Abstract
Tocotrienol rich fraction (TRF) is an extract of palm oil, which consists of 25% alpha tocopherol (α-TCP) and 75% tocotrienols. TRF has been shown to possess potent antioxidant, anti-inflammatory, anticancer, neuroprotection, and cholesterol lowering activities. Glutamate is the main excitatory amino acid neurotransmitter in the central nervous system of mammalian, which can be excitotoxic, and it has been suggested to play a key role in neurodegenerative disorders like Parkinson's and Alzheimer's diseases. In this present study, the effects of vitamin E (TRF and α-TCP) in protecting astrocytes against glutamate injury were elucidated. Astrocytes induced with 180 mM of glutamate lead to significant cell death. However, glutamate mediated cytotoxicity was diminished via pre and post supplementation of TRF and α-TCP. Hence, vitamin E acted as a potent antioxidant agent in recovering mitochondrial injury due to elevated oxidative stress, and enhanced better survivability upon glutamate toxicity.
KEY WORDS: vitamin E, tocotrienol rich fraction, alpha tocopherol, glutamate, astrocytes
INTRODUCTION
The expansion of an in vitro model for the early stages of neurodegenerative disease is a current inevitability. Neurodegenerative disease is one of the leading causes of death throughout the world [1]. It has been considered as one of the major problems for our aging society and well-defined as a group of illnesses of the nervous system, which comprises of brain, spinal cord, as well as peripheral nerves [1]. Degenerative nerve diseases result in the deterioration of several human body activities like talking, balancing, moving, breathing, and cardiac function [2]. Oxidative stress and reactive oxygen species (ROS) have been implicated in the development of neurodegenerative diseases [3]. An in vitro model of these processes would improve our understanding of the development of neurodegenerative diseases, and enhance the development of further treatments.
Astrocytes are predominant cell types in the brain [4] and play a critical role in maintaining synaptic transmission, antioxidant defense, metabolic and ionic homeostasis, and trophic support, as well as protection of neurons [5]. Glutamate is a principal excitatory amino acid neurotransmitter, which is a messenger molecule that is released when nerve cells pass signals to each other and to their target organ. Like all neurotransmitters, glutamate harbor at specific recognition molecules on the receiving neuron, and plays an important role in most forms of neurodegenerative diseases, especially when there is an increased concentration of extracellular glutamate [6].
Since the brain consists of easily oxidized lipid and has a large oxygen consumption rate, they are consistently deficient of antioxidant contents. Brain is susceptible towards oxidative injury, which will further damage the cell lipid, protein, and DNA [7]. Oxidative stress also plays a role in the modulation of critical cellular functions, such as apoptosis program activation, ion transport, and calcium mobilization, which lead to cell death [8,9]. Thus, several studies have been carried out to prevent nerve cell death caused by oxidative stress through the administration of free radical scavenging antioxidant, such as vitamin E. Vitamin E is a well-known chain-breaking antioxidant, with the ability to increase the viability of neuronal cells that had undergone glutamate injury [10].
Vitamin E is composed of eight different isoforms, four tocopherols (α-, β-, γ-, δ-), and four tocotrienols (α-, β-, γ-, δ-), which have been identified with neuroprotective properties. In human, the presence of alpha-tocopherol transfer protein (α-TTP) renders the bioavailability of alpha tocopherol (α-TCP) to be higher than α-tocotrienol. Despite its low concentration, tocotrienol is more effective than tocopherol in protecting cells from oxidative stress [11]. It is of a particular interest that the slight structural differences between tocopherol and tocotrienol can account for the greater physiological activities found in tocotrienol.
Tocotrienol rich fraction (TRF) is an extract of palm oil, and consists of 25% of α-TCP and 75% tocotrienols. TRF has been shown to possess potent antioxidant [12,13], anti-inflammatory [14], anticancer [15-17], neuroprotection [10,18], and cholesterol-lowering [19-21] activities. Crude palm oil extracted from the oil palm fruits (Elaeis guineensis) mostly comprises huge volume of tocotrienols (about 800 mg/kg), which mostly contain γ-tocotrienol, α-tocotrienol, and δ-tocotrienol. Other sources for tocotrienols are from barley, rice bran, rye, and wheat germ. Refined palm oil contains about 350-440 ppm of vitamin E, which consists of tocopherol (30%) and tocotrienol (70%) [22].
The effects of tocotrienols and tocopherols against glutamate injury in neuronal cells have been extensively studied [22]; however, to our knowledge, there is still lack of information on their effects in astrocytes. It is expected that the prophylactic and preventive functions of tocotrienols and tocopherol in neurodegeneration could be achieved and there could be possibilities of effective nutrition based therapeutics usages.
In addition, the use of vitamin E for the management of Alzheimer's disease is progressively becoming a topic of interest. Vitamin E treatment has been shown to slow the development of Alzheimer's disease [23], and might offer a therapeutically relevant solution. Vitamin E also prevents oxidative stress related cell death [24]. Although the effects of vitamin E on neuronal cells have been well documented, knowledge on astrocytes is still lacking and the concern on astrocytes could promote better protection to neuronal cells. Our brain is made up of billions of neurons, which are loyally supported by glial cells (astrocytes). Therefore, in order to function well, these neurons need astrocytes, as astrocytes support the function of neuronal in transmitting messages.
MATERIALS AND METHODS
Media, chemicals, and reagents
Human glioblastoma cells (DBTRG-05MG) were obtained from ATCC (Manassas, VA, USA). RPMI 1640 culture media, trypsin, fetal bovine serum (FBS), penicillin/streptomycin, and phosphate buffered saline (PBS) were acquired from Invitrogen (Carlsbad, CA, USA). 3-(4,5-dimethylthiazol-2-yl)-5-(carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium powder was gained from Phytotechnology Laboratories (Flint St, Overland Park, KS). Dimethyl sulfoxide (DMSO), Rhodamine 123, propidium iodide (PI), and other chemicals were obtained from Sigma (St. Louis, MO, USA).
