Abstract
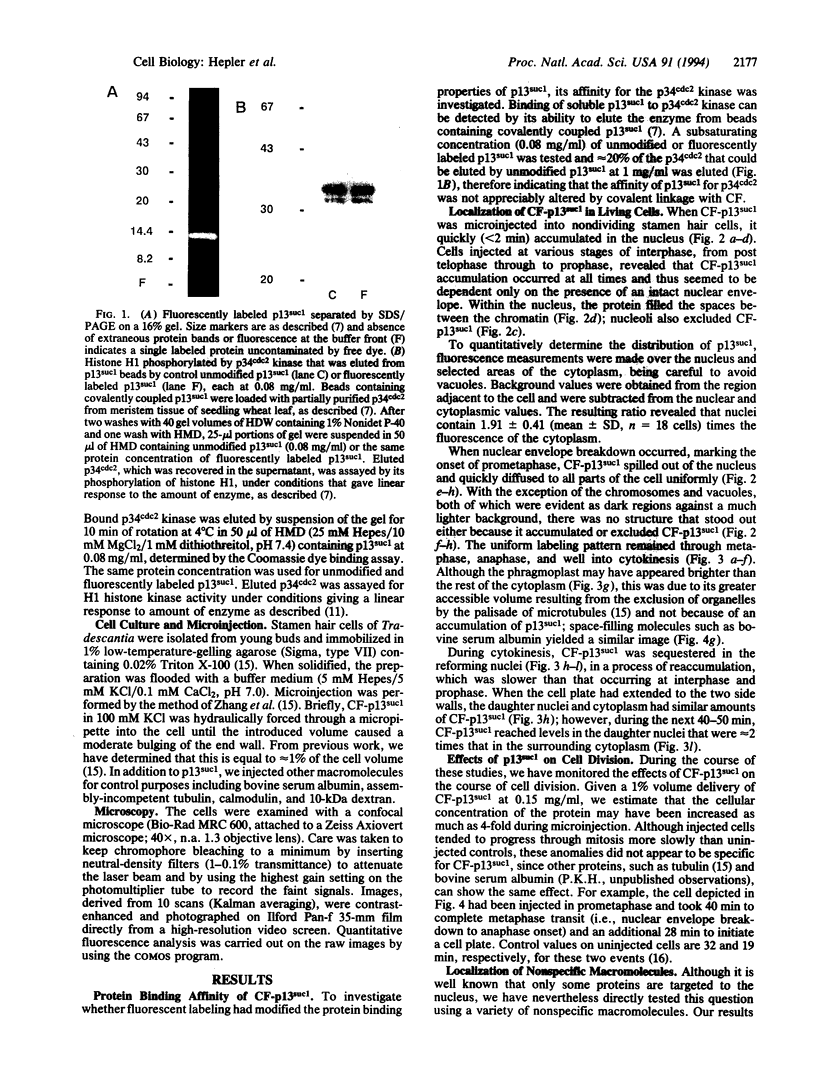
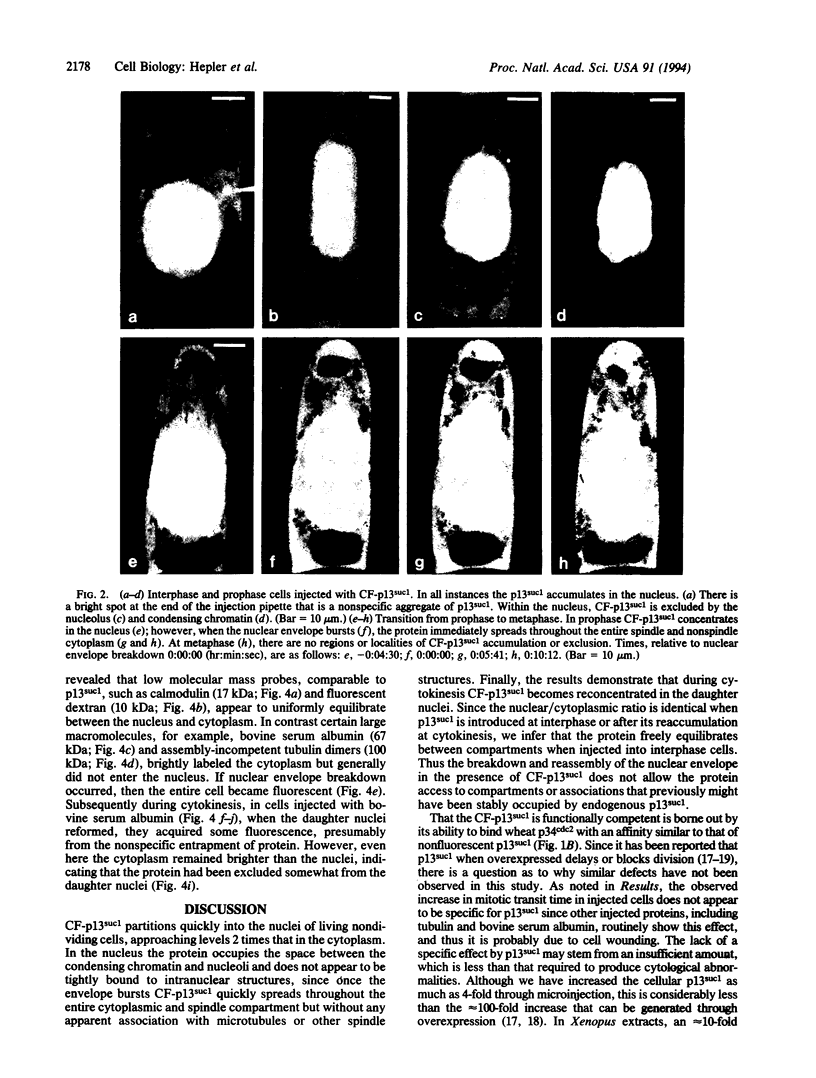
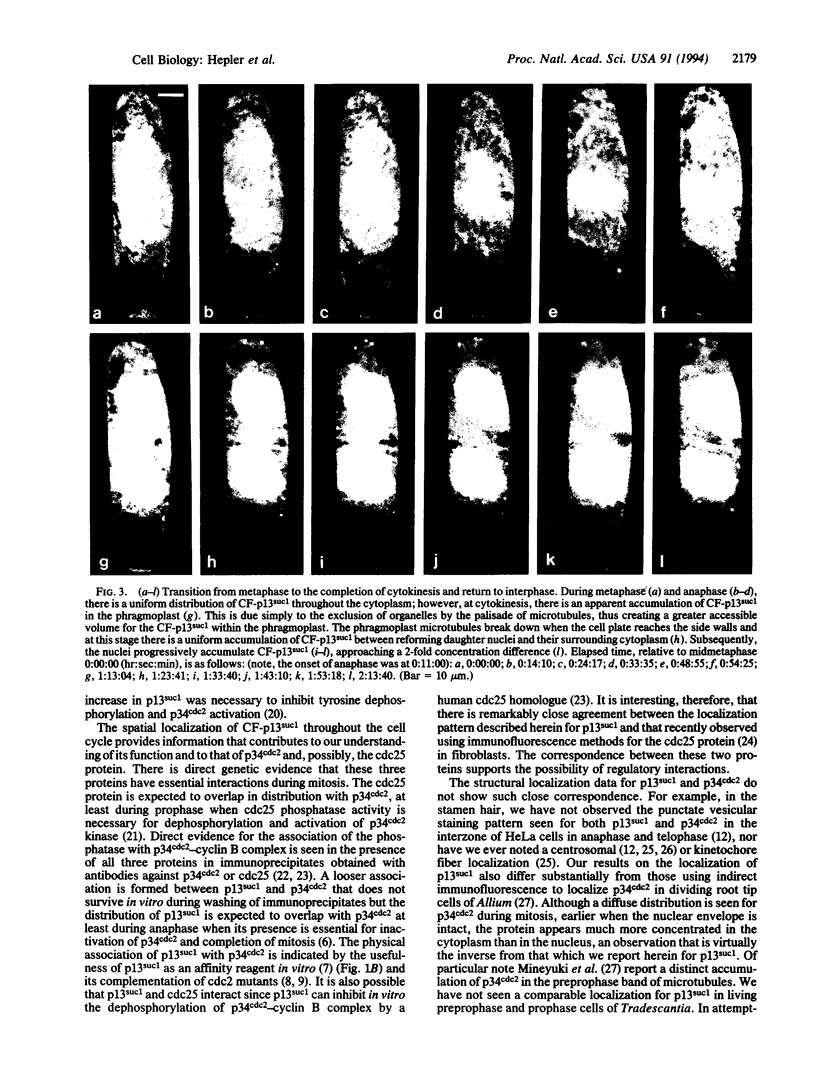
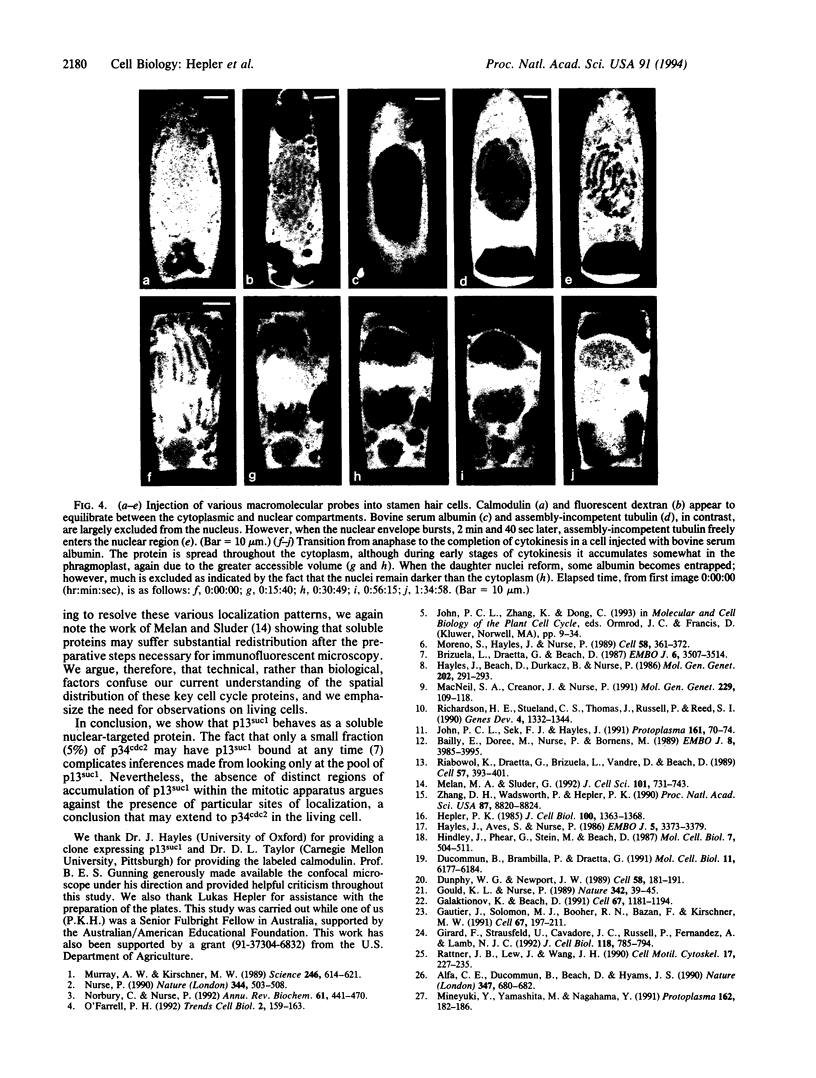
Stamen hair cells of Tradescantia virginiana have been microinjected with p13suc1 labeled with carboxyfluorescein (CF) and studied throughout the division cycle in living cells by using the confocal laser scanning microscope. The protein, p13suc1, is essential for the rapid inactivation of the key mitotic catalyst, p34cdc2 kinase, at anaphase and for completion of nuclear division. During interphase or prophase, CF-p13suc1 concentrates quickly (< 2 min) in nuclei, reaching levels that are approximately 2-fold greater than those in the cytoplasm. At nuclear envelope breakdown, CF-p13suc1 permeates throughout the entire spindle and nonspindle cytoplasm. The protein is excluded from the tightly condensed chromosomes but otherwise no regions accumulate or exclude the protein. It remains evenly distributed throughout metaphase, anaphase, and well into cytokinesis; however, during telophase CF-p13suc1 reconcentrates in the daughter nuclei.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfa C. E., Ducommun B., Beach D., Hyams J. S. Distinct nuclear and spindle pole body population of cyclin-cdc2 in fission yeast. Nature. 1990 Oct 18;347(6294):680–682. doi: 10.1038/347680a0. [DOI] [PubMed] [Google Scholar]
- Bailly E., Dorée M., Nurse P., Bornens M. p34cdc2 is located in both nucleus and cytoplasm; part is centrosomally associated at G2/M and enters vesicles at anaphase. EMBO J. 1989 Dec 20;8(13):3985–3995. doi: 10.1002/j.1460-2075.1989.tb08581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizuela L., Draetta G., Beach D. p13suc1 acts in the fission yeast cell division cycle as a component of the p34cdc2 protein kinase. EMBO J. 1987 Nov;6(11):3507–3514. doi: 10.1002/j.1460-2075.1987.tb02676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducommun B., Brambilla P., Draetta G. Mutations at sites involved in Suc1 binding inactivate Cdc2. Mol Cell Biol. 1991 Dec;11(12):6177–6184. doi: 10.1128/mcb.11.12.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy W. G., Newport J. W. Fission yeast p13 blocks mitotic activation and tyrosine dephosphorylation of the Xenopus cdc2 protein kinase. Cell. 1989 Jul 14;58(1):181–191. doi: 10.1016/0092-8674(89)90414-5. [DOI] [PubMed] [Google Scholar]
- Galaktionov K., Beach D. Specific activation of cdc25 tyrosine phosphatases by B-type cyclins: evidence for multiple roles of mitotic cyclins. Cell. 1991 Dec 20;67(6):1181–1194. doi: 10.1016/0092-8674(91)90294-9. [DOI] [PubMed] [Google Scholar]
- Gautier J., Solomon M. J., Booher R. N., Bazan J. F., Kirschner M. W. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991 Oct 4;67(1):197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- Girard F., Strausfeld U., Cavadore J. C., Russell P., Fernandez A., Lamb N. J. cdc25 is a nuclear protein expressed constitutively throughout the cell cycle in nontransformed mammalian cells. J Cell Biol. 1992 Aug;118(4):785–794. doi: 10.1083/jcb.118.