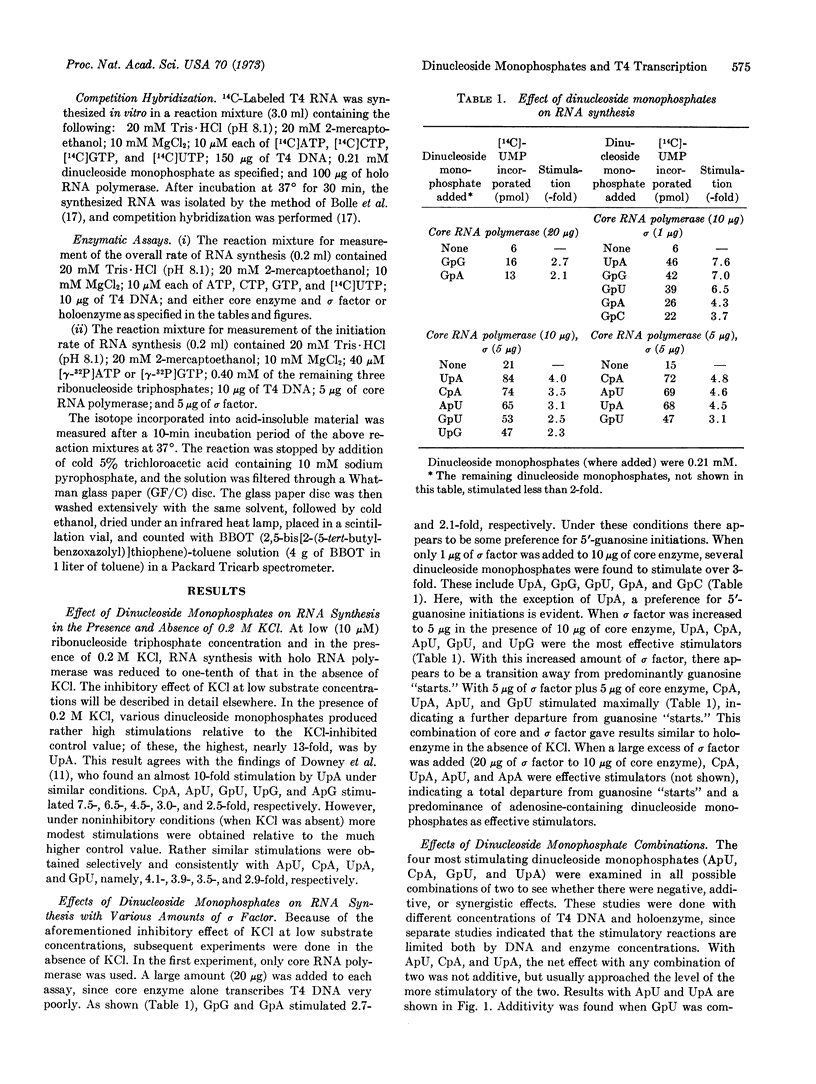
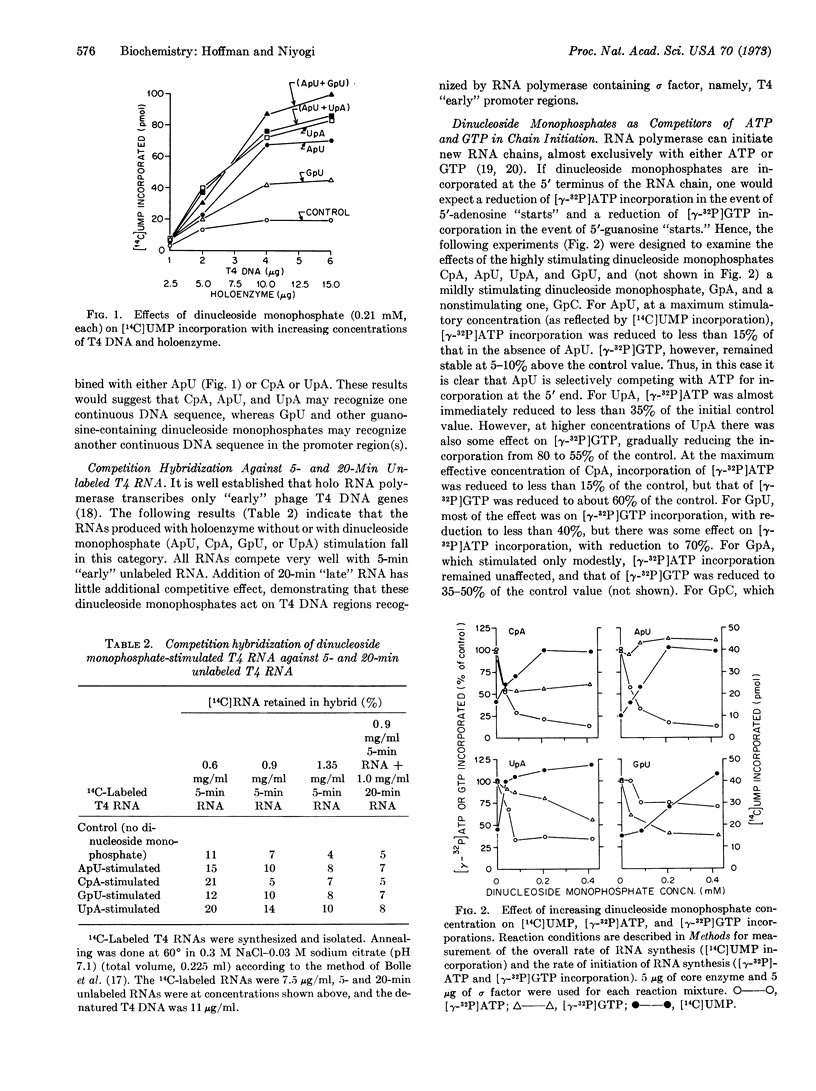
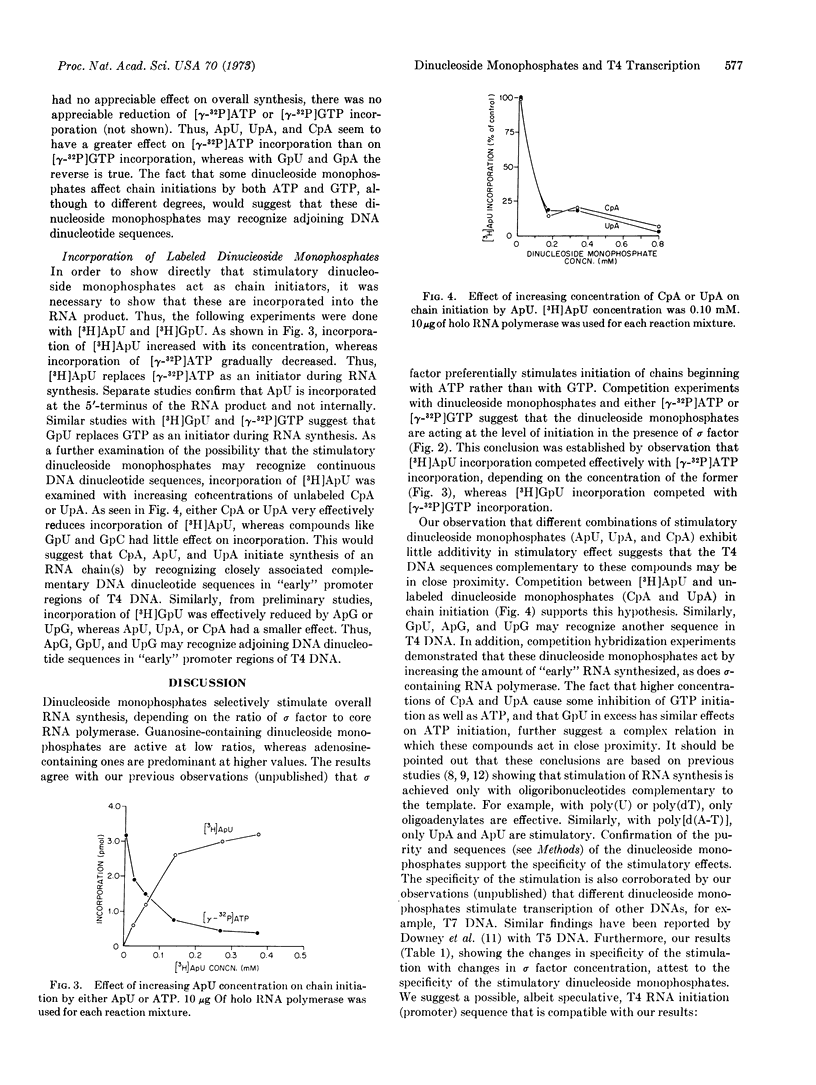
The effects of dinucleoside monophosphates on the transcription of phage T4 DNA by E. coli RNA polymerase have been examined at various concentrations of the sigma subunit and extremely low concentration of ribonucleoside triphosphate. The following conclusions were reached: (i) Labeled specific dinucleoside monophosphates are incorporated as chain initiators. (ii) When the ratio of sigma factor to core enzyme is small, there is a general stimulation by most 5′-guanosyl dinucleoside monophosphates. (iii) When the ratio is increased or holoenzyme is present, ApU, CpA, UpA, and GpU are the most effective stimulators. (iv) At high concentrations of sigma factor, only certain adenosine-containing dinucleoside monophosphates (ApU, CpA, UpA, and ApA) stimulate the reaction. (v) Competition hybridization studies indicate that the RNAs stimulated by dinucleoside monophosphates (ApU, CpA, UpA, and GpU) are of the T4 “early” type. (vi) Studies involving both combinations of stimulatory dinucleoside monophosphates and competitive effects of these compounds on chain initiation by ATP and GTP suggest that the stimulatory dinucleoside monophosphates act as chain initiators and may recognize part of a continuous sequence in a promoter region. Studies based on the incorporation of 3H-labeled stimulatory dinucleoside monophosphates support the above conclusions.