MTT assay
Once the treated cells were incubated for 24 hours, MTT assay was carried out to determine the percentage of cells viable upon vitamin E supplementation against glutamate insult. This assay was carried out in two different conditions: pre-and post-treatments. Pre-treatment is defined as astrocytes were exposed to 100, 200, and 300 ng/mL of TRF and α-TCP before glutamate injury. Meanwhile, in post-treatment, the cells had undergone glutamate challenge before they were treated with various types and concentrations of vitamin E. The procedure of MTT assay was carried out by adding 50 µL of MTT into each well and was incubated for 4 hours. After that, all the contents of the well were removed with a syringe before 100 µL of DMSO was added into each well. The data related to absorbance, which reflected the viability of the cells, were taken at 570 nm with a background value at 630 nm by using the microplate reader [11]. The graph of the viability of the cells against vitamin E was plotted.
Mitochondria membrane potential assay (MMP assay)
The treated cells were incubated for 24 hours, and MMP assay was conducted as another indicator for the survival of cell upon the supplementation of vitamin E and glutamate challenge. On the third day (after seeding and treatment), the cells were washed with PBS and were stained with 50 µL Rhodamine 123 for 30 minutes. Rhodamine 123 is known as a fluorescent detection dye, as it will bind to the mitochondria of cells and inhibit electron transport chain (ETC). Thus, in healthy mitochondria, more Rhodamine 123 was needed in order to stop the process of ETC and to give high density of fluorescent detection. After 30 minutes of incubation with dye, the cells were washed with PBS and were read through a fluorescent microplate reader at a wavelength of 485 nm and emission at 530 nm [25]. The graph of mitochondrial membrane potentiality against vitamin E was plotted.
Thiobarbituric acid-reactive substance (TBARS) assay
The treated cells were washed with PBS twice and were trypsinized. The collected cells were sonicated in ice in a sonicator for a minute. After the addition of TBA and TCA, the cells were vortexed and were heated at 80°C for 40 minutes. After heating, the mixture was cooled to room temperature, and 50µL of 1-butanol was added. Then, the supernatant was transferred to respective eppendorf tubes, and was centrifuged at 6,000 rpm for 5 minutes at 4°C. After that, the pinkish supernatant was transferred to respective cuvette, and was read by a spectrophotometer at 535 nm to measure the concentration of malondialdehyde. The purpose of TBARS assay was to study the effects of TRF and α-TCP in quenching lipid peroxidation in the glutamate injured astrocytes.
Annexin V-FITC and PI staining assay
Quantitative morphological analysis was executed via Annexin V FITC apoptosis detection kit according to the protocol provided by the manufacturer. Astrocytes were seeded in 6 well plates at a density of 5 × 105 cells/mL. On the following day, the astrocytes were pre-and post-treated with various concentrations of vitamin E. The harvested cells were resuspended in 1x binding buffer. Subsequently, 5 µL of Annexin V-FITC, and 5 µL of PI were added into 100 µL of cells suspension, which were then incubated for 15 minutes in the dark. Next, it was followed by an addition of 400 µL ice-cold 1X binding buffer, and the solution was mixed gently. The samples were quantitatively analyzed using a flow cytometer (LSR Fortessa, USA).
Scanning electron microscopy
The astrocytes that were exposed to 200 ng/mL of TRF and α-TCP were subjected to scanning electron microscopy analysis to observe the morphological changes upon glutamate challenge. The cells that were grown on coverslips in 6 well plates were transferred to petri dishes and were fixed in 4% glutaraldehyde (Agar scientific, UK) for 4 hours at 4°C. Then, the samples were washed (0.1 M sodium cacodylate buffer), were post-fixed (1% osmium tetroxide Agar Scientific, UK), and were dehydrated (20%-90% alcohol, ChemAR® Systerm, Malaysia) before they were placed on a critical point dryer for 30 mins (Bal-tec CPD 030, Germany). After that, mounting was carried out by sticking the samples onto stubs. Finally, the specimens were gold coated in a sputter coater (Bal-tec SCD 005, Germany), prior to view under a variety of pressures via scanning electron microscope (Leo 1455 VPSEM attached with energy dispersive X-ray (EDX).
Cell cycle analysis (RNase/PI assay)
Astrocytes were collected 24 hours after pre-and post-treatment of vitamin E and were washed with PBS. The pellets were fixed in 70% ice cold ethanol and were kept overnight. Ethanol was removed through centrifugation (1200 rpm, 5 mins) and the pellets were washed thoroughly with PBS twice. The astrocytes pellets were resuspended in 425 µL of PBS, 50 µL of 1mg/mL RNase, and 25 µL of 1 mg/mL PI. This was followed by incubation of pellet mixture for 30 mins in dark, and the DNA contents of the cells were analyzed using a flow cytometer (BD Facs Calibur, USA) with cell quest pro software.
Data analysis
All the data retrieved were reported as the mean ± SEM. As for statistical analysis, one way analysis of variance (ANOVA) was used and Tukey's test was carried out for comparison in each treatment concentration using SPSS (Version 17.0, SPSS Inc., Chicago, IL, USA). A p-value less than 0.05 was considered as statistically significant.
RESULTS
Cell viability of glutamate-injured astrocytes against vitamin E treatment
The microvolume of tetrazolium test (MTT) assay is a potential indicator of the viability of cells, as it was used to evaluate the activity of enzyme within the mitochondria, which can reduce the yellow MTT solution to purple formazan [26]. The effects of TRF and α-TCP upon glutamate induced cytotoxicity were evaluated via MTT cell viability assay. Exposure to 180 mM of glutamate in astrocytes caused inhibition of cell viability approximately about 60%. In this study, TRF and α-TCP were pre-incubated for 5 minutes for pre-treatment purposes. Short pre-incubation time (5 mins) was used to compare the efficiency between TRF and α-TCP uptake. Pre-treatment with 100, 200, and 300 ng/mL of TRF and α-TCP increased the viability of the cells significantly with an average of 50.72%, 52.96%, 49.02%, and 58.94%, 60.24%, 59.50% respectively (Figure 1). This suggests that TRF and α-TCP, at low concentration and short pre-incubation period, exert potential prophylactic effect against the toxicity of glutamate in astrocyte. The proliferation rate for 100, 200, and 300 ng/mL of TRF treated cells were 53.13%, 60.81%, and 58.79% respectively, and it had been noticed in post-treatment as the TRF and α-TCP were given after 30 minutes incubation of glutamate. Meanwhile, α-TCP exhibited 59.46%, 60.12%, and 57.29% of cell survival after glutamate challenge (Figure 1).
