Abstract
Autologous tissue-engineered blood vessels (TEBVs) generated using adult stem cells have shown promising results, but many preclinical evaluations do not test the efficacy of stem cells from patient populations likely to need therapy (i.e., elderly and diabetic humans). Two critical functions of these cells will be (i) secreting factors that induce the migration of host cells into the graft and (ii) differentiating into functional vascular cells themselves. The purpose of this study was to analyze whether adipose-derived mesenchymal stem cells (AD-MSCs) sourced from diabetic and elderly patients have a reduced ability to promote human smooth muscle cell (SMC) migration and differentiation potential toward SMCs, two important processes in stem cell-based tissue engineering of vascular grafts. SMC monolayers were disrupted in vitro by a scratch wound and were induced to close the wound by exposure to media conditioned by AD-MSCs from healthy, elderly, and diabetic patients. Media conditioned by AD-MSCs from healthy patients promoted the migration of SMCs and did so in a dose-dependent manner; heating the media to 56°C eliminated the media's potency. AD-MSCs from diabetic and elderly patients had a decreased ability to differentiate into SMCs under angiotensin II stimulation; however, only AD-MSCs from elderly donors were unable to promote SMC migration. Gender and body–mass index of the patients showed no effect on either critical function of AD-MSCs. In conclusion, AD-MSCs from elderly patients may not be suitable for autologous TEBVs due to inadequate promotion of SMC migration and differentiation.
Introduction
Utilizing adult mesenchymal stem cells (MSCs) represents a critical step in the clinical translation of many autologous tissue-engineered technologies. MSCs offer several advantages over primary cells such as their ease of isolation, self-renewal capacity, differentiation potential, and ability to secrete a wide spectrum of factors with varying functional effects.1–7 Indeed, MSCs have seen a wide-spread use in developing tissue-engineered cardiovascular8–11 and musculoskeletal constructs.12,13 In many tissue-engineered designs, these cells are coaxed to differentiate during culture into a desired cell phenotype, so that the construct will directly mimic native tissue.14–17 Alternatively, MSCs can be utilized in a paracrine nature for their secreted factors. In vascular tissue engineering, this paracrine signaling appears to be an important mechanism18–22 by which tissue-engineered blood vessels (TEBVs) remodel. Recent studies have shown they possess a dynamic environment that is repopulated with host cells,18–32 such as by the inward migration of vascular smooth muscle cells (SMCs).
Despite the success of autologous vascular tissue engineering in reducing intimal hyperplasia and thrombosis,8 translatability to the clinic has been limited by improper testing of cell sources. Many preclinical investigations do not test the efficacy of cells from clinically realistic patient populations who would routinely see this type of therapy, opting for healthy human or animal cells instead.18–22,24,25,33–63 In addition, many of these healthy human cells are purchased from companies as opposed to being taken from an array of individuals, representative of a realistic patient population. While taken together, these studies have shown that cellularizing tissue-engineered grafts significantly improves the patency and regeneration over their acellular counterparts; these models hold limited relevance for clinical translation since in practice, cells would need to be harvested from patients at high cardiovascular risk such as diabetics64,65 and the elderly.66–68 In addition, these high-risk groups possess pathologic conditions such as a hyperglycemic environment69–72 and age-associated senescence,73–85 respectively, which can decrease the ability of their stem cells to proliferate, differentiate into specific mesenchymal cell types, and promote angiogenesis. Whereas bone marrow has been shown to be a source of stem cells, it is particularly important to consider the use of adipose-derived mesenchymal stem cells (AD-MSCs) due to their ease of isolation and abundance. Whereas there have been studies noting various changes in AD-MSC properties with aging74–76,78,79,82,83,85 and diabetes,71 it is unclear how donor demographics affect functions related to vascular engineering such as the ability to secrete factors to induce SMC migration and their potential to differentiate into SMCs. Also, investigating if donor demographics alter the factors secreted by AD-MSCs will have a broad impact as the AD-MSC secretome has been used for a variety of applications.82,85–89
In this study, we use human AD-MSCs to test the hypothesis that cells sourced from high-risk populations (i.e., diabetic, elderly) will have a decreased efficacy to promote the migration of SMCs and ability to differentiate into SMCs themselves. We test this in vitro using a scratch wound assay and angiotensin II (AngII)-induced differentiation, respectively. Understanding the effect of donor demographics on the ability of human MSCs to produce SMC promigratory factors and differentiate into SMC is critical to the design of functional TEBVs and could have a wide impact of the stem cell therapy field.
Materials and Methods
Conditioned media from AD-MSCs
AD-MSCs were harvested from the adipose tissue of human patients using previously described methods.76,90 Only information on patient age, gender, body–mass index (BMI), and diabetic status was linked to the harvested cells to protect patient confidentiality, in accordance with an approved Institutional Review Board exempt protocol. Briefly, adipose tissue was isolated from patients, which was minced and digested in a collagenase solution (1 mg/mL, type II) for 30 min. The solution was then filtered through a gauze, centrifuged, resuspended in an NH4Cl erythrocyte lysis buffer (154 mM), and centrifuged again to obtain a cell pellet. The cell pellet was plated and cultured to obtain AD-MSCs. AD-MSCs were then classified into groups that represented a healthy status (<45 years of age, nondiabetic), a diabetic status (<45 years of age, diabetic), or an elderly status (>60 years of age, nondiabetic). To avoid confounding variables when comparing between groups, the age or diabetic condition was held constant and only female donors were used. However, to compare on the basis of gender, cells from different healthy male donors were also analyzed.
