Abstract
The utility of procollagen type 1 N-terminal propeptide (P1NP) in the management of metabolic bone diseases remains a subject of debate since the reference ranges are not rigorously established and fail to account for many of the preanalytical variables. We aimed to establish reference intervals for P1NP level in healthy and osteoporotic postmenopausal females stratified by age, body mass index and menopausal duration. We also aimed to assess the relationship between P1NP and BMD. This cross-sectional study enrolled 183 postmenopausal females who were divided in osteoporosis group (N=93) and control group (N=90) with preserved bone mass based on BMD assessed by DXA. In the osteoporosis group median P1NP was significantly higher (51.7 ng / mL; 95%CI 43.2-53.7) compared to control group (38.9 ng/mL; 95%CI 34.2-43.9)(p<0.01). After controlling for age, BMI and years since menopause, there was significant inverse association between BMD and P1NP at the femoral neck (r=-0.18), total hip (r=-0.207) and lumbar spine (r=-0.236). There was no significant difference in P1NP concentration across quartiles of age in postmenopausal females. P1NP was significantly lower in obese postmenopausal females with preserved bone mass compared to normal weight and overweight females in control and in osteoporosis group.
In conclusion, we showed that P1NP is inversely associated with BMD even after controlling for age, BMI and years since menopause. Although, P1NP is significantly higher in postmenopausal females with osteoporosis compared to postmenopausal females with preserved bone mass its low specificity does not warrant its utility is diagnosing osteoporosis.
KEY WORDS: procollagen type 1 N-terminal propeptide, postmenopausal females, osteoporosis, bone mineral density
INTRODUCTION
Bone mineral density (BMD) assessed by dual-energy X-ray absorptiometry (DXA) is the most commonly used measure in the assessment of osteoporotic status, risk for fragility fracture and in the management of postmenopausal osteoporosis [i]. BMD by DXA provides a static measure of skeletal status: a snapshot of the cumulative effects of different factors on the assessed skeletal site over the time, but does not provide a dynamic estimate of skeletal activity, which could provide insight into the changes the skeleton may undergo in the future [2]. Bone is a dynamically and metabolically active organ that is continuously subjected to two processes: resorption and formation, collectively called bone turnover or bone remodeling. Bone turnover markers (BMTs), released into bloodstream during remodeling process can aid in the management of postmenopausal osteoporosis as they provide dynamic information regarding skeletal status that is independent from, and often complementary to, BMD measurements [3]. Formation and resorption are usually tightly coupled in time and space; thus, any such marker reflects the overall rate of bone turnover. Several studies have looked at various BTMs and their contribution to fracture risk, but the results of these studies have been inconsistent, not the least due to the use of different markers and different methodologies for their assessment [4]. Current recommendation for the standardization of BTM measurements in future studies is to use serum carboxy terminal telopeptide of collagen type I (s-CTX) as the standard bone resorption marker and serum procollagen type I N-terminal propeptide (P1NP) as the standard bone formation marker [4]. Automated assays for BTMs are now commercially available and are a relatively non-invasive means of assessing bone remodeling activity in routine clinical practice. Traditionally most laboratory results are reported with a reference interval. The use of reference intervals with BTMs is limited as BTMs consistently demonstrate large degrees of variability between individuals and between age and physiological maturity [2,5]. These intervals are even wider in the postmenopausal group, which limits the use of a normal reference interval in the interpretation of BTMs in this population [2]. Very few studies are available with the reference ranges for the newer, automated assays of bone turnover such as procollagen type I N-terminal propeptide (P1NP). Type I collagen is the main protein of bone matrix and is cleaved to N-terminal (P1NP) and C-terminal (P1CP) propeptides of Type I collagen during bone formation. P1NP is released into circulation and offer several practical advantages including its low diurnal variability and stability at room temperature. Its circulating levels are not significantly influenced by food intake and, consequently, patients do not need to be fasting [6]. The utility of P1NP in the management of metabolic bone diseases in individual patients remains a subject of much debate [7] since the reference ranges currently reported by commercial labs are often not rigorously established and fail to account for many of the preanalytical variable such as age, duration of postmenopausal period and sessional variations. Therefore, it is essential to establish a valid reference range stratified by the known determinants of BTMs, such as age, and body mass index (BMI) for clinical use. Once reference ranges for BTMs are established, comparisons of BTMs between healthy individuals and osteoporotic patients can be performed [8]. In this study, we aimed to establish reference intervals for P1NP level in healthy and osteoporotic women stratified by age, body mass index and menopausal duration and investigate the utility of P1NP measurement as a marker of osteoporosis in postmenopausal women. We also aimed to assess the relationship between BTMs and BMD in postmenopausal women.