FIGURE 1.
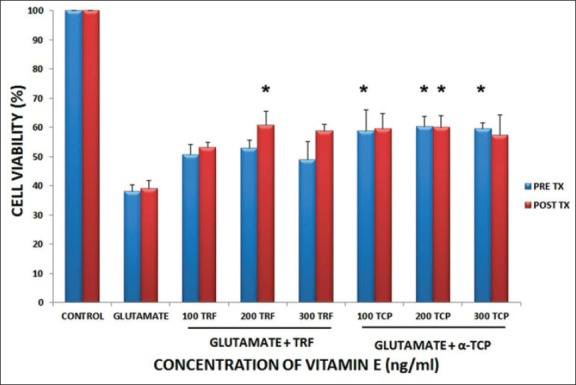
Effect of vitamin E pre and post treatment on astrocytes against 180 mM glutamate on cell viability. Data is presented as mean ± SEM of 3 independent experiments (n=3 in each experiment). *p<0.05, vitamin E treated groups compared with glutamate treated group.
Effects of vitamin E in preserving mitochondrial membrane potential of astrocytes after glutamate excitotoxicity
A pre-treatment study of mitochondrial membrane potential (MMP) assay elucidated the effects of vitamin E at 100, 200, and 300 ng/mL in preventing mitochondrial injury from glutamate toxicity. Figure 2 shows the results of pre-treatment of this assay with TRF and α-TCP, which indicated that MMP reached at 75.58%, 68.17%, and 69.92%, and at 54.41%, 75.69%, and 66.56% respectively. TRF and α-TCP, at low concentration and 24 hours of pre-incubation, exerted better prophylactic properties against the toxicity of glutamate in astrocytes. Nevertheless, 100 ng/mL of TRF and 200 ng/mL of α-TCP gave the highest MMP value.
FIGURE 2.
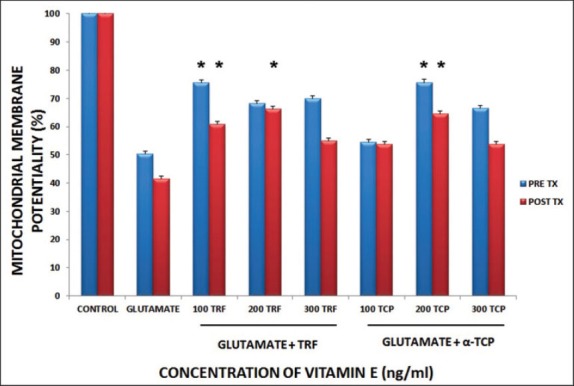
Pre and post treatment of vitamin E against glutamate injured astrocytes on mitochondrial membrane potentiality. Data is presented as mean ± SEM of 3 independent experiments (n=3 in each experiment). *p<0.05, vitamin E treated groups compared with glutamate treated group.
Next, a post-treatment of MMP assay was carried out to determine recovery effects of vitamin E upon glutamate insult in astrocytes. From the analysis carried out (Figure 2), both TRF and α-TCP showed increased MMP upon glutamate challenge. The MMP reached 60.81%, 66.28%, and 54.88% for TRF with 100, 200, and 300 ng/mL respectively. α-TCP treatment showed MMP values of 53.78%, 64.44%, and 53.77% at 100, 200, and 300 ng/mL respectively. Both TRF and α-TCP at low concentrations, which were 100 to 300 ng/mL, were able to prevent the decrease in the level of MMP for glutamate injured astrocytes.
Reduction of lipid peroxidation in glutamate treated astrocytes upon vitamin E treatment
The resultant oxidative stress was evaluated by identifying the level of lipid peroxidation via Thiobarbituric acid reactive substances (TBARS) assay. Measurement of malondialdehyde (MDA) was used as an indicator of lipid peroxidation. From the results depicted in Table 1, the concentration of MDA per protein in pre-treated astrocytes decreased significantly to 0.2031, 0.1947, and 0.1061 of MDA (µM/mg) at 100, 200 and 300 ng/mL TRF treatment respectively when compared to positive control. As for 100-300 ng/mL α-TCP, the MDA concentration decreased to 0.3040, 0.1643, and 0.1239 (µM/mg). In the pre-treatment study of TBARS assay, 300 ng/mL of TRF exhibited high potential in reducing MDA concentration per protein. Overall, Vitamin E pre-treated astrocytes displayed lower MDA concentration per protein than glutamate injured cells, which proved the potential prophylactic effects of TRF and α-TCP [27].
TABLE 1.
Effect of different doses of vitamin E (ng/mL) on astrocytes injured with 180 mM glutamate
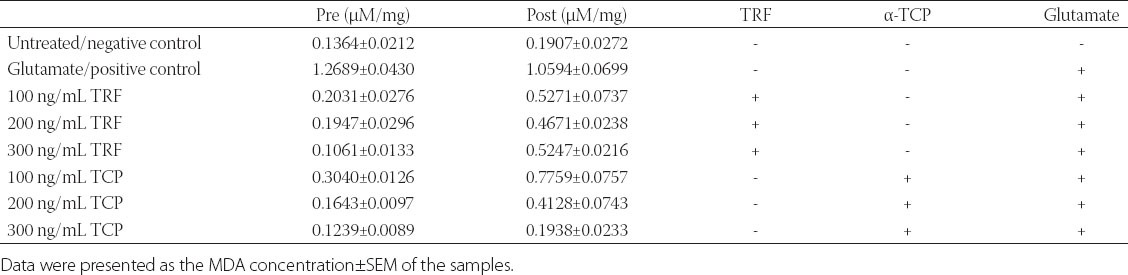
Furthermore, the post-treatment of TRF and α-TCP showed reduction in the concentrations of MDA per protein (µM/mg) against glutamate induced injury in astrocytes. The concentrations of MDA reduced to 0.5271, 0.4671, and 0.5247 MDA (µM/mg) at 100, 200, and 300 ng TRF treatment respectively, as compared to neurotoxic agent glutamate induced sample. As for α-TCP, the values of MDA decreased to 0.7759, 0.4128, and 0.1938 at 100-300ng/mL α-TCP. In the post-treatment study, 200 ng/mL of TRF and 300 ng/mL of α-TCP demonstrated a significant difference with the positive control. Post-treated astrocytes showed prominent reduction in the concentration of MDA per protein in both TRF and α-TCP treated groups.