All AD-MSCs were cultured in 75-cm2 tissue culture flasks (Corning) with defined culture media [1:1 Dulbecco's modified Eagle's medium (DMEM, #11965; Gibco) to DMEM/F12 (#113300; Gibco) with 10% fetal bovine serum (#S11550; Atlanta Biologics), antibiotics (1% Pen/Strep, 0.5% Fungizone, 0.1% Gentamycin), and 10 μL dexamethasone] mixed with 25% Preadipocyte Growth Medium (#C-27410, #C-39425; PromoCell). To obtain conditioned media, culture media were replenished when flasks were near confluence and termed conditioned after 2 days when collected. Upon collection, the conditioned media were centrifuged to remove any cells or fragments and stored at−80°C until use. For most experiments, conditioned media were diluted with culture media to 100,000 conditioning cells/mL; however, for dose dependence studies, media were diluted from 500,000 conditioning cells/mL (dose 1:0) at dilutions of 1:2, 1:4, 1:9, and 1:19. To heat inactivate, the conditioned media were raised to a temperature of 56°C for 30 min. For all experiments, AD-MSCs between passage number 2 and 6 were used.
Culture of SMCs
Human SMCs were purchased from ATCC (#PCS-100-012) and grown in 75-cm2 culture flasks with SMC Growth Media (#311-500, #311-GS; Cell Applications). SMCs were removed from culture flasks with 0.25% trypsin-EDTA (#25200; Gibco) and placed in 24-well plates (TPP) at 10,000 cells/cm2 to achieve confluent monolayers 1 day before experimentation.
Scratch wound assay
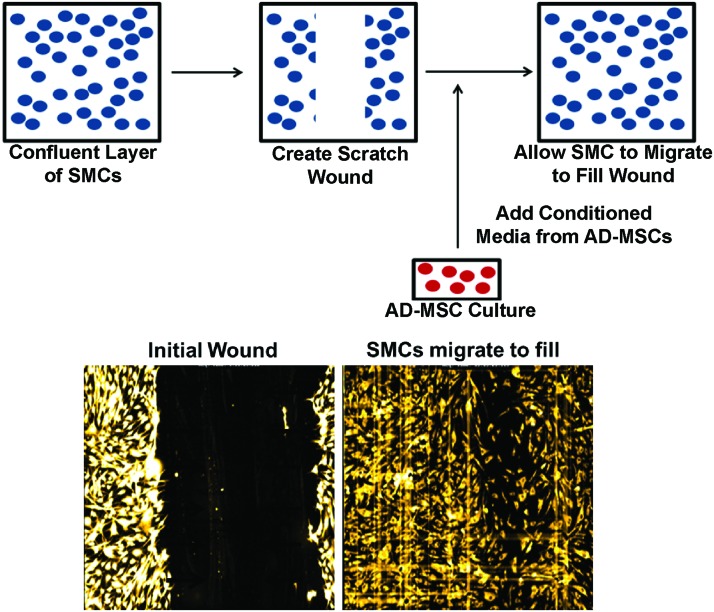
Confluent layers of SMCs within each well had their culture media removed and were scratched with a single stroke of a 200-μL pipette tip to make a wound [scratch wound length: 682±105 μm (avg±SD, n=48)]. SMCs were then washed in a 1× Hanks' balanced salt solution to remove any cellular debris. SMCs were then incubated with SMC media containing 1 μL/mL of Cell Tracker Red (#C34552; Invitrogen) for 30 min to load cells for fluorescent visualization. The media were then replaced with AD-MSC conditioned media and SMCs were then allowed to migrate over 24 h (experimental schematic: Fig. 1). Nonconditioned AD-MSC media were used as a control to show the effect of AD-MSC secreted factors in the conditioned media to promote migration.
FIG. 1.
Scratch wound assay. Smooth muscle cells (SMCs) are induced to migrate through a scratch wound assay while being stimulated with adipose-derived mesenchymal stem cell (AD-MSC) conditioned media over the course of 24 h. Top: Schematic of migration assay. Bottom: Images of fluorescently labeled SMCs directly after scratch wound (left), and after migrating into wound area (right). Color images available online at www.liebertpub.com/tea
Cells were placed in a closed thermo-controlled (37°C) stage-top incubator (Tokai Hit Co.) atop the motorized stage of an inverted Nikon TiE fluorescent microscope (Nikon, Inc.) equipped with a 10×, 0.5NA plan apochromat lens (Nikon, Inc.). Cell Tracker Red was excited using a Lumencor diode-pumped light engine (SpectraX, Lumencor, Inc.) and detected using a DsRed longpass filter set (Chroma Technology Corp.) and ORCA-Flash4.0 sCMOS camera (Hamamatsu Corporation). NIS Elements software (version 4.0) was used to automatically image cell migration every 2 h while typical cell culture conditions were maintained (20% O2, 5% CO2, and 37°C). The resulting images were analyzed by measuring the area of the wound normalized to the area at the initial time point for each well. The migration rate was calculated by averaging the difference in normalized area between the first four time points (equivalent to 8 h) and was normalized to nonconditioned controls. In addition, images of healthy, diabetic, elderly, and control conditions were acquired every 15 min and joined sequentially as a time-lapse video to show migration of each side-by-side (Supplementary Video SV1; Supplementary Data are available online at www.liebertpub.com/tea).
ELISA on AD-MSC conditioned media
An enzyme-linked immunosorbent assay (ELISA) kit was utilized to detect the levels of vascular endothelial growth factor (VEGF) within the conditioned media of AD-MSC donors (#DVE00; R&D Systems). All protocols were followed according to the manufacturer's instructions. To get a greater sense of the population variability of VEGF present within conditioned media, a higher number of donors (n=12) were utilized for this assay. All donors investigated remained in the healthy AD-MSC group (i.e., <45 years of age, nondiabetic) and spanned a wide range of BMI (avg±SD: 27.3±4.3, range: 21.3–34.7).
