Abstract
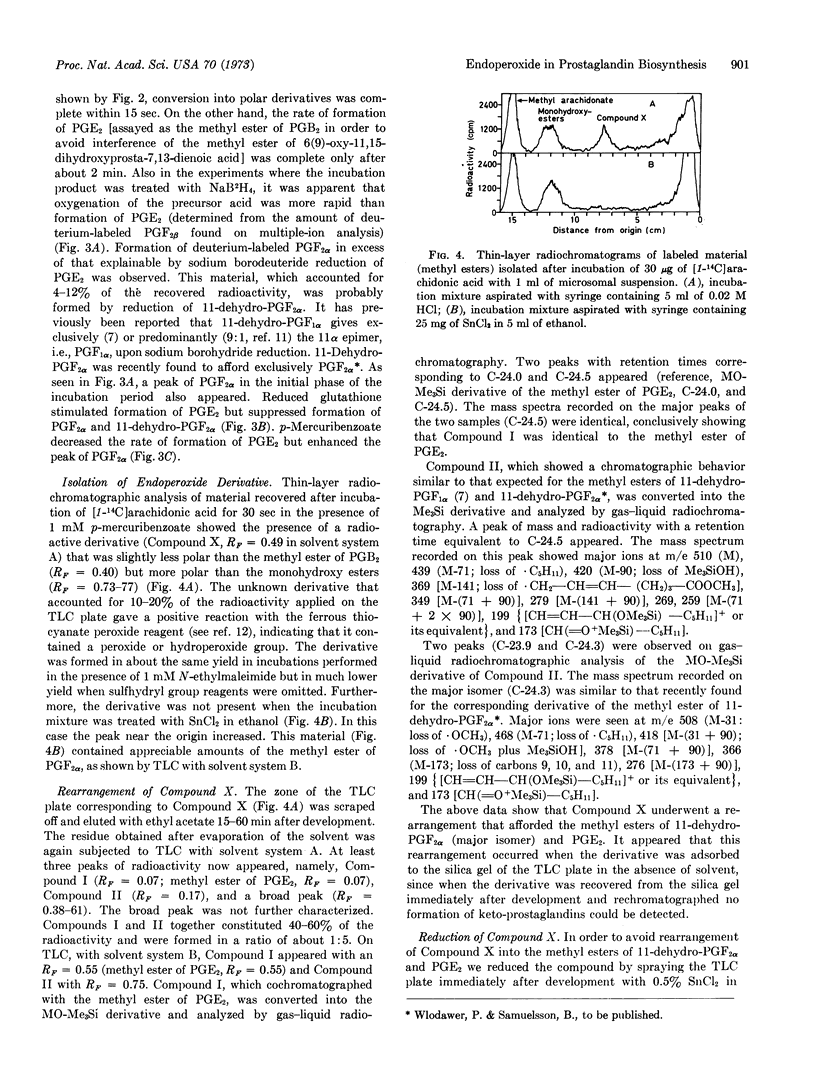
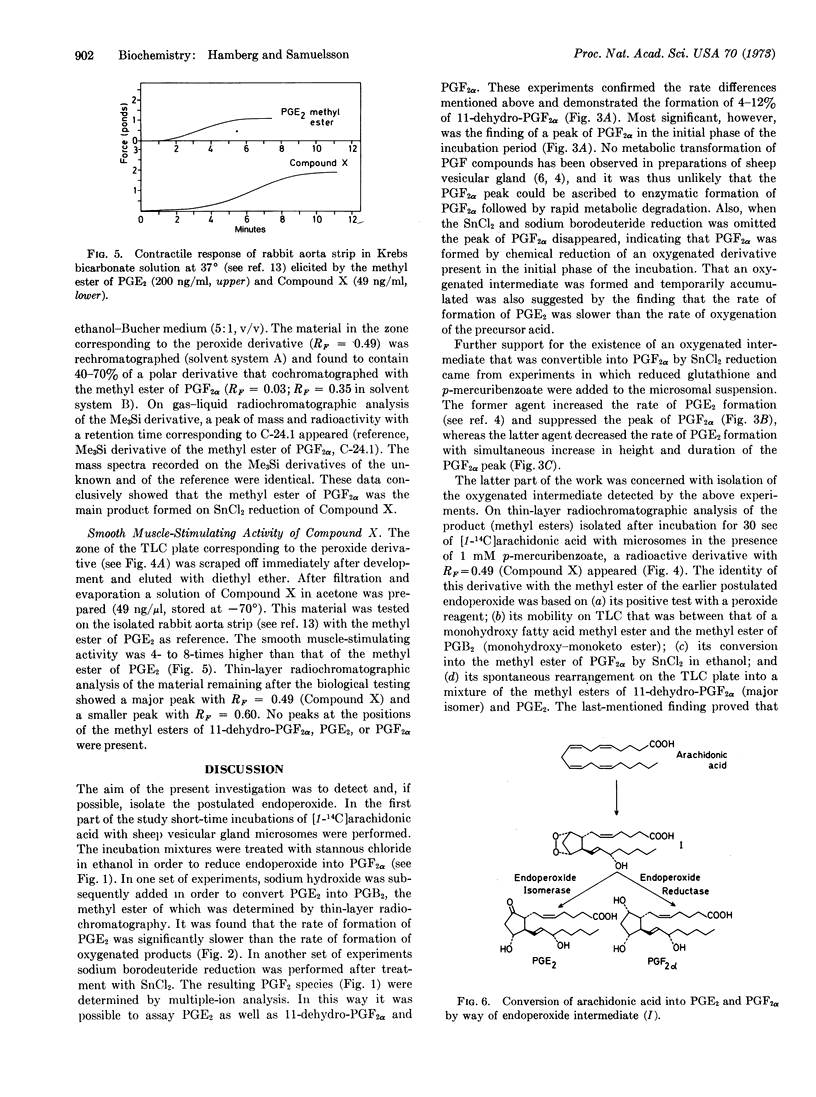
An earlier proposed endoperoxide intermediate in the biosynthesis of prostaglandins was detected in short-time incubations of arachidonic acid with the microsomal fraction of homogenates of sheep vesicular glands. Conversion of the endoperoxide into prostaglandin E2 was stimulated by reduced glutathione but suppressed by p-mercuribenzoate and N-ethylmaleimide. The methyl ester of an unknown compound was isolated by solvent extraction and thin-layer chromatography after short-time incubation of arachidonic acid with the microsomal fraction and p-mercuribenzoate. This derivative was identical to the methylester of the endoperoxide, as shown by its conversion into the methyl esters of 11-dehydroprostaglandin F2α and prostaglandin E2 by spontaneous rearrangement and its conversion into the methyl ester of prostaglandin F2α by mild chemical reduction. The smooth muscle-stimulating activity of the endoperoxide ester on the isolated rabbit aortas trip was 4- to 8-times higher than that of the methyl ester of prostaglandin E2.
Keywords: fatty acid oxygenase, endoperoxide isomerase, endoperoxide reductase, rabbit aorta-contracting substance
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Foss P. S., Sih C. J., Takeguchi C., Schnoes H. Biosynthesis and chemistry of 9 ,15(S)-dihydroxy-11-oxo-13-trans-prostenoic acid. Biochemistry. 1972 Jun 6;11(12):2271–2277. doi: 10.1021/bi00762a010. [DOI] [PubMed] [Google Scholar]