TRF and α-TCP prevented glutamate induced cell death in astrocytes
In the pre-treatment study of annexin V-FITC apoptosis detection assay, the control showed 90.87% of viable cells, while glutamate treated group exhibited reduction in cell viability to 39.77%. Both TRF and α-TCP treated samples showed increment in cell viability compared to glutamate treated group. Concentration of 200 ng/mL of TRF portrayed the highest viability rate of 61.30% in this pre-treatment study. The number of cells that had undergone apoptosis and necrosis were lower in the untreated sample, as compared to glutamate induced model. By pre-treating the astrocytes with various concentrations of vitamin E, it can be clearly seen from Figure 3 that apoptotic and necrotic rates in vitamin E supplemented samples were lesser than those in glutamate injured group.
FIGURE 3.

Pre-treatment of TRF and α-TCP upon glutamate challenge on cell viability, apoptosis and necrosis. Results are the mean ± SEM in triplicates. *p<0.05, vitamin E treated groups compared with glutamate treated group.
A post-treatment of this apoptosis test showed 92.47% of cell viability for untreated cells, whereas the cell viability decreased to 43.83% for glutamate induced astrocytes. Both TRF and α-TCP treated samples increased the percentage of cell viability. As for TRF and α-TCP, concentration of 200 ng/mL exhibited higher percentage of cell viability in post-treatment. On the other hand, the glutamate treated cells had undergone higher apoptosis and necrosis rates than the untreated cells. The post-treated astrocytes with TRF and α-TCP had improved cell viability and decreased the number of cells that went through apoptosis and necrosis phases, as shown in Figure 4. Representative flowcytometric quadrants are shown in Figure 5
FIGURE 4.
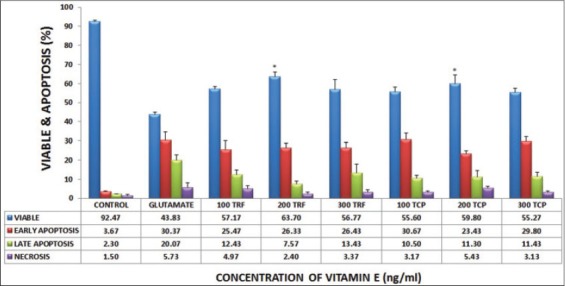
Effects of various concentrations of TRF and αTCP in DBTRG-05MG cell injured with 180 mM glutamate. * shown significant results with glutamate challenged astrocytes. Data were presented as mean± SEM of the samples.
FIGURE 5.
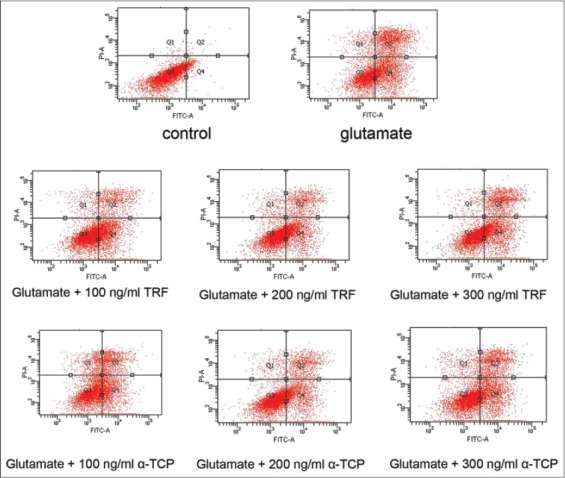
Determination of viable, apoptotic and necrotic cell death after exposure of astrocytes to 180 mM of glutamate by Annexin V-FITC flowcytometric staining assay. Q1: late necrosis; Q2: late apoptosis; Q3: viable cells; Q4: early apoptosis. Results are representative quadrants of 3 independent experiments.
Morphological analysis of astrocytes via scanning electron microscopy
Apart from flowcytometric annexin V-FITC apoptosis detection assay analysis, the effects of vitamin E were also examined morphologically via scanning electron microscopy. Visible difference was noticed in the morphology of untreated sample with glutamate treated and vitamin E supplemented groups. The untreated cells possess smooth, finite, and rigid surface area with good cell membrane integrity (Figure 6a). Cells treated with 180 mM glutamate alone showed damage evidenced by blebbing, rounded appearance, irregular plasmalemma, and loss of refraction fibers (Figure 6b). Cells that were pre-and post-treated with 200 ng/mL TRF and α-TCP had similar appearance to control cells (untreated), although many contained fragmented processes and blebs. Cellular debris was also noted (6c-6f).
FIGURE 6.