AD-MSC differentiation into SMCs
To induce differentiation of AD-MSCs into SMCs, AD-MSCs were plated on PLL-coated coverslips (GG-22-pll; Neuvitro) at a density of 10,000 cells per coverslip. They were cultured in media (MEMα, #12561-056; Life Technologies) containing AngII (1 μM, #A9525; Sigma) with 10% serum.91 After 4 days of culture, AD-MSCs were fixed in 4% paraformaldehyde and evaluated using a standard immunofluorescent chemistry protocol to detect calponin (1:250, #ab46794; Abcam), myosin heavy chain (1:250, #ab77967; Abcam), and smoothelin (1:250, #ab8969), to quantify percent expressing cells. F-actin was fluorescently labeled with FITC-phalloidin (1:250, #P5282; Sigma) to assess the cell shape (approaching a spindle-like SMC morphology). Calponin is expressed in aortic SMCs across their phenotype diversity, whereas myosin heavy chain and smoothelin are selective for mature contractile SMCs.92–94 All staining intensities were quantified using NIS Elements software. Primary human aortic SMCs (#PCS-100-102; ATCC) were used as positive controls for SMC staining.
Statistical analyses
All statistical analyses were done utilizing Minitab software (version 16) to perform a t-test, ANOVA, or linear regression. Statistical significance was accepted at p<0.05.
Results
AD-MSC secreted factors can promote SMC migration
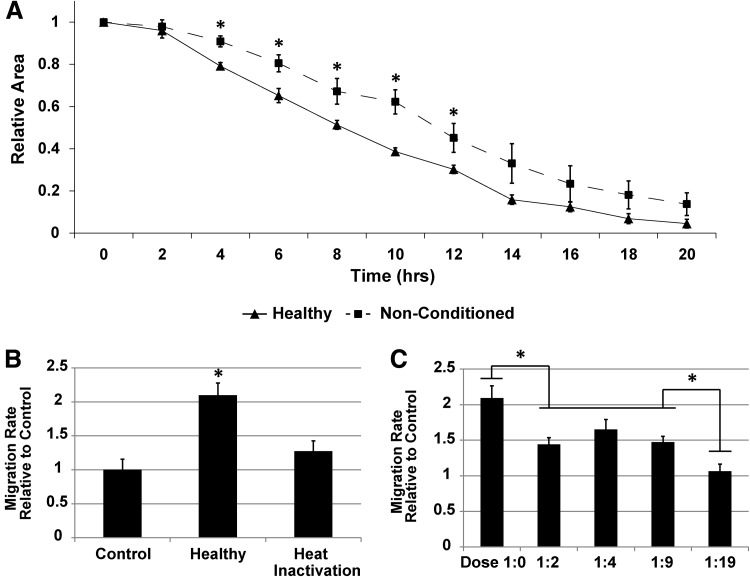
To investigate the ability of AD-MSCs to exert a paracrine promigratory effect on SMCs, we harvested human AD-MSCs from a variety of patients to obtain their conditioned media and employed a scratch wound assay with a confluent monolayer of human SMCs. Upon stimulation with AD-MSC conditioned media from healthy donors, SMCs showed an increased wound closure rate when compared with nonconditioned AD-MSC media (Fig. 2A and Supplementary Video SV1). These data were also expressed as the migration rate per hour over the first 8 h, and conditioned media displayed a significant increase in rate over nonconditioned controls (Fig. 2B). In addition, the factors promoting SMC migration were heat labile, as heating of conditioned media to 56°C removed the promigratory effect (Fig. 2B). Upon dilution of AD-MSC conditioned media to varying levels of conditioning cells/mL, a dose-dependent effect was observed (Fig. 2C). A dose of 1:19 (20-fold dilution) showed a similar effect to nonconditioned media.
FIG. 2.
AD-MSC secreted factors can promote SMC migration. SMCs are induced to migrate through a scratch wound assay while being stimulated with AD-MSC conditioned media over the course of 24 h. AD-MSCs promote the migration of SMCs compared to nonconditioned controls (A). These data were quantified by measuring the normalized wound area over time. These data were converted to migration rate per hour relative to controls, expressing a significant difference between AD-MSC conditioned media and nonconditioned controls (B). In addition, heat inactivating conditioned media caused a significant loss in functionality, indicating that SMC promigratory effects happen at least, in part, on a protein level. Diluting AD-MSC conditioned media to varying levels of conditioning cells/mL produced a dose-dependent effect (dose 1:0=500,000 conditioning cells/mL) (C). Data are presented as mean±SEM with *, significant difference at p<0.05. n=4 was used per group in all experiments.
To identify one factor that could be responsible for this paracrine effect on migration, we quantified VEGF levels in the conditioned media. VEGF was shown to be present at 4091±1903 pg/106 AD-MSCs.
AD-MSCs from diabetic donors can promote the migration of SMCs but have a decreased ability to differentiate into SMCs
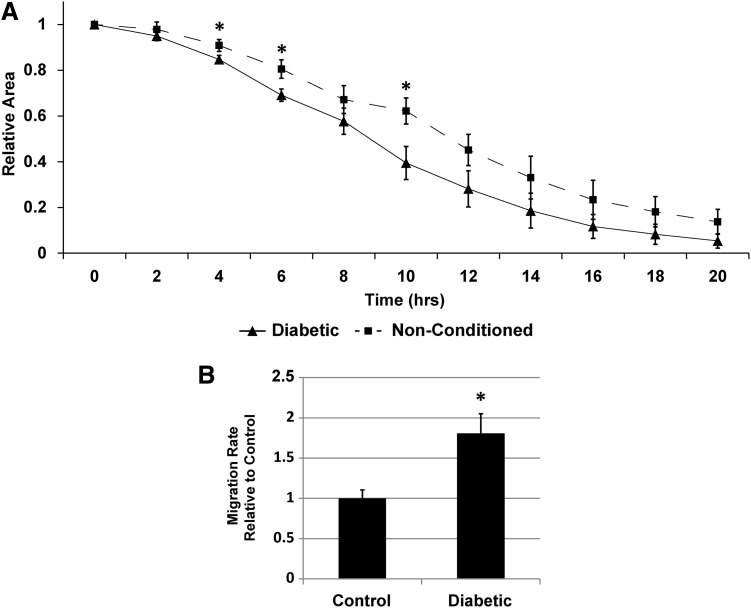
Determining if AD-MSCs from clinically realistic patient groups can perform two main functions utilized in vascular tissue engineering—inducing SMC migration and differentiating directly into SMCs—is critical to the development of a stem cell-based vascular graft. To first assess if AD-MSCs from diabetic patients (i.e., a cohort of patients at high cardiovascular risk) would have a reduced ability to produce SMC promigratory secreted factors, we stimulated SMCs with conditioned media from the AD-MSCs of this patient population. Media conditioned by diabetic AD-MSCs induced faster wound closure than nonconditioned media (Fig. 3A, B and Supplementary Video SV1) in a similar manner to healthy AD-MSCs. This shows that a diabetic origin does not affect the functionality of these cells. Also, it is noteworthy that all AD-MSCs (healthy, diabetic, and elderly) were cultured in high glucose growth media (∼17 mM that is equivalent to 306 mg/dL, which is above the diabetic threshold), which suggests that the diabetic hyperglycemic environment does not limit the ability of AD-MSCs to produce SMC promigratory factors.