- Granström E., Lands W. E., Samuelsson B. Biosynthesis of 9-alpha,15-dihydroxy-11-ketoprost-13-enoic acid. J Biol Chem. 1968 Aug 10;243(15):4104–4108. [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. On the mechanism of the biosynthesis of prostaglandins E-1 and F-1-alpha. J Biol Chem. 1967 Nov 25;242(22):5336–5343. [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Oxygenation of unsaturated fatty acids by the vesicular gland of sheep. J Biol Chem. 1967 Nov 25;242(22):5344–5354. [PubMed] [Google Scholar]
- Pace-Asciak C., Wolfe L. S. A novel prostaglandin derivative formed from arachidonic acid by rat stomach homogenates. Biochemistry. 1971 Sep 28;10(20):3657–3664. doi: 10.1021/bi00796a004. [DOI] [PubMed] [Google Scholar]
- Piper P. J., Vane J. R. Release of additional factors in anaphylaxis and its antagonism by anti-inflammatory drugs. Nature. 1969 Jul 5;223(5201):29–35. doi: 10.1038/223029a0. [DOI] [PubMed] [Google Scholar]
- SAMUELSSON B. ON THE INCORPORATION OF OXYGEN IN THE CONVERSION OF 8, 11, 14-EICOSATRIENOIC ACID TO PROSTAGLANDIN E1. J Am Chem Soc. 1965 Jul 5;87:3011–3013. doi: 10.1021/ja01091a043. [DOI] [PubMed] [Google Scholar]
- Samuelsson B. Biosynthesis of prostaglandins. Fed Proc. 1972 Sep-Oct;31(5):1442–1450. [PubMed] [Google Scholar]
- Sweeley C. C., Elliott W. H., Fries I., Ryhage R. Mass spectrometric determination of unresolved components in gas chromatographic effluents. Anal Chem. 1966 Oct;38(11):1549–1553. doi: 10.1021/ac60243a023. [DOI] [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Wlodawer P., Samuelsson B., Albonico S. M., Corey E. J. Selective inhibition of prostaglandin synthetase by a bicyclo[2.2.1]heptene derivative. J Am Chem Soc. 1971 Jun 2;93(11):2815–2816. doi: 10.1021/ja00740a056. [DOI] [PubMed] [Google Scholar]