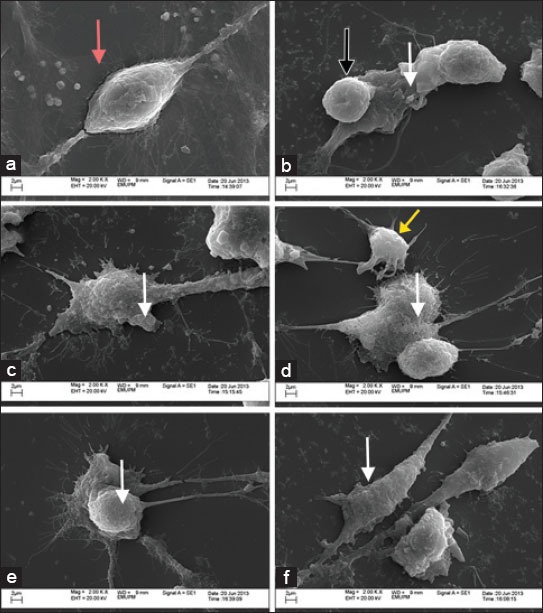
Scanning electron micrographs. (a) Scanning electron micrograph of control/untreated astrocyte showing retraction fibers (red arrow). (b) Scanning electron micrograph of 180 mM glutamate treated astrocyte for 24 hours. Cells appear rounded (black arrow) and evident blebbing (white arrow). (c-f) Scanning electron micrographs for pre and post treatment of 200 ng/mL of TRF and α-TCP upon glutamate challenge. Although cellular debris was noted (yellow arrow), many cells appeared with intact cell membrane but cellular blebbing was noted in some cells (white arrow).
Neuroprotective effects of vitamin E on cell cycle phases after exposure to glutamate toxicity
Based on Table 2, the cell cycle is divided into three distinct phases, which are G1, S, and G2 or M phases. In the pre-treatment study, the untreated sample illustrated the percentages of cell accumulation for astrocytes with 46.68%, 36.63%, and 16.69% in G1, S, and G2/M phases respectively. Meanwhile, for glutamate injured cells, the results of cell accumulation were 37.25%, 23.11%, and 39.64%. Vitamin E in pre-treated astrocytes resulted in the increase of cell population in S and G2/M phases when compared to glutamate induced sample.
TABLE 2.
Pre and post treatment of TRF and α-TCP upon glutamate toxicity on astrocyte cell population in cell cycle phases. Data is presented as mean±SEM of 3 independent experiments (n=3 in each experiment).
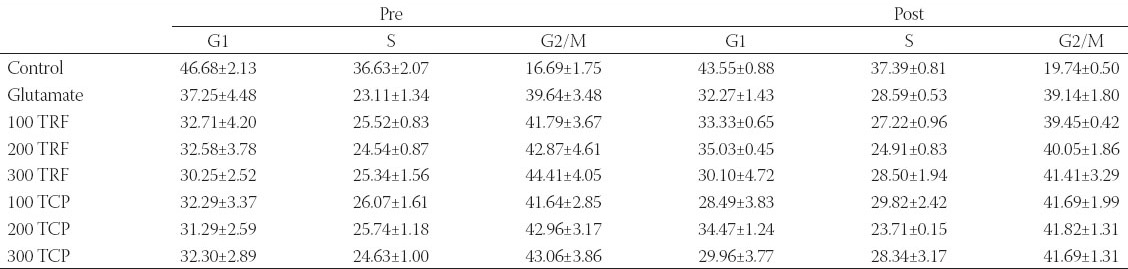
In the post-treatment study, the control expressed 43.55%, 37.39%, and 19.74% of cell accumulation, whereas glutamate treated sample populated 32.27%, 28.59%, and 39.14% of astrocytes in G1, S, and G2/M phases accordingly. Cell accumulation with the addition of TRF and α-TCP increased the quantity of cells in G2/M and S phases. Similar pattern of findings were obtained for both pre-and post-treatment studies.
DISCUSSION
The MTT findings reflexed that TRF and α-TCP at low concentrations, which were 100 to 300 ng/mL, restored the glutamate-injured astrocytes from injury. Previous studies on neuronal cells reported that 100 nM of α-tocotrienol introduced after 60 minutes of glutamate exposure, but not 90 minutes, showed almost complete protection [10]. In addition, 100 µg/mL and 250 µg/mL of vitamin E protected PC12 neuronal cell from glutamate toxicity in vitamin E co-treatment with increment of more than 20% of cell viability [11,28]. Furthermore, in both pre-and post-treatment studies, 200 ng/mL of α-TCP showed significant neuroprotective effects due to its higher bioavailability and greater uptake via α-TTP. According to a study conducted by Saito et al., 2010 [11], longer incubation time allowed better cytoprotective effect of tocopherol than tocotrienol. Injured astrocytes may utilize tocopherol in advance due to higher affinity to α-TTP. Apart from that, both TRF and α-TCP exerted similar protective effects with 20% of increased cell viability. This finding was consistent with the previous studies that reported tocotrienol and tocopherol showed similar capacities for cytoprotection against glutamate challenge [11].
Besides, in both pre-and post-treatment studies of astrocytes, 100 ng/mL of TRF and 200 ng/mL α-TCP exhibited significant difference in mitochondrial membrane protection compared to glutamate insulted astrocytes. Lipid-soluble antioxidant retained in membrane more effectively than hydrophilic antioxidant [11]. Hence, vitamin E can give better neuroprotective effects towards damaged mitochondria of astrocytes. Moreover, TRF possesses a special conformation in the membrane of phospholipid bilayer due to its unsaturated phytyl tail [22]. Other than that, the side chain features also enable more efficient penetration into tissues with high level of saturated fatty acid, such as the brain. Nanomolar concentrations of α-tocotrienol, in contrast with α-TCP, have the ability to protect against glutamate-induced neuronal death by suppressing inducible pp60 c-Src kinase activation [22].
Lipid peroxidation assay measured the concentration of MDA as the level of lipid peroxidation. The pre-and post-treatments of TRF and α-TCP showed potential protection against glutamate challenge. Both TRF and α-TCP treated cells had low MDA concentration and the difference was significant in comparison with glutamate injured astrocytes. This study also specified prophylactic effect of vitamin E in scavenging ROS. Concentration of 300 ng/mL of TRF presented high potential in reducing MDA concentration per protein in pre-treatment study of TBARS assay. The efficiency of TRF is highly related to its better distribution in fatty layers of membrane. TRF did manage to penetrate through the cell membrane efficiently, hence, could protect the cells from oxidative stress caused by glutamate [29]. A previous study conducted by Long et al., [30] showed that the accumulation of MDA while aging can cause mitochondrial dysfunction by inhibiting mitochondrial respiration and enzyme activity. Thus, with supplementation of various doses of TRF and α-TCP, the concentration of MDA decreases, and subsequently, causes cell viability augmentation.