Keywords: dinucleoside monophosphate stimulation, sigma factor, dinucleoside monophosphate initiation
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler K., Beyreuther K., Fanning E., Geisler N., Gronenborn B., Klemm A., Müller-Hill B., Pfahl M., Schmitz A. How lac repressor binds to DNA. Nature. 1972 Jun 9;237(5354):322–327. doi: 10.1038/237322a0. [DOI] [PubMed] [Google Scholar]
- Bautz E. K., Bautz F. A., Dunn J. J. E. coli sigma factor: a positive control element in phage T4 development. Nature. 1969 Sep 6;223(5210):1022–1024. doi: 10.1038/2231022a0. [DOI] [PubMed] [Google Scholar]
- Bautz E. K., Bautz F. A. Initiation of RNA synthesis: the function of sigma in the binding of RNA polymerase to promoter sites. Nature. 1970 Jun 27;226(5252):1219–1222. doi: 10.1038/2261219a0. [DOI] [PubMed] [Google Scholar]
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: synthesis and relative stability of early and late RNA. J Mol Biol. 1968 Feb 14;31(3):325–348. doi: 10.1016/0022-2836(68)90413-0. [DOI] [PubMed] [Google Scholar]
- Bremer H., Konrad M. W., Gaines K., Stent G. S. Direction of chain growth in enzymic RNA synthesis. J Mol Biol. 1965 Sep;13(2):540–553. doi: 10.1016/s0022-2836(65)80116-4. [DOI] [PubMed] [Google Scholar]
- Brody E. N., Geiduschek E. P. Transcription of the bacteriophage T4 template. Detailed comparison of in vitro and in vivo transcripts. Biochemistry. 1970 Mar 17;9(6):1300–1309. doi: 10.1021/bi00808a002. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Burgess R. R., Travers A. A., Dunn J. J., Bautz E. K. Factor stimulating transcription by RNA polymerase. Nature. 1969 Jan 4;221(5175):43–46. doi: 10.1038/221043a0. [DOI] [PubMed] [Google Scholar]