FIG. 3.
AD-MSCs from diabetic patients produce SMC promigratory secreted factors. Investigating diabetic patient AD-MSCs for the ability of their secreted factors to promote SMC migration, showed an increased migration compared to nonconditioned media [(A) wound closure over time; (B) wound closure rate per hour]. This increased migration rate was similar to that seen with healthy AD-MSCs. Data are presented as mean±SEM with *, significant difference at p<0.05. n=4 was used per group in all experiments.
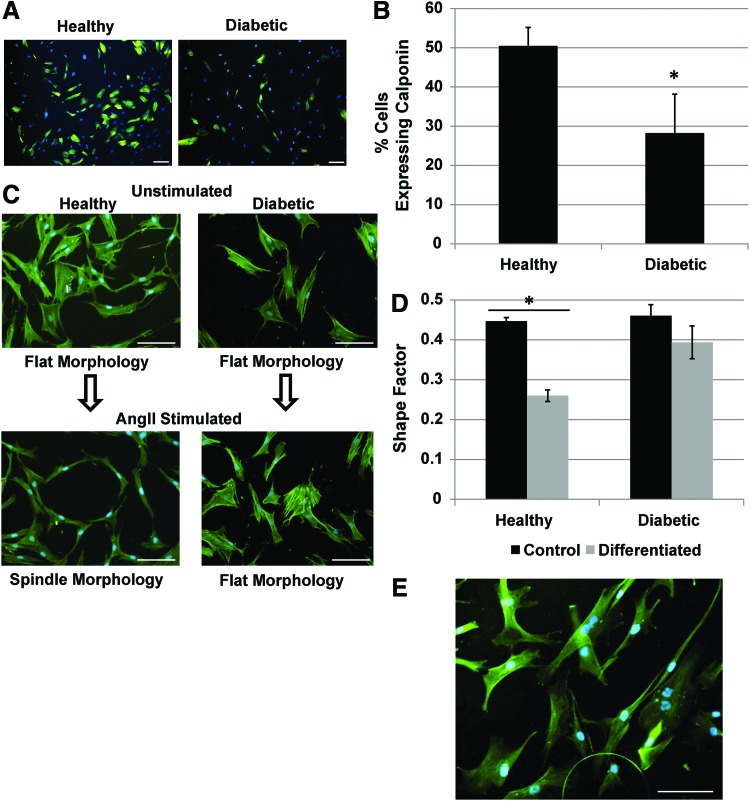
To assess if AD-MSCs from diabetic patients had a reduced ability to differentiate into SMCs, they were stimulated with AngII. Diabetic cells displayed a less efficacious differentiation compared to controls indicated by a significantly lower expression of the SMC marker calponin (Fig. 4A, B) and a failure to adopt an SMC spindle-like morphology (Fig. 4C, D). Unstimulated AD-MSCs do not express calponin (data not shown) and maintain a flat morphology (Fig. 4C, D), whereas SMCs ubiquitously express calponin and a spindle-like morphology (Fig. 4E). In addition, no positive staining was seen with myosin heavy chain or smoothelin with healthy or diabetic donor AD-MSCs in either stimulated or unstimulated states, although SMCs stained positively in the same assay (data not shown).
FIG. 4.