In the study of vitamin E, astrocytes were treated with 180 mM of glutamate that caused approximately 60% of cell death. Flowcytometric annexin V-FITC analysis revealed that glutamate injured cells showed lower cell viability and higher apoptotic rate, meanwhile the untreated sample exhibited higher cell viability and lower amount of cell death. Therefore, this results indicated that 180 mM glutamate was toxic to astrocytes. However, the pre-and post-treatments of various concentrations of vitamin E presented better survivability and low cell death rates against glutamate neurotoxicity in astrocytes. α-TCP and TRF protected the cells from rapidly undergoing cell death induced by glutamate by preventing PS translocation, thus, the cell membrane remained intact. Previous research findings showed that at a concentration less than 10 µM, γ-tocotrienol has been reported to improve cell viability significantly against H2O2-induced apoptosis in primary astrocytes [31], primary cerebellar neurons [32], as well as in primary rat cortical neurons, and human neuroblastoma cell line [33]. The morphological findings obtained from scanning electron microscopy were well in accordance to the other research studies [34].
In addition, astrocytes treated with 180 mM of glutamate may induce impairment of DNA, protein, and chromatin, and subsequently, result in oxidative stress. Oxidative stress could be one of the mechanisms responsible for cell cycle re-entry [35]. In this study, astrocytes may re-enter the cell cycle to repair the damages occurred due to glutamate insult. Otherwise, badly injured cells might initiate cell death if the damage is too extensive to be repaired [36]. From the results obtained, it showed that vitamin E acts as a potent antioxidant as it can actually enhance the synthesis of DNA (S phase) and the recovery/repair of DNA (G2/M) in glutamate injured cells.
On the other hand, the role of astrocytes in promoting neuronal survival and recovery, following a cerebral insult, is becoming increasingly appreciated. Astrocyte, a subtype of glial cell, is known to protect neuronal cells against oxidative stress through transcriptional upregulation of glutathione synthesis and removal of extracellular glutamate [37-39]. Besides, studies have shown that the death of astrocytes after ischemia or reperfusion may strongly affect neuronal survival due to the absence of trophic and metabolic support to neuronal cells and astrocytic glutamate uptake [40]. Therefore, the cytoprotective effect of vitamin E against glutamate induced astrocytes is of our interest in order to maintain homeostasis for the neuronal cells in the brain.
There are several limitations of this study. Mechanisms of action of both TRF and α-TCP in elucidating astrocytes recovery upon glutamate insult need to be strongly validated via genomic studies. However, further studies are currently conducted by our research group to determine the effects of vitamin E in down regulating the expression of traumatic brain injury markers in glutamate induced astrocytes.
In conclusion, revealing a perfect therapy/compound that can protect people who are suffering from nerve disorders is the big concern worldwide. Currently, studies have revealed that astrocyte, supportive cell of neuronal, play an important role in the survival of neuronal cells. Earlier, more focus was given to find substance or compound that can provide protection for the neurons against oxidative stress. Only in the last few years, scientists have found out that astrocytes play a more important role rather than just to provide support to the neurons. This study demonstrated that vitamin E (both TRF and α-TCP) is competent in preventing glutamate induced injury and death in astrocytes. In the nervous system, the astrocytes are in close interaction with the neuronal cells, and therefore, by ensuring the survival of astrocytes from oxidative stress, it is expected that the neurons are also protected. Hence, understanding the dosages, as well as the prophylactic role of vitamin E, is rather crucial in minimizing neurodegeneration that is offered by nutrition-based therapy. It is obvious that palm TRF and α-TCP possess the prospective to be developed as a measure for the management of neurodegenerative diseases.
DECLARATION OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This research was financially supported by Faculty of Medicine and Health Sciences Grant Scheme (vote 9300337), University Putra Malaysia.
REFERENCES
- [1].Tabrizi S. Neurodegenerative diseases neurobiology pathogenesis and therapeutics. J Neurol Neurosurg and Psychiatry. 2006;77(2):284. http://dx.doi.org/10.1136/jnnp.2005.072710 . [Google Scholar]
- [2].Dibble LE, Lange M. Predicting falls in individuals with Parkinson disease: a reconsideration of clinical balance measures. J Neurol Phys Ther. 2006;30(2):60–7. doi: 10.1097/01.npt.0000282569.70920.dc. http://dx.doi.org/10.1097/01.NPT.0000282569.70920.dc . [DOI] [PubMed] [Google Scholar]