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. L., Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989 Nov 2;342(6245):39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- Hayles J., Aves S., Nurse P. suc1 is an essential gene involved in both the cell cycle and growth in fission yeast. EMBO J. 1986 Dec 1;5(12):3373–3379. doi: 10.1002/j.1460-2075.1986.tb04653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayles J., Beach D., Durkacz B., Nurse P. The fission yeast cell cycle control gene cdc2: isolation of a sequence suc1 that suppresses cdc2 mutant function. Mol Gen Genet. 1986 Feb;202(2):291–293. doi: 10.1007/BF00331653. [DOI] [PubMed] [Google Scholar]
- Hepler P. K. Calcium restriction prolongs metaphase in dividing Tradescantia stamen hair cells. J Cell Biol. 1985 May;100(5):1363–1368. doi: 10.1083/jcb.100.5.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindley J., Phear G., Stein M., Beach D. Sucl+ encodes a predicted 13-kilodalton protein that is essential for cell viability and is directly involved in the division cycle of Schizosaccharomyces pombe. Mol Cell Biol. 1987 Jan;7(1):504–511. doi: 10.1128/mcb.7.1.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeill S. A., Creanor J., Nurse P. Isolation, characterisation and molecular cloning of new mutant alleles of the fission yeast p34cdc2+ protein kinase gene: identification of temperature-sensitive G2-arresting alleles. Mol Gen Genet. 1991 Sep;229(1):109–118. doi: 10.1007/BF00264219. [DOI] [PubMed] [Google Scholar]
- Melan M. A., Sluder G. Redistribution and differential extraction of soluble proteins in permeabilized cultured cells. Implications for immunofluorescence microscopy. J Cell Sci. 1992 Apr;101(Pt 4):731–743. doi: 10.1242/jcs.101.4.731. [DOI] [PubMed] [Google Scholar]