AD-MSC differentiation into SMC is decreased for diabetic patients. Diabetic AD-MSCs displayed a less efficacious SMC differentiation compared to healthy AD-MSCs in terms of the expression of calponin [(A) immunofluorescence for calponin (green) with counterstained nuclei (blue)]. This was quantified by percentage of calponin expressing cells (B). In addition, upon differentiation, AD-MSCs did not acquire an SMC spindle morphology [(C) immunofluorescence for F-actin (green) with counterstained nuclei (blue)]. This was quantified by measuring the shape factor of the cells (4π×area/perimeter2, ∼0=ellipsoid, 1=circular) (D). SMCs inherently express calponin (green) and have a spindle-like morphology (E). Data are presented as mean±SEM with *, significant difference at p<0.05. n=4 was used per group for shape factor experiments. For calponin expression, n=7 was used for the healthy group and n=4 for the diabetic group. All scale bars=100 μm. Color images available online at www.liebertpub.com/tea
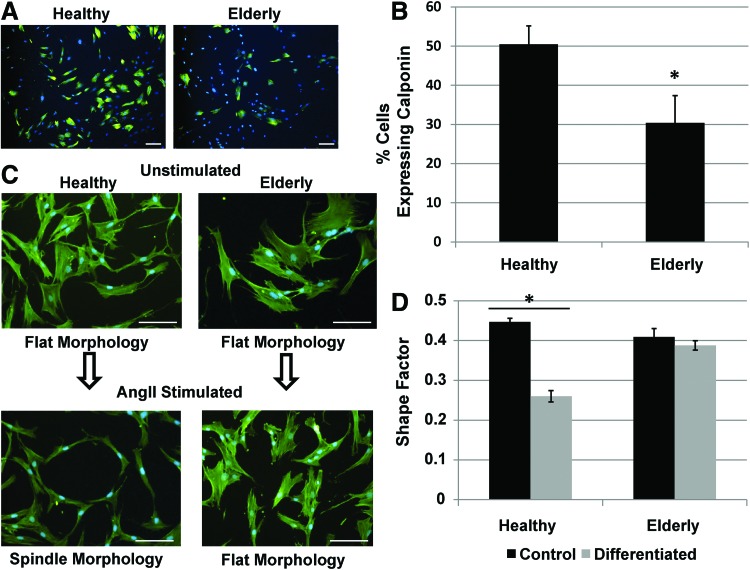
AD-MSCs from elderly donors have both a decreased ability to promote the migration of SMCs and differentiate into SMCs
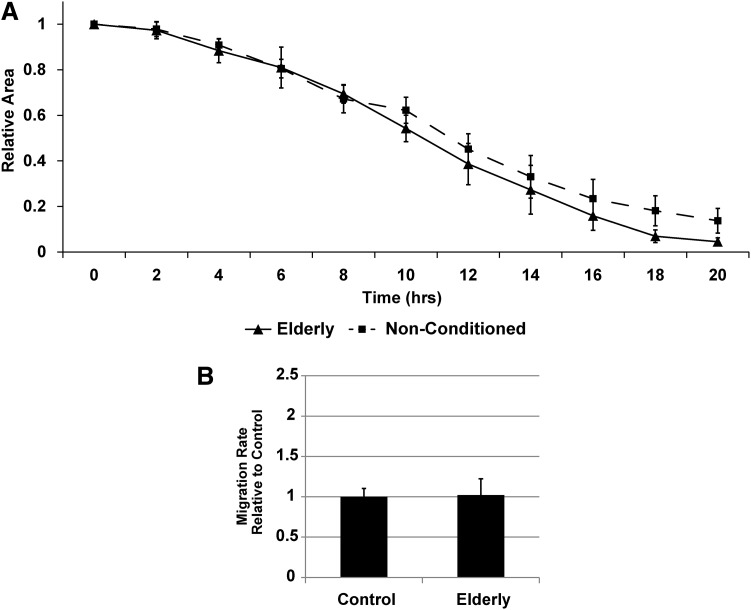
Utilizing a scratch wound assay, AD-MSC secreted factors from elderly donors (i.e., another cohort of patients at high cardiovascular risk) were unable to promote the migration of SMCs (Fig. 5A, B and Supplementary Video SV1). The differentiation potential of elderly donor AD-MSCs toward SMCs was also found to be reduced based on the expression of calponin (Fig. 6A, B) and maintainance of a flat morphology (Fig. 6C, D) under AngII stimulation. AD-MSCs from elderly donors did not stain positively for myosin heavy chain or smoothelin in either stimulated or unstimulated states (data not shown).
FIG. 5.
AD-MSCs from elderly patients do not produce SMC promigratory secreted factors. When comparing between healthy and elderly patient AD-MSCs, those from elderly patients displayed an inability of their secreted factors to produce SMC promigratory effects [(A) wound closure over time; (B) wound closure rate per hour]. Data are presented as mean±SEM with no significant differences found at p<0.05. n=4 was used per group in all experiments.
FIG. 6.
AD-MSC differentiation into SMC is decreased for elderly patients. Elderly AD-MSCs displayed a reduced ability to differentiate into SMCs under angiotensin II (AngII) stimulation for 4 days. AngII-stimulated AD-MSCs showed a significantly lower expression of calponin [(A) immunofluorescence for calponin (green) with counterstained nuclei (blue)]. This was quantified by number of calponin expressing cells (B). In addition, they did not acquire an SMC spindle morphology [(C) immunofluorescence for F-actin (green) with counterstained nuclei (blue)]. This was quantified by measuring the cell shape factor (4π×area/perimeter2, ∼0=ellipsoid, 1=circular) (D). Data are presented as mean±SEM with *, significant difference at p<0.05. n=4 was used per group for shape factor experiments. For calponin expression, n=7 was used for the healthy group and n=5 for the elderly group. All scale bars=100 μm. Color images available online at www.liebertpub.com/tea
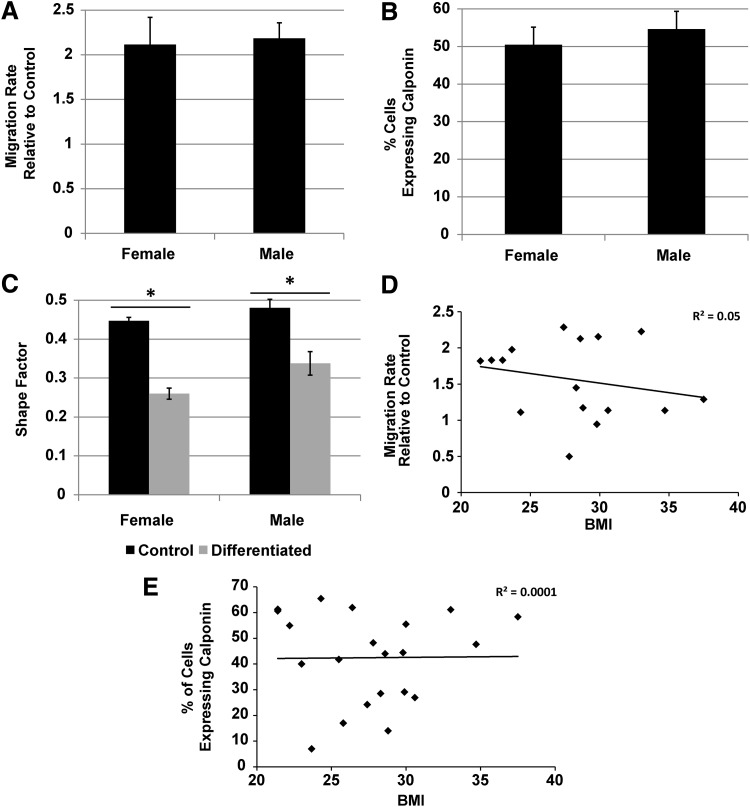
Gender and BMI do not affect the ability of AD-MSCs to promote the migration of SMCs or the ability to differentiate into SMCs
As all the donors utilized for the scratch wound assay were females, male donors from the healthy demographic were acquired and compared directly to the healthy female group to assess potential gender differences. The ability to produce SMC promigratory factors did not appear to be gender specific (Fig. 7A), as there was no difference in the migration rate observed between the groups. In addition, the gender did not affect the ability of AD-MSCs to differentiate into SMCs as both male and female donors displayed equivalent results in terms of expression of calponin postdifferentiation (Fig. 7B) and change to a SMC-like morphology in the presence of AngII (Fig. 7C). The BMI was also investigated in both assays by performing a regression analysis on the migration rate (Fig. 7D) and calponin expression (Fig. 7E) postdifferentiation, respectively. No significant trend was seen between either set of data (migration rate: R2=0.05, p=0.4; calponin expression: R2=0.001, p=0.96). Also, we correlated the concentration of VEGF (pg/106 cells) with BMI and showed no significant trend (data not shown, R2=0.01, p=0.74). From these data, altered abilities in AD-MSC function appear to be dependent on diabetes and age, but not gender or BMI.