- [3].Shukla V, Mishra SK, Pant HC. Oxidative stress in neurodegeneration. Adv Pharmacol Sci. 2011;2011:572–634. doi: 10.1155/2011/572634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rock RB, Gekker G, Hu S, Sheng WS, Cheeran M, Lokensgard JR, et al. Role of microglia in central nervous system infections. Clin Microbiol Rev. 2004;17(4):942–64. doi: 10.1128/CMR.17.4.942-964.2004. table of contents. http://dx.doi.org/10.1128/CMR.17.4.942-964.2004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang Y, Qin ZH. Molecular and cellular mechanisms of excitotoxic neuronal death. Apoptosis. 2010;15(11):1382–402. doi: 10.1007/s10495-010-0481-0. http://dx.doi.org/10.1007/s10495-010-0481-0 . [DOI] [PubMed] [Google Scholar]
- [6].Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130(4S Suppl):1007S–15S. doi: 10.1093/jn/130.4.1007S. Epub 2000/03/29. [DOI] [PubMed] [Google Scholar]
- [7].Higuchi Y. Glutathione depletion-induced chromosomal DNA fragmentation associated with apoptosis and necrosis. J Cell Mol Med. 2004;8(4):455–64. doi: 10.1111/j.1582-4934.2004.tb00470.x. http://dx.doi.org/10.1111/j.1582-4934.2004.tb00470 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Scandalios JG. Oxidative stress responses--what have genome-scale studies taught us? Genome Biol. 2002;3(7):1019. doi: 10.1186/gb-2002-3-7-reviews1019. http://dx.doi.org/10.1186/gb-2002-3-7-reviews1019 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Emerit J, Edeas M, Bricaire F. Neurodegenerative diseases and oxidative stress. Biomed Pharmacother. 2004;58(1):39–46. doi: 10.1016/j.biopha.2003.11.004. http://dx.doi.org/10.1016/j.biopha.2003.11.004 . [DOI] [PubMed] [Google Scholar]
- [10].Sen CK, Khanna S, Roy S, Packer L. Molecular basis of vitamin E action. Tocotrienol potently inhibits glutamate-induced pp60(c-Src) kinase activation and death of HT4 neuronal cells. J Biol Chem. 2000;275(17):13049–55. doi: 10.1074/jbc.275.17.13049. http://dx.doi.org/10.1074/jbc.275.17.13049 . [DOI] [PubMed] [Google Scholar]
- [11].Saito Y, Nishio K, Akazawa YO, Yamanaka K, Miyama A, Yoshida Y, et al. Cytoprotective effects of vitamin E homologues against glutamate-induced cell death in immature primary cortical neuron cultures: Tocopherols and tocotrienols exert similar effects by antioxidant function. Free Radic Biol Med. 2010;49(10):1542–9. doi: 10.1016/j.freeradbiomed.2010.08.016. http://dx.doi.org/10.1016/j.freeradbiomed.2010.08.016 . [DOI] [PubMed] [Google Scholar]
- [12].Maniam S, Mohamed N, Shuid AN, Soelaiman IN. Palm tocotrienol exerted better antioxidant activities in bone than alpha-tocopherol. Basic Clin Pharmacol Toxicol. 2008;103(1):55–60. doi: 10.1111/j.1742-7843.2008.00241.x. http://dx.doi.org/10.1111/j.1742-7843.2008.00241.x . [DOI] [PubMed] [Google Scholar]
- [13].Serbinova E, Kagan V, Han D, Packer L. Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radic Biol Med. 1991;10(5):263–75. doi: 10.1016/0891-5849(91)90033-y. http://dx.doi.org/10.1016/0891-5849(91)90033-Y . [DOI] [PubMed] [Google Scholar]
- [14].Wu SJ, Liu PL, Ng LT. Tocotrienol-rich fraction of palm oil exhibits anti-inflammatory property by suppressing the expression of inflammatory mediators in human monocytic cells. Molecular nutrition &food research. 2008;52(8):921–9. doi: 10.1002/mnfr.200700418. http://dx.doi.org/10.1002/mnfr.200700418 . [DOI] [PubMed] [Google Scholar]
- [15].Wu SJ, Ng LT. Antioxidant and antihepatoma activities of palm oil extract. Journal of Food Lipids. 2007;14(2):122–37. http://dx.doi.org/10.1111/j.1745-4522.2007.00075.x . [Google Scholar]
- [16].Takahashi K, Loo G. Disruption of mitochondria during tocotrienol-induced apoptosis in MDA-MB-231 human breast cancer cells. Biochem Pharmacol. 2004;67(2):315–24. doi: 10.1016/j.bcp.2003.07.015. http://dx.doi.org/10.1016/j.bcp.2003.07.015 . [DOI] [PubMed] [Google Scholar]
- [17].Goh SH, Hew NF, Norhanom AW, Yadav M. Inhibition of tumour promotion by various palm-oil tocotrienols. Int J Cancer. 1994;57(4):529–31. doi: 10.1002/ijc.2910570415. http://dx.doi.org/10.1002/ijc.291057041 . [DOI] [PubMed] [Google Scholar]
- [18].Osakada F, Hashino A, Kume T, Katsuki H, Kaneko S, Akaike A. Alpha-tocotrienol provides the most potent neuroprotection among Vitamin E analogs on cultured striatal neurons. Neuropharmacology. 2004;47(6):904–15. doi: 10.1016/j.neuropharm.2004.06.029. http://dx.doi.org/10.1016/j.neuropharm.2004.06.029 . [DOI] [PubMed] [Google Scholar]
- [19].Minhajuddin M, Beg ZH, Iqbal J. Hypolipidemic and antioxidant properties of tocotrienol rich fraction isolated from rice bran oil in experimentally induced hyperlipidemic rats. Food Chem Toxicol. 2005;43(5):747–53. doi: 10.1016/j.fct.2005.01.015. http://dx.doi.org/10.1016/j.fct.2005.01.015 . [DOI] [PubMed] [Google Scholar]
- [20].Mutalib MSA, Khaza’ai H, Wahle KWJ. Palm-tocotrienol rich fraction (TRF) is a more effective inhibitor of LDL oxidation and endothelial cell lipid peroxidation than α-tocopherol in vitro. Food Research International. 2003;36(5):405–13. http://dx.doi.org/10.1016/S0963-9969(02)00173-4 . [Google Scholar]
- [21].Qureshi AA, Bradlow BA, Brace L, Manganello J, Peterson DM, Pearce BC, et al. Response of hypercholesterolemic subjects to administration of tocotrienols. Lipids. 1995;30(12):1171–7. doi: 10.1007/BF02536620. http://dx.doi.org/10.1007/BF02536620 . [DOI] [PubMed] [Google Scholar]