- Downey K. M., Jurmark B. S., So A. G. Determination of nucleotide sequences at promoter regions by the use of dinucleotides. Biochemistry. 1971 Dec 21;10(26):4970–4975. doi: 10.1021/bi00802a021. [DOI] [PubMed] [Google Scholar]
- Downey K. M., So A. G. Studies on the kinetics of ribonucleic acid chain initiation and elongation. Biochemistry. 1970 Jun 9;9(12):2520–2525. doi: 10.1021/bi00814a019. [DOI] [PubMed] [Google Scholar]
- Jones O. W., Berg P. Studies on the binding of RNA polymerase to polynucleotides. J Mol Biol. 1966 Dec 28;22(2):199–209. doi: 10.1016/0022-2836(66)90126-4. [DOI] [PubMed] [Google Scholar]
- Krakow J. S., Daley K., Karstadt M. Azotobacter vinelandii RNA polymerase. VII. Enzyme transitions during unprimed r[I-C] synthesis. Proc Natl Acad Sci U S A. 1969 Feb;62(2):432–437. doi: 10.1073/pnas.62.2.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. Y., Riggs A. D. Lac repressor binding to DNA not containing the lac operator and to synthetic poly dAT. Nature. 1970 Dec 19;228(5277):1184–1186. doi: 10.1038/2281184a0. [DOI] [PubMed] [Google Scholar]
- Maitra U., Hurwitz H. The role of DNA in RNA synthesis, IX. Nucleoside triphosphate termini in RNA polymerase products. Proc Natl Acad Sci U S A. 1965 Sep;54(3):815–822. doi: 10.1073/pnas.54.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIYOGI S. K., STEVENS A. STUDIES OF THE RIBONUCLEIC ACID POLYMERASE FROM ESCHERICHIA COLI. IV. EFFECT OF OLIGONUCLEOTIDES ON THE RIBONUCLEIC ACID POLYMERASE REACTION WITH SYNTHETIC POLYRIBONUCLEOTIDES AS TEMPLATES. J Biol Chem. 1965 Jun;240:2593–2598. [PubMed] [Google Scholar]