MATERIALS AND METHODS
Participants
This cross-sectional study enrolled 183 postmenopausal females, referred to the Clinics for Nuclear Medicine at Clinical Centre University of Sarajevo (CCUS) by their primary health care practitioner for osteoporosis screening. The study was approved by the Ethics Committee at Faculty of Medicine, University of Sarajevo and by the Research Ethics Committee at the CCUS and performed from November 2010 – January 2012. Postmenopausal status was initially defined as absence of menstruation for at least 12 months and confirmed by elevated serum FSH levels (> 40 UI/L). Participants were excluded if they reported taking medications known to affect the skeleton such as corticosteroids; if they were on calcium and vitamin D supplements; whether they had ever been exposed to bisphosphonate therapy or if they were receiving hormone replacement therapy or had received it within the last year before the start of the study. Also participants who self-reported having diabetes mellitus, breast cancer or any other disease affecting bone metabolism such as endocrinopathies associated with secondary osteoporosis suspected after the laboratory testing. The definite sample size consisted of 183 postmenopausal females who were based on BMD values obtained by DXA divided in two groups:
Osteoporosis group (OG) included 93 newly diagnosed postmenopausal females with osteoporosis in whom bone mineral density T score values were <-2.5 at the lumbar spine and/or at any part of the hip.
Control group (CG) included 90 postmenopausal females with preserved bone mass in whom bone mineral density total T score values were > -1 both at the lumbar spine and the hip. All participants informed written consent after the explanation of the study procedure. All procedures in this study were conducted in accordance with the guidelines of The Declaration of Helsinki.
Procedures
The health and lifestyle questionnaires were administered to the participants by the investigator. Demographic data and information on health and lifestyle of participants were collected from the questionnaire. Questions included smoking, alcohol consumption, exercise habits, current and past history of diseases, short family history. Details of medication and previous fractures were also indicated on the questionnaire. Additional information such as age at menarche, menopausal status, use of hormone replacement therapy and use of contraceptives were obtained.
Anthropometric measurements
The subjects’ height and weight were measured while they wore indoor clothes and no shoes. Height was measured to the nearest 0.5 centimeters using a wall-mounted stadiometer. Weight was measured to the nearest 0.1 kg on a seca digital scale. Body mass index (BMI) was calculated as weight (kg) divided by height2 (m). BMI values in the range 19 - 25 kg/m2 were considered normal weight, while subjects with BMI ≥25 kg/m2 and ≥30 kg/m2 were the cutoff levels for overweight and obese subjects, respectively.
P1NP measurement
Blood samples were collected by venipuncture into 5 mL serum separator tube by the clinical laboratory phlebotomist at the Institute of Nuclear Medicine at CCUS between 8:00 and 11:00 AM to minimize the effects of circadian variation after at least a 12-h fast. Blood sampling was performed no longer than two week after the bone mineral density measurement. For the measurement of P1NP the tube was immediately put on ice and kept cool until serum separation. Tubes were centrifuged after 1 hour at room temperature. Serum levels of total P1NP were determined using electrochemiluminescence immunoassay “ECLIA” on Elecsys 2010 (Roche Diagnostic GmbH) at the Clinics for Nuclear Medicine at the CCUS. This assay detects both intact mono- and trimetric forms (total P1NP). Referral values using this method for the P1NP are 16.27-73.87 ng/mL.
DXA measurement
Bone mineral density was measured with Hologic QDR 4500 DXA equipment (Hologic Inc., Waltham, MD, USA) at the Clinics for Radiology at Clinical Center University of Sarajevo. Values of bone mineral density are expressed as BMC (g) and areal BMD (g/cm2) and then converted into T-scores and Z-scores. The bone mineral density was measured at the lumbar spine (L1–L4) and all regions of the hip including total hip, femoral neck, trochanter and intertrochanteric shaft. For the lumbar spine measurement, the patient was position supine on the scanner table with arms resting on the table-top with the knees flexed over a 90° and placed on a support pad. Hip measurements were always performed on the left side, unless there was a previous fracture or joint replacement. Precision coefficients of variation of the method for hip and lumbar spine is 1% and for neck and trochanter 2.5%
Statistical analysis
Data were analyzed using SPSS 11.0 (SPSS Inc., Chicago, IL, USA). The anthropometric characteristics were presented as the means ± SD. P1NP was determined to be not normally distributed by the Kolmogorov-Smirnov test. P1NP reference range was presented as medians with 95% confidence intervals (95% CI) (the reference range between the 2.5 th and 97.5 th percentiles) for the whole study population and for different subgroups (osteoporosis status, age, BMI and BMD). Levels of P1NP was compared between subgroups using the Mann-Whitney test or Kruskal-Wallis test. Differences were considered significant at p<0.05. An ROC curve was constructed for P1NP. The sensitivity, specificity, positive and negative predictive values of P1NP were calculated with several cut-off levels. We performed multiple regression analysis between P1NP and BMD, controlling age, BMI and years since menopause.
RESULTS
The median serum P1NP concentration measured in 183 postmenopausal females was 43.9 (30.9-58.6) ng/mL; ranging from 7.96-128.0 ng/mL. The referral values in our laboratory for postmenopausal females without HRT are 20.3-76.3 ng/mL with the median of 42.9 ng/mL. Serum P1NP below lower and over upper reference range was measured in io (5%) and 16 (8%) postmenopausal females respectively. There was no significant difference in P1NP concentration across quartiles of age in postmenopausal females (Table 1). In our study there was significant difference in P1NP levels across quartiles of BMD for lumbar spine, total hip and femoral neck. s-P1NP level in the 4th and 3rd quartile of lumbar spine BMD was significantly lower (36.8 (30.8-44.4) and 39.4 (33.7-46.5) ng/mL, respectively) compared to ist and 2nd quartile (49.4 (40.6-54.6) and 52.6 (36.1-59.6) ng/mL, respectively). Also, s-P1NP level was significantly lower in the 4th quartile (35.2 (28.3-42.3) ng/mL) compared to lower three quartiles of total hip BMD (Figure 1).
TABLE 1.
s-P1NP concentration in postmenopausal females (N=183) across quartiles of age.
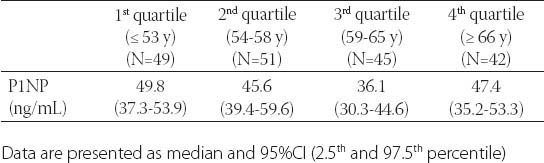
FIGURE 1.
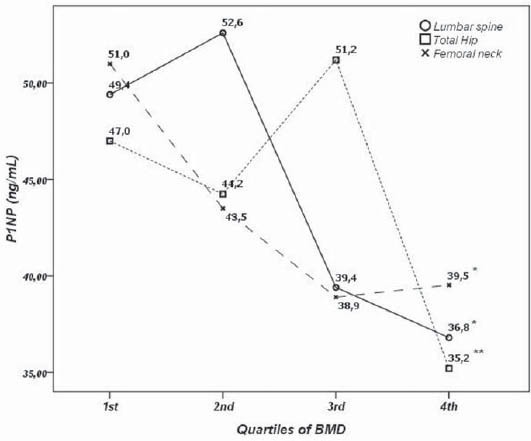
s-P1NP concentration across quartiles of lumbar spine, total hip and femoral neck bone mineral density. *-p<0.01
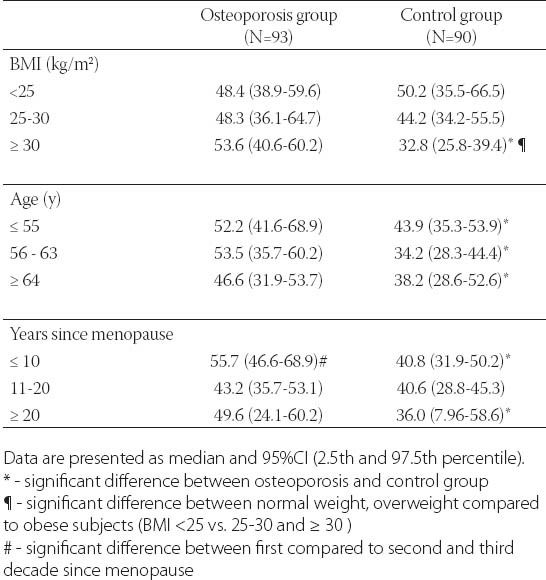
After controlling for age, BMI and years since menopause, there was significant negative correlation between BMD and s-P1NP at the femoral neck (r=-0.18; p<0.05), total hip (r=- 0.207; p<0.01) and lumbar spine (r=-0.236; p<0.01)(Table 2). s-P1NP level in postmenopausal females with and without osteoporosis were within reference range but the median serum P1NP concentration in postmenopausal females with osteoporosis was significantly higher (51.7 ng/mL; 95%CI 43.2-53.7 ng/mL) compared to healthy postmenopausal females with preserved bone mass (38.9 ng/mL; 95%CI 34.2-43.9 ng/mL)(p<0.01)(Figure 2). The area under the ROC curve was 0.634 with 95%CI (0.554-0.714), which suggested that changes in the P1NP levels might have a direct relation to osteoporosis (Figure 3). However, when the cut-offvalue of P1NP was accepted as 37.1 ng/mL, the sensitivity and specificity were poor 70.8% and 38.5% respectively. On the other hand, when a value of 22.1 ng/mL was used as the cut-off point, the sensitivity increased to 93.9% while specificity greatly decreased. In the osteoporosis group there was no significant difference in median s-P1NP values between different BMI values. However, in obese postmenopausal females without osteoporosis, P1NP was significantly lower compared to normal and overweight subjects and compared to obese females with osteoporosis (Table 3). In the osteoporosis group, P1NP during the first decade since menopause (55.7 95%CI (46.6-68.9) ng/mL) was significantly higher compared to the second and third decade in the same group (p<0.01). Females with osteoporosis had significantly higher levels of s-P1NP during their first and the third decade (>20 years) since menopause compared to females with preserved bone mass (Table 3).
TABLE 2.
Standardized correlation coefficients between s-P1NP and BMD in 183 postmenopausal women.
FIGURE 2.
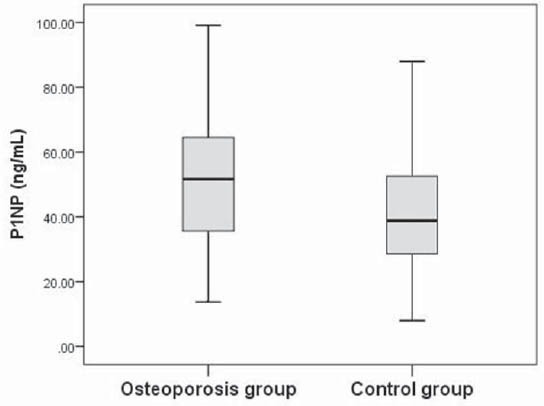
Serum P1NP levels in the osteoporosis and control groups of postmenopausal females. The solid horizontal lines denote the median value, the box represents the 25% and 75% interquartile ranges and the whiskers represent minimum and maximum values.
FIGURE 3.
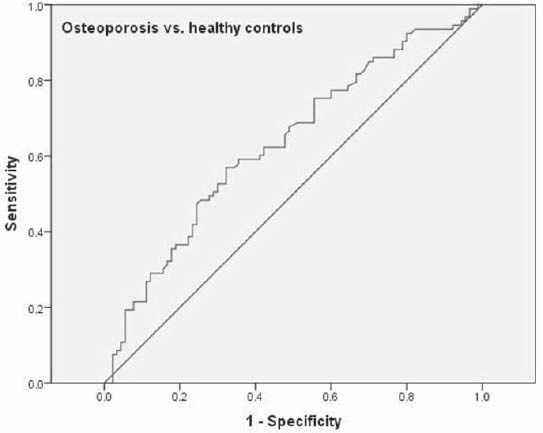
Receiver operating characteristic (ROC) curve of serum P1NP level for differentiation between postmenopausal females with osteoporosis and healthy controls.
TABLE 3.
P1NP concentration in postmenopausal females with osteoporosis and in healthy controls presented by different age, BMI and years since menopause ranges.
DISCUSSION
Type I collagen, which constitutes 90% of bone proteins, is synthesized as type I procollagen. During the extracellular processing of type I procollagen, there is cleavage of the amino terminal [N-terminal propeptide of type I collagen (P1NP)] and carboxy terminal propeptide (P1CP). These propeptides circulate in blood, where they are markers of bone formation. In contrast to serum P1CP, which is a single protein, P1NP circulates as different forms, including the intact authentic trimeric P1NP, a monomer, and several fragments [9]. Intact P1NP is mainly metabolized by the endothelial cells of the liver whereas clearance of monomeric P1NP depends on kidney function. In infants and children, the concentration is much higher than in adults. Serum P1NP is a useful indicator of disease activity in Paget’s disease of bone, in bone metastases of osteoblastic nature, and in the follow-up of treatment of osteoporosis. The IFCC and IOF recently recommended the use of P1NP as a reference marker for bone formation in studies concerning fracture risk assessment and treatment response [10]. Different assays can measure both monomeric and trimeric forms. Different methodologies used to measure P1NP (intact, monomers etc.) are the reasons, which make it difficult to compare the results from other studies and to understand clinical utility of bone turnover markers in clinical practice [11]. The lack of assay standardisation is a matter of concern, making difficult the comparison of results obtained by different methods and/or in different laboratories [12]. Moreover, there is a paucity of published reference ranges particularly using the newer, automated assays of BTMs. Therefore, National Bone Health Alliance (NBHA) is conducting a project to standardize bone turnover marker sample collection procedures in the USA, establish a USA reference range for one bone formation (serum procollagen type I N propeptide, P1NP) and one bone resorption (serum C-terminal telopeptide of type I collagen, s-CTX) marker, and standardize bone turnover marker assays used in clinical laboratories and stress the importance of harmonization for future research [13]. In this cross-sectional study we included 183 postmenopausal females in whom we measured P1NP level using the new automated assay. Median P1NP in postmenopausal females included in our study was within reference range (43.9 ng/mL) as provided by the manufacturer. In a study by Glover et al. [14] which included 637 premenopausal women from four countries (United Kingdom, France, Belgium, United States) reference intervals for bone turnover markers were established. The study revealed that the median P1NP level in women from France was 44.5 ng/mL and higher compared to median P1NP levels of women from Belgium (40.2 ng/mL), the United States (33.7 ng/mL), and the United Kingdom (35.0 ng/mL) suggesting that there exist differences between the levels of bone turnover in women living in geographically diverse locations. In another recently published, cross-sectional registry study, which included 194 healthy, premenopausal, European Caucasian women serum P1NP reference range was established (17.3-83.4ng/mL) [11]. The median P1NP concentration in our postmenopausal females was very similar to the median values obtained from premenopausal females from European countries. When we compared the P1NP between osteoporosis and healthy postmenopausal females, serum P1NP level was significantly higher in females with osteoporosis compared to females with preserved bone mass (51.7 ng/mL 95%CI (43.2-53.7) vs. 38.9 ng/mL; 95%CI (34.2-43.9)). But, when we analyzed sensitivity and specificity of P1NP in discriminating between postmenopausal females with osteoporosis and preserved bone mass, the marker was of poor value. When we set the cut-off value of 22.1 ng/mL the sensitivity was 93.9% but the specificity was poor. Garnero et al. [15] found that in postmenopausal women with osteoporosis, concentrations of total P1NP were 74% higher than in premenopausal women. In postmenopausal osteoporosis, levels of bone resorption markers above the upper limit of the premenopausal range are associated with an increased risk of hip, vertebral, and non-vertebral fracture, independent of BMD. Therefore, the combined use of BMD measurement and biochemical markers is helpful in risk assessment, especially in those women who are not identified as at risk by BMD measurement alone [16]. Many pre-analytical variables such as age, menopause, gender, body mass index are known to affect measures of BTMs [3]. In addition, medical conditions such as metabolic bone diseases or recent fractures and medications such as antiresorptives, corticosteroids, anticonvulsants, oral contraceptives (OCs) can also affect marker measurements. The median P1NP in postmenopausal females below the age of 53 y was 49.8, in the age group 54-58 y P1NP was 45.6 and in females above 66 y it was 47.4 ng/mL. We did not observe significant difference in P1NP levels across age quartiles which is also confirmed in a study by Glover et al. [14] who also did not observed the difference in bone turnover markers across different ages, but the study included only premenopausal women. Also our results are similar to those obtained by Hu et al. [8] who used the same automated assay for P1NP measurement. In their study median P1NP for the age group 50-54 y was 52.72, slightly decreased but not significantly in the older age groups (55-59 y - 50.21 and 60-66 y - 47.07 ng/mL). In their study, P1NP was significantly higher in the postmenopausal females compared to premenopausal females. When we divided postmenopausal females in those with osteoporosis and with preserved bone mass based on DXA measurement, significant differences in P1NP values were observed across age groups. In females < 55 y of age P1NP was significantly higher in osteoporosis compared to control group (52.2 (41.6-68.9) vs. 43.9 (35.3-53.9) ng/mL which was also observed in females older than 66 y of age (46.6 (31.9-53.7) vs. 38.2 (28.6-52.6) ng/mL; p<0.05). In our study we found that P1NP and the BMD of lumbar spine, femoral neck, and total hip were significantly inversely correlated. The effects remained after being adjusted for age, height, weight, and years since menopause. Our results are in line with the results from Hu et al. [8] who also observed negative association between bone turnover markers and BMD. After menopause, an increased bone turnover is related to bone loss by an imbalance between bone formation and bone resorption. During the first three decades of life, bone formation predominates over bone resorption, while after the age of 40 years, the remodeling process is not in balance and bone resorption predominates over bone formation. Individuals with a high rate of bone loss are at risk of developing osteoporosis and fracture. In our normal weight and overweight postmenopausal females no significant differences in P1NP values between osteoporosis and control group was observed. However, in obese control subjects P1NP was significantly lower compared to obese postmenopausal females with osteoporosis and compared to normal weight and overweight postmenopausal females. In our previous report we have shown that the levels of bone markers decrease rapidly with antiresorptive therapies [17] but we did not measure P1NP levels. Considering the increased levels found in patients with osteoporosis, the measurement of P1NP before the initiation of the therapy and during the follow up could aid in monitoring the treatment efficiency. Serum P1NP show responsiveness to treatment and low within-subject variability. Thus, the P1NP measurement usually enables the identification of the majority of responders to treatment [18]. The BTMs levels reached after 3–6 months of therapy have been shown to be more strongly associated with fracture outcome than changes in BMD [16]. Preliminary studies indicate that monitoring changes of bone formation markers could also be useful to monitor anabolic therapies, including intermittent parathyroid hormone administration and, possibly, to improve adherence to treatment. Thus, repeated measurements of bone markers during therapy may help improve the management of osteoporosis in patients. Our study has several strengths and limitations. Strengths include the large sample size of extensively characterized study participants, measured BMD at hip and spine and stratification of the population by osteoporosis and post-menopausal status. On the other hand our reference ranges are method-sensitive and may not be applicable to other methods of P1NP measurement. In addition, our sample is restricted to Caucasian subjects. It may therefore not be appropriate to apply our reference ranges to other ethnicities.
CONCLUSION
In conclusion, we established robust reference intervals for P1NP in postmenopausal females. P1NP is inversely associated with BMD even after controlling for age, BMI and years since menopause. Although, P1NP is significantly higher in postmenopausal females with osteoporosis compared to postmenopausal females with preserved bone mass its low specificity does not warrant its utility is diagnosing osteoporosis. Our study also showed that P1NP is significantly higher in postmenopausal females with osteoporosis compared to healthy subjects during the first decade since menopause.
DECLARATION OF INTEREST
Authors declare no conflict of interest
REFERENCES
- [1].Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY. Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24(1):23–57. doi: 10.1007/s00198-012-2074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Brown JP, Albert C, Nassar BA, Adachi JD, Cole D, Davison KS, et al. Bone turnover markers in the management of postmenopausal osteoporosis. Clin Biochem. 2009;42(10-11):929–942. doi: 10.1016/j.clinbiochem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- [3].Vasikaran S, Eastell R, Bruyere O, Foldes AJ, Garnero P, Gries-macher A, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22(2):391–420. doi: 10.1007/s00198-010-1501-1. [DOI] [PubMed] [Google Scholar]
- [4].Lee J, Vasikaran S. Current Recommendations for Laboratory Testing and Use of Bone Turnover Markers in Management of Osteoporosis. Ann Lab Med. 2012;32(2):105–112. doi: 10.3343/alm.2012.32.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rauchenzauner M, Schmid A, Heinz-Erian P, Kapelari K, Falkens-ammer G, Griesmacher A, et al. Sex-and age-specific reference curves for serum markers of bone turnover in healthy children from 2 months to 18 years. J Clin Endocrinol Metab. 2007;92(2):443–449. doi: 10.1210/jc.2006-1706. [DOI] [PubMed] [Google Scholar]
- [6].Clowes JA, Hannon RA, Yap TS, Hoyle NR, Blumsohn A, Eastell R. Effect of feeding on bone turnover markers and its impact on biological variability of measurements. Bone. 2002;30(6):886–890. doi: 10.1016/s8756-3282(02)00728-7. [DOI] [PubMed] [Google Scholar]
- [7].de Papp AE, Bone HG, Caulfield MP, Kagan R, Buinewicz A, Chen E, et al. A cross-sectional study of bone turnover markers in healthy premenopausal women. Bone. 2007;40(5):1222–1230. doi: 10.1016/j.bone.2007.01.008. [DOI] [PubMed] [Google Scholar]
- [8].Hu WW, Zhang Z, He JW, Fu WZ, Wang C, Zhang H, et al. Establishing reference intervals for bone turnover markers in the healthy shanghai population and the relationship with bone mineral density in postmenopausal women. Int J Endocrinol 2013. 2013:513925. doi: 10.1155/2013/513925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Terreni A, Pezzati P. Biochemical markers in the follow-up of the osteoporotic patients. Clin Cases Miner Bone Metab. 2012;9(2):80–84. [PMC free article] [PubMed] [Google Scholar]
- [10].Koivula MK, Risteli L, Risteli J. Measurement of aminoterminal propeptide of type I procollagen (PINP) in serum. Clin Biochem. 2012;45(12):920–927. doi: 10.1016/j.clinbiochem.2012.03.023. [DOI] [PubMed] [Google Scholar]
- [11].Eastell R, Garnero P, Audebert C, Cahall DL. Reference intervals of bone turnover markers in healthy premenopausal women: results from a cross-sectional European study. Bone. 2012;50(5):1141–1147. doi: 10.1016/j.bone.2012.02.003. [DOI] [PubMed] [Google Scholar]
- [12].Terreni A, Pezzati P. Biochemical markers in the follow-up of the osteoporotic patients. Clin Cases Miner Bone Metab. 2012;9(2):80–84. [PMC free article] [PubMed] [Google Scholar]
- [13].Bauer D, Krege J, Lane N, Leary E, Libanati C, Miller P, et al. National Bone Health Alliance Bone Turnover Marker Project: current practices and the need for US harmonization, standardization, and common reference ranges. Osteoporos Int. 2012;23(10):2425–2433. doi: 10.1007/s00198-012-2049-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Glover SJ, Gall M, Schoenborn-Kellenberger O, Wagener M, Gar- nero P, Boonen S, et al. Establishing a reference interval for bone turnover markers in 637 healthy, young, premenopausal women from the United Kingdom, France, Belgium, and the United States. J Bone Miner Res. 2009;24(3):389–397. doi: 10.1359/jbmr.080703. [DOI] [PubMed] [Google Scholar]
- [15].Garnero P, Vergnaud P, Hoyle N. Evaluation of a Fully Automated Serum Assay for Total N-Terminal Propeptide of Type I Collagen in Postmenopausal Osteoporosis. Clin Chem. 2008;54(1):1188–196. doi: 10.1373/clinchem.2007.094953. [DOI] [PubMed] [Google Scholar]
- [16].Garnero P. Biomarkers for osteoporosis management: utility in diagnosis, fracture risk prediction and therapy monitoring. Mol Diagn Ther. 2008;12(3):157–170. doi: 10.1007/BF03256280. [DOI] [PubMed] [Google Scholar]
- [17].Kučukalic-Selimović E, Valjevać A, Hadžović-Dzuvo A, Skopljak-Beganović A, Alimanovic-Alagić R, Brković A. Evaluation of bone remodelling parameters after one year treatment with alendronate in postmenopausal women with osteoporosis. Bosn J Basic Med Sci. 2011;11(1):41–45. doi: 10.17305/bjbms.2011.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rogers A, Glover SJ, Eastell R. A randomised, double-blinded, placebo-controlled, trial to determine the individual response in bone turnover markers to lasofoxifene therapy. Bone. 2009;45(6):1044–1052. doi: 10.1016/j.bone.2009.07.089. [DOI] [PubMed] [Google Scholar]