FIG. 7.
Gender and body–mass index (BMI) do not affect AD-MSC secreted SMC promigratory factors or AD-MSC differentiation into SMCs. Comparing gender of AD-MSCs from both healthy male and female donors produced equivalent SMC promigratory responses (A). In addition, gender produced equivalent results when AD-MSCs were stimulated to differentiate into SMCs with AngII based on calponin expression (B) and change in cell morphology (C). Performing a regression analysis utilizing BMI from all donors produced no correlation for either migration rate (D) or percentage of cells expressing calponin (E). Data for bar graphs are represented as mean±SEM with *, signficant difference at p<0.05. n=4 was used per group for migration and shape factor experiments. For calponin expression, n=7 was used for the female group and n=5 for the male group.
Discussion
The ability of an implanted scaffold to become populated with functional cellular constituents is a critical factor for success in tissue engineering applications. For example, not only does a TEBV with more SMCs indicate a higher degree of vascular maturity,50 including medial cellularity, myosin heavy chain expression, and contractility, but SMC content also correlates with desirable mechanical properties such as tensile strength and stiffness.39,41 These mechanical properties are critical for the long-term success of a TEBV as they can be predictive of failure by aneurysmal dilation/rupture and intimal hyperplasia.95 As one of the main design strategies in tissue engineering is to utilize a biodegradable scaffold that is replaced with host tissue, if sufficient vascular cells and matrices are not recruited and produced to compensate the mechanical loss due to scaffold degradation, the TEBV could fail in the long term. Including MSCs that can guide this process is critical to avoiding failure.
Many recent studies have begun to investigate the mechanisms by which TEBVs remodel to achieve a mature native-like vessel. Studies utilizing cell tracking have shown that seeded cells are eventually replaced with migrating host SMCs, with the seeded cells being necessary for providing initial antithrombogenicty and initiating the remodeling process.18–22 Providing further evidence for this phemonmenon, implanted scaffolds with low porosity28,30,32 and even TEBVs with already intact SMC layers24 have been shown to be repopulated with host vessel wall cells. In addition, vascular engineering methods have begun to take advantage of host recellularization by creating acellular approaches, utilizing bound growth factors or drug delivery methods,19,23,96 or designing porous scaffolds to facilitate this cellular infiltration.31,46,97,98 In all of these cases, establishing a mature TEBV populated by SMCs requires recruitment of host cells.
In this study, we show that donor demographics in human AD-MSCs can play a role in their ability to produce secreted factors that are responsible for SMC migration and for them to differentiate into SMCs, both of which are important for remodeling and maturation of TEBVs. Age is critically important to both the abilities of AD-MSCs to produce secreted factors and differentiate into SMCs, whereas a diabetic condition only affected the ability of AD-MSCs to differentiate. However, gender and BMI did not seem to play a role in either stem cell function, which is surprising since female AD-MSCs proliferate at a higher rate than male ones99,100 and BMI is negatively correlated with the AD-MSC differentiation capacity.101
One area of interest that was not yet investigated in the migration experiments is the effects of each AD-MSC demographic on their respective SMC demographic, such as the effect of diabetic AD-MSC conditioned media on diabetic SMCs. However, SMCs sourced from diabetic and elderly humans have been previously shown to have increased102 and decreased103 migration, respectively. This parallels the above conclusions that the combination of AD-MSC and SMC of diabetic patients is promigratory, whereas the combination of the elderly is not.
As we have utilized human cells in this study, particularly those from clinically realistic groups, and shown functional differences in a mechanism of action by which TEBVs remodel and the ability of AD-MSCs to form SMCs, this represents a critical step in the clinical translation of TEBVs. We have shown that elderly AD-MSCs may cause poor TEBV remodeling in terms of their ability to recruit SMCs. While these cells may have auxiliary functions such as providing resistance against thrombosis,20 this clearly could reduce the effectiveness of an autologous TEBV therapy. This an important concern as more than 4 million people in the United States are over 65 years old104 and these represent one of the highest cardiovascular risk groups.67 In addition, as many TEBV approaches utilize AD-MSCs to differentiate into SMCs,14–17 those utilizing elderly and diabetic cells could be heavily affected due to the reduced differentiation potential for both groups. One point worth noting in this regard is that with 1 μM AngII stimulation for 4 days, AD-MSCs do not achieve complete differentation into SMCs, as evidenced by the absence of staining for myosin heavy chain or smoothelin (both late differentiation markers).