- Niyogi S. K. Effect of sigma factor and oligoribonucleotides on the transcription of well-defined templates with the ribonucleic acid polymerase of Escherichia coli. J Mol Biol. 1972 Mar 14;64(3):609–618. doi: 10.1016/0022-2836(72)90086-1. [DOI] [PubMed] [Google Scholar]
- Shishido K., Ikeda Y. Preferential binding of E. coli RNA polymerase to the AT-rich regions of bacteriophage f1 DNA. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1420–1428. doi: 10.1016/s0006-291x(71)80244-9. [DOI] [PubMed] [Google Scholar]
- Stevens A. Studies of the ribonucleic acid polymerase from Escherichia coli. V. Studies of its complexes with polyribonucleotides. J Biol Chem. 1969 Jan 25;244(2):425–429. [PubMed] [Google Scholar]
- Sugiura M., Okamoto T., Takanami M. RNA polymerase sigma-factor and the selection of initiation site. Nature. 1970 Feb 14;225(5233):598–600. doi: 10.1038/225598a0. [DOI] [PubMed] [Google Scholar]
- Travers A. A., Burgessrr Cyclic re-use of the RNA polymerase sigma factor. Nature. 1969 May 10;222(5193):537–540. doi: 10.1038/222537a0. [DOI] [PubMed] [Google Scholar]
- di Mauro E., Synder L., Marino P., Lamberti A., Coppo A., Tocchini-Valentini G. P. Rifampicin sensitivity of the components of DNA-dependent RNA polymerase. Nature. 1969 May 10;222(5193):533–537. doi: 10.1038/222533a0. [DOI] [PubMed] [Google Scholar]