- Moreno S., Hayles J., Nurse P. Regulation of p34cdc2 protein kinase during mitosis. Cell. 1989 Jul 28;58(2):361–372. doi: 10.1016/0092-8674(89)90850-7. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Kirschner M. W. Dominoes and clocks: the union of two views of the cell cycle. Science. 1989 Nov 3;246(4930):614–621. doi: 10.1126/science.2683077. [DOI] [PubMed] [Google Scholar]
- Norbury C., Nurse P. Animal cell cycles and their control. Annu Rev Biochem. 1992;61:441–470. doi: 10.1146/annurev.bi.61.070192.002301. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. Cell cycle control: many ways to skin a cat. Trends Cell Biol. 1992 Jun;2(6):159–163. doi: 10.1016/0962-8924(92)90034-k. [DOI] [PubMed] [Google Scholar]
- Rattner J. B., Lew J., Wang J. H. p34cdc2 kinase is localized to distinct domains within the mitotic apparatus. Cell Motil Cytoskeleton. 1990;17(3):227–235. doi: 10.1002/cm.970170309. [DOI] [PubMed] [Google Scholar]
- Riabowol K., Draetta G., Brizuela L., Vandre D., Beach D. The cdc2 kinase is a nuclear protein that is essential for mitosis in mammalian cells. Cell. 1989 May 5;57(3):393–401. doi: 10.1016/0092-8674(89)90914-8. [DOI] [PubMed] [Google Scholar]
- Richardson H. E., Stueland C. S., Thomas J., Russell P., Reed S. I. Human cDNAs encoding homologs of the small p34Cdc28/Cdc2-associated protein of Saccharomyces cerevisiae and Schizosaccharomyces pombe. Genes Dev. 1990 Aug;4(8):1332–1344. doi: 10.1101/gad.4.8.1332. [DOI] [PubMed] [Google Scholar]
- Zhang D., Wadsworth P., Hepler P. K. Microtubule dynamics in living dividing plant cells: confocal imaging of microinjected fluorescent brain tubulin. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8820–8824. doi: 10.1073/pnas.87.22.8820. [DOI] [PMC free article] [PubMed] [Google Scholar]