Abstract
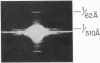
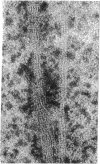
Deoxyhemoglobin from patients homozygous for sickle-cell anemia (deoxyhb S) aggregates into long straight fibers. These may extend through most of the length of the sickled cell, forming either square or hexagonally packed bundles with lattice constants of 170-180 Å. Each fiber is a tube made up of six thin filaments, which are wound around the tubular surface with a helical pitch of about 3000 Å. Each filament is a string of single hemoglobin molecules linked end to end at intervals of 62 Å in dry and 64 Å in wet fibers. Molecules in neighboring filaments are in longitudinal register so that they form flat hexagonal rings; these rings are stacked so that successive ones are rotated about the fiber axis by 7.3°. The whole structure repeats after about eight rings. In this structure each molecule makes contact with four neighbors. The likely orientation of the molecules and points of contact between them are discussed. Similar filaments are also observed in normal deoxygenated erythrocytes, but in much lower concentration and aggregated into fibers of irregular diameter. No filaments appear in oxygenated sickle, or normal, adult cells, nor in oxygenated or deoxygenated fetal cells.
Keywords: electron microscopy, x-ray diffraction, molecular arrangement, helices
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BESSIS M., NOMARSKI G., THIERY J. P., BRETON-GORIUS J. Etude sur la falciformation des globules rouges au microscope polarisant et au microscope électronique. II. L'intérieru du globule; comparaison avec les cristaux intra-globulaires. Rev Hematol. 1958 Apr-Jun;13(2):249–270. [PubMed] [Google Scholar]
- Bertles J. F., Rabinowitz R., Döbler J. Hemoglobin interaction: modification of solid phase composition in the sickling phenomenon. Science. 1970 Jul 24;169(3943):375–377. doi: 10.1126/science.169.3943.375. [DOI] [PubMed] [Google Scholar]
- Blackwell R. Q., Oemijati S., Pribadi W., Weng M. I., Liu C. S. Hemoglobin G Makassar: beta-6 Glu leads to Ala. Biochim Biophys Acta. 1970 Sep 29;214(3):396–401. [PubMed] [Google Scholar]
- Bookchin R. M., Nagel R. L., Ranney H. M. The effect of beta 73 Asn on the interactions of sickling hemoglobins. Biochim Biophys Acta. 1970 Nov 17;221(2):373–375. doi: 10.1016/0005-2795(70)90279-5. [DOI] [PubMed] [Google Scholar]
- Cerami A., Manning J. M. Potassium cyanate as an inhibitor of the sickling of erythrocytes in vitro. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1180–1183. doi: 10.1073/pnas.68.6.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döbler J., Bertles J. F. The physical state of hemoglobin in sickle-cell anemia erythrocytes in vivo. J Exp Med. 1968 Apr 1;127(4):711–714. doi: 10.1084/jem.127.4.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLUG A., BERGER J. E. AN OPTICAL METHOD FOR THE ANALYSIS OF PERIODICITIES IN ELECTRON MICROGRAPHS, AND SOME OBSERVATIONS ON THE MECHANISM OF NEGATIVE STAINING. J Mol Biol. 1964 Dec;10:565–569. doi: 10.1016/s0022-2836(64)80081-4. [DOI] [PubMed] [Google Scholar]
- Magdoff-Fairchild B., Swerdlow P. H., Bertles J. F. Intermolecular organization of deoxygenated sickle haemoglobin determined by x-ray diffraction. Nature. 1972 Sep 22;239(5369):217–219. doi: 10.1038/239217a0. [DOI] [PubMed] [Google Scholar]
- Muirhead H., Cox J. M., Mazzarella L., Perutz M. F. Structure and function of haemoglobin. 3. A three-dimensional fourier synthesis of human deoxyhaemoglobin at 5.5 Angstrom resolution. J Mol Biol. 1967 Aug 28;28(1):117–156. doi: 10.1016/s0022-2836(67)80082-2. [DOI] [PubMed] [Google Scholar]
- Murayama M. Molecular mechanism of red cell "sickling". Science. 1966 Jul 8;153(3732):145–149. doi: 10.1126/science.153.3732.145. [DOI] [PubMed] [Google Scholar]
- PERUTZ M. F., MITCHISON J. M. State of haemoglobin in sickle-cell anaemia. Nature. 1950 Oct 21;166(4225):677–679. doi: 10.1038/166677a0. [DOI] [PubMed] [Google Scholar]
- PERUTZ R. R., LIQUORI A. M., EIRICH F. X-ray and solubility studies of the haemoglobin of sickle-cell anaemia patients. Nature. 1951 Jun 9;167(4258):929–931. doi: 10.1038/167929a0. [DOI] [PubMed] [Google Scholar]
- Ranney H. M. The interactions of sickle hemoglobin. Biochimie. 1972;54(5):633–638. doi: 10.1016/s0300-9084(72)80154-8. [DOI] [PubMed] [Google Scholar]
- SINGER K., SINGER L. Studies on abnormal hemoglobins. VIII. The gelling phenomenon of sickle cell hemoglobin: its biologic and diagnostic significance. Blood. 1953 Nov;8(11):1008–1023. [PubMed] [Google Scholar]
- Stetson C. A., Jr The state of hemoglobin in sickled erythrocytes. J Exp Med. 1966 Feb 1;123(2):341–346. doi: 10.1084/jem.123.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. G., Heagan B. Gels of normal and sickled hemoglobin: comparative study. J Exp Med. 1970 Jun 1;131(6):1079–1092. doi: 10.1084/jem.131.6.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. G. The fine structure of sickled hemoglobin in situ. Blood. 1968 May;31(5):561–579. [PubMed] [Google Scholar]