We have also shown that the ability of AD-MSCs to induce SMC migration is decreased upon heat inactivation indicating that these secreted factors likely operate on a protein level. One likely explanation could be that growth factors secreted by MSCs are responsible for this promigratory function. Indeed, we have shown that one known factor to promote SMC migration, VEGF, is present in our conditioned media. However, many growth factors produced by AD-MSCs are promigratory for SMCs105 and can also induce other mitogenic, proteolytic, extracellular matrix producing, inflammatory, and angiogenic effects.5 This is concerning for studies that target elderly patients, as the same growth factors that lead to the deficiency in promoting SMC migration may also affect other applications beyond TEBVs. Also, with AD-MSC secreted factors showing a dose-dependent response, the need to optimize the number of cells utilized in cell therapy approaches is clear.
In conclusion, by utilizing conditioned media from AD-MSCs to induce SMC migration, we have shown that age and not diabetes, gender, or BMI results in an inability. In addition, this effect is dose dependent and functions on a protein level. Also, we have shown that differentiation of AD-MSCs from diabetic and elderly patients is decreased but no effect was seen due to gender or BMI. With respect to TEBVs, AD-MSCs from elderly patients may be suboptimal in autologous TEBVs, as they lack the ability to induce host recellularization.
Supplementary Material
Acknowledgments
This work was supported by the American Heart Association (AHA #12PRE12050163 to J.T.K.) and the National Institutes of Health (R21 #EB016138 to D.A.V.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Ryu Y.-J., Cho T.-J., Lee D.-S., Choi J.-Y., and Cho J.Phenotypic characterization and in vivo localization of human adipose-derived mesenchymal stem cells. Mol Cells 35,557, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gimble J.M., Katz A.J., and Bunnell B.A.Adipose-derived stem cells for regenerative medicine. Circ Res 100,1249, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mizuno H.Adipose-derived stem cells for tissue repair and regeneration: ten years of research and a literature review. J Nippon Med Sch 76,56, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Rehman J., Traktuev D., Li J., Merfeld-Clauss S., Temm-Grove C.J., Bovenkerk J.E., Pell C.L., Johnstone B.H., Considine R.V., and March K.L.Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109,1292, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Kinnaird T., Stabile E., Burnett M., Lee C., Barr S., Fuchs S., and Epstein S.Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res 94,678, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Gnecchi M., Zhang Z., Ni A., and Dzau V.J.Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res 103,1204, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schinköthe T., Bloch W., and Schmidt A.In vitro secreting profile of human mesenchymal stem cells. Stem Cells Dev 17,199, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Krawiec J.T., and Vorp D.A.Adult stem cell-based tissue engineered blood vessels: a review. Biomaterials 33,3388, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Wei H.-J., Chen C.-H., Lee W.-Y., Chiu I., Hwang S.-M., Lin W.-W., Huang C.-C., Yeh Y.-C., Chang Y., and Sung H.-W.Bioengineered cardiac patch constructed from multilayered mesenchymal stem cells for myocardial repair. Biomaterials 29,3547, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Schoen F.J.Heart valve tissue engineering: quo vadis? Curr Opin Biotechnol 22,698, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Tedder M.E., Simionescu A., Chen J., Liao J., and Simionescu D.T.Assembly and testing of stem cell-seeded layered collagen constructs for heart valve tissue engineering. Tissue Eng Part A 17,25, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinert A.F., Rackwitz L., Gilbert F., Nöth U., and Tuan R.S.Concise review: the clinical application of mesenchymal stem cells for musculoskeletal regeneration: current status and perspectives. Stem Cells Transl Med 1,237, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richardson S.M., Hoyland J.A., Mobasheri R., Csaki C., Shakibaei M., and Mobasheri A.Mesenchymal stem cells in regenerative medicine: opportunities and challenges for articular cartilage and intervertebral disc tissue engineering. J Cell Physiol 222,23, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Rodríguez L.V., Alfonso Z., Zhang R., Leung J., Wu B., and Ignarro L.J.Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc Natl Acad Sci U S A 103,12167, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang C., Yin S., Cen L., Liu Q., Liu W., Cao Y., and Cui L.Differentiation of adipose-derived stem cells into contractile smooth muscle cells induced by transforming growth factor-β1 and bone morphogenetic protein-4. Tissue Eng Part A 16,1201, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Wang C., Cen L., Yin S., Liu Q., Liu W., Cao Y., and Cui L.A.small diameter elastic blood vessel wall prepared under pulsatile conditions from polyglycolic acid mesh and smooth muscle cells differentiated from adipose-derived stem cells. Biomaterials 31,621, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Harris L.J., Abdollahi H., Zhang P., McIlhenny S., Tulenko T.N., and DiMuzio P.J.Differentiation of adult stem cells into smooth muscle for vascular tissue engineering. J Surg Res 168,306, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hjortnaes J., Gottlieb D., Figueiredo J.-L., Melero-Martin J., Kohler R.H., Bischoff J., Weissleder R., Mayer J.E., and Aikawa E.Intravital molecular imaging of small-diameter tissue-engineered vascular grafts in mice: a feasibility study. Tissue Eng Part C Methods 16,597, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roh J.D., Sawh-Martinez R., Brennan M.P., Jay S.M., Devine L., Rao D.A., Yi T., Mirensky T.L., Nalbandian A., and Udelsman B.Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci U S A 107,4669, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashi C.K., Zhu Y., Yang G.-Y., Young W.L., Hsiao B.S., Wang K., Chu B., and Li S.Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc Natl Acad Sci U S A 104,11915, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hibino N., Yi T., Duncan D.R., Rathore A., Dean E., Naito Y., Dardik A., Kyriakides T., Madri J., and Pober J.S.A critical role for macrophages in neovessel formation and the development of stenosis in tissue-engineered vascular grafts. FASEB J 25,4253, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hibino N., Villalona G., Pietris N., Duncan D.R., Schoffner A., Roh J.D., Yi T., Dobrucki L.W., Mejias D., and Sawh-Martinez R.Tissue-engineered vascular grafts form neovessels that arise from regeneration of the adjacent blood vessel. FASEB J 25,2731, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu J., Wang A., Tang Z., Henry J., Li-Ping Lee B., Zhu Y., Yuan F., Huang F., and Li S.The effect of stromal cell-derived factor-1α/heparin coating of biodegradable vascular grafts on the recruitment of both endothelial and smooth muscle progenitor cells for accelerated regeneration. Biomaterials 33,8062, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelm J.M., Emmert M.Y., Zürcher A., Schmidt D., Begus Nahrmann Y., Rudolph K.L., Weber B., Brokopp C.E., Frauenfelder T., and Leschka S.Functionality, growth and accelerated aging of tissue engineered living autologous vascular grafts. Biomaterials 33,8277, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Dahl S.L., Kypson A.P., Lawson J.H., Blum J.L., Strader J.T., Li Y., Manson R.J., Tente W.E., DiBernardo L., and Hensley M.T.Readily available tissue-engineered vascular grafts. Sci Transl Med 3,68, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Lepidi S., Grego F., Vindigni V., Zavan B., Tonello C., Deriu G., Abatangelo G., and Cortivo R.Hyaluronan biodegradable scaffold for small-caliber artery grafting: preliminary results in an animal model. Eur J Vasc Endovasc Surg 32,411, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Torikai K., Ichikawa H., Hirakawa K., Matsumiya G., Kuratani T., Iwai S., Saito A., Kawaguchi N., Matsuura N., and Sawa Y.A self-renewing, tissue-engineered vascular graft for arterial reconstruction. J Thorac Cardiovasc Surg 136,37, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Yokota T., Ichikawa H., Matsumiya G., Kuratani T., Sakaguchi T., Iwai S., Shirakawa Y., Torikai K., Saito A., and Uchimura E.In situ tissue regeneration using a novel tissue-engineered, small-caliber vascular graft without cell seeding. J Thorac Cardiovasc Surg 136,900, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Pavcnik D., Obermiller J., Uchida B.T., Van Alstine W., Edwards J.M., Landry G.J., Kaufman J.A., Keller F.S., and Rösch J.Angiographic evaluation of carotid artery grafting with prefabricated small-diameter, small-intestinal submucosa grafts in sheep. Cardiovasc Intervent Radiol 32,106, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Soletti L., Nieponice A., Hong Y., Ye S.H., Stankus J.J., Wagner W.R., and Vorp D.A.In vivo performance of a phospholipid-coated bioerodable elastomeric graft for small-diameter vascular applications. J Biomed Mater Res A 96,436, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu W., Allen R.A., and Wang Y.Fast-degrading elastomer enables rapid remodeling of a cell-free synthetic graft into a neoartery. Nat Med 18,1148, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lovett M., Eng G., Kluge J., Cannizzaro C., Vunjak-Novakovic G., and Kaplan D.L.Tubular silk scaffolds for small diameter vascular grafts. Organogenesis 6,217, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho S.-W., Lim S.H., Kim I.-K., Hong Y.S., Kim S.-S., Yoo K.J., Park H.-Y., Jang Y., Chang B.C., and Choi C.Y.Small-diameter blood vessels engineered with bone marrow-derived cells. Ann Surg 241,506, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho S.-W., Lim J.E., Chu H.S., Hyun H.-J., Choi C.Y., Hwang K.-C., Yoo K.J., Kim D.-I., and Kim B.-S.Enhancement of in vivo endothelialization of tissue-engineered vascular grafts by granulocyte colony-stimulating factor. J Biomed Mater Res A 76,252, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Lim S.H., Cho S.-W., Park J.-C., Jeon O., Lim J.M., Kim S.-S., and Kim B.-S.Tissue-engineered blood vessels with endothelial nitric oxide synthase activity. J Biomed Mater Res B Appl Biomater 85,537, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Roh J.D., Brennan M.P., Lopez-Soler R.I., Fong P.M., Goyal A., Dardik A., and Breuer C.K.Construction of an autologous tissue-engineered venous conduit from bone marrow-derived vascular cells: optimization of cell harvest and seeding techniques. J Pediatr Surg 42,198, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Cho S.-W., Kim I.-K., Kang J.M., Song K.W., Kim H.S., Park C.H., Yoo K.J., and Kim B.-S.Evidence for in vivo growth potential and vascular remodeling of tissue-engineered artery. Tissue Eng Part A 15,901, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Liu J.Y., Swartz D.D., Peng H.F., Gugino S.F., Russell J.A., and Andreadis S.T.Functional tissue-engineered blood vessels from bone marrow progenitor cells. Cardiovasc Res 75,618, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Matsumura G., Miyagawa-Tomita S., Shin'oka T., Ikada Y., and Kurosawa H.First evidence that bone marrow cells contribute to the construction of tissue-engineered vascular autografts in vivo. Circulation 108,1729, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Hibino N., Shin'oka T., Matsumura G., Ikada Y., and Kurosawa H.The tissue-engineered vascular graft using bone marrow without culture. J Thorac Cardiovasc Surg 129,1064, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Matsumura G., Ishihara Y., Miyagawa-Tomita S., Ikada Y., Matsuda S., Kurosawa H., and Shin'oka T.Evaluation of tissue-engineered vascular autografts. Tissue Eng 12,3075, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Brennan M.P., Dardik A., Hibino N., Roh J.D., Nelson G.N., Papademitris X., Shinoka T., and Breuer C.K.Tissue engineered vascular grafts demonstrate evidence of growth and development when implanted in a juvenile animal model. Ann Surg 248,370, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mirensky T.L., Hibino N., Sawh-Martinez R.F., Yi T., Villalona G., Shinoka T., and Breuer C.K.Tissue-engineered vascular grafts: does cell seeding matter? J Pediatr Surg 45,1299, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao Y., Zhang S., Zhou J., Wang J., Zhen M., Liu Y., Chen J., and Qi Z.The development of a tissue-