- [22].Sen CK, Rink C, Khanna S. Palm oil-derived natural Vitamin E alpha-tocotrienol in brain health and disease. J Am Coll Nutr. 2010;29(3 Suppl):314S–23S. doi: 10.1080/07315724.2010.10719846. http://dx.doi.org/10.1080/07315724.2010.10719846 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mangialasche F, Kivipelto M, Mecocci P, Rizzuto D, Palmer K, Winblad B, et al. High plasma levels of vitamin E forms and reduced Alzheimer's disease risk in advanced age. J Alzheimers Dis. 2010;20(4):1029–37. doi: 10.3233/JAD-2010-091450. [DOI] [PubMed] [Google Scholar]
- [24].Aggarwal BB, Sundaram C, Prasad S, Kannappan R. Tocotrienols, the vitamin E of the 21st century: its potential against cancer and other chronic diseases. Biochem Pharmacol. 2010;80(11):1613–31. doi: 10.1016/j.bcp.2010.07.043. http://dx.doi.org/10.1016/j.bcp.2010.07.043 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang J, Rajakulendran N, Amirsadeghi S, Vanlerberghe GC. Impact of mitochondrial alternative oxidase expression on the response of Nicotiana tabacum to cold temperature. Physiol Plant. 2011;142(4):339–51. doi: 10.1111/j.1399-3054.2011.01471.x. Epub 2011/03/16. [DOI] [PubMed] [Google Scholar]
- [26].van Meerloo J, Kaspers GJ, Cloos J. Cell sensitivity assays: the MTT assay. Methods Mol Biol. 2011;731:237–45. doi: 10.1007/978-1-61779-080-5_20. http://dx.doi.org/10.1007/978-1-61779-080-5_20 . [DOI] [PubMed] [Google Scholar]
- [27].Nguyen HT, Olson J, DeVries C, Savage W, Holtzman D. Scanning electron microscopic study of lead effects on cerebral astrocytes in primary culture. Scan Electron Microsc. 1982;(Pt 2):891–6. [PubMed] [Google Scholar]
- [28].Schubert D, Kimura H, Maher P. Growth factors and vitamin E modify neuronal glutamate toxicity. Proc Natl Acad Sci U S A. 1992;89(17):8264–7. doi: 10.1073/pnas.89.17.8264. http://dx.doi.org/10.1073/pnas.89.17.8264 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Khanna S, Roy S, Parinandi NL, Maurer M, Sen CK. Characterization of the potent neuroprotective properties of the natural vitamin E alpha-tocotrienol. J Neurochem. 2006;98(5):1474–86. doi: 10.1111/j.1471-4159.2006.04000.x. http://dx.doi.org/10.1111/j.1471-4159.2006.04000.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Long J, Wang X, Gao H, Liu Z, Liu C, Miao M, et al. Malonaldehyde acts as a mitochondrial toxin: Inhibitory effects on respiratory function and enzyme activities in isolated rat liver mitochondria. Life Sci. 2006;79(15):1466–72. doi: 10.1016/j.lfs.2006.04.024. http://dx.doi.org/10.1016/j.lfs.2006.04.024 . [DOI] [PubMed] [Google Scholar]
- [31].Mazlan M, Sue Mian T, Mat Top G, Zurinah Wan Ngah W. Comparative effects of alpha-tocopherol and gamma-tocotrienol against hydrogen peroxide induced apoptosis on primary-cultured astrocytes. J Neurol Sci. 2006;243(1-2):5–12. doi: 10.1016/j.jns.2005.10.006. http://dx.doi.org/10.1016/j.jns.2005.10.006 . [DOI] [PubMed] [Google Scholar]
- [32].Then SM, Mazlan M, Mat Top G, Wan Ngah WZ. Is Vitamin E toxic to neuron cells? Cell Mol Neurobiol. 2009;29(4):485–96. doi: 10.1007/s10571-008-9340-8. http://dx.doi.org/10.1007/s10571-008-9340-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Then WZWN SM, Top G. M, Mazlan M. Comparison of the effects of α-tocopherol and γ-tocotrienol against oxidative stress in two different neuronal cultures. Sains Malaysiana. 2010;39(1):145–56. [Google Scholar]
- [34].Hardej D, Trombetta LD. The effects of ebselen on cisplatin and diethyldithiocarbamate (DDC) cytotoxicity in rat hippocampal astrocytes. Toxicol Lett. 2002;131(3):215–26. doi: 10.1016/s0378-4274(02)00056-5. http://dx.doi.org/10.1016/S0378-4274(02)00056-5 . [DOI] [PubMed] [Google Scholar]
- [35].Folch J, Junyent F, Verdaguer E, Auladell C, Pizarro JG, Beas-Zarate C, et al. Role of cell cycle re-entry in neurons: a common apoptotic mechanism of neuronal cell death. Neurotox Res. 2012;22(3):195–207. doi: 10.1007/s12640-011-9277-4. http://dx.doi.org/10.1007/s12640-011-9277-4 . [DOI] [PubMed] [Google Scholar]
- [36].Schwartz EI, Smilenov LB, Price MA, Osredkar T, Baker RA, Ghosh S, et al. Cell cycle activation in postmitotic neurons is essential for DNA repair. Cell Cycle. 2007;6(3):318–29. doi: 10.4161/cc.6.3.3752. http://dx.doi.org/10.4161/cc.6.3.3752 . [DOI] [PubMed] [Google Scholar]
- [37].Dringen R, Hirrlinger J. Glutathione pathways in the brain. Biol Chem. 2003;384(4):505–16. doi: 10.1515/BC.2003.059. http://dx.doi.org/10.1515/BC.2003.059 . [DOI] [PubMed] [Google Scholar]
- [38].Iwata-Ichikawa E, Kondo Y, Miyazaki I, Asanuma M, Ogawa N. Glial cells protect neurons against oxidative stress via transcriptional up-regulation of the glutathione synthesis. J Neurochem. 1999;72(6):2334–44. doi: 10.1046/j.1471-4159.1999.0722334.x. http://dx.doi.org/10.1046/j.1471-4159.1999.0722334.x . [DOI] [PubMed] [Google Scholar]
- [39].Gegelashvili G, Schousboe A. High affinity glutamate transporters: regulation of expression and activity. Mol Pharmacol. 1997;52(1):6–15. doi: 10.1124/mol.52.1.6. [DOI] [PubMed] [Google Scholar]
- [40].Szydlowska K, Gozdz A, Dabrowski M, Zawadzka M, Kaminska B. Prolonged activation of ERK triggers glutamate-induced apoptosis of astrocytes: neuroprotective effect of FK506. J Neurochem. 2010;113(4):904–18. doi: 10.1111/j.1471-4159.2010.06656.x. http://dx.doi.org/10.1111/j.1471-4159.2010.06656.x . [DOI] [PubMed] [Google Scholar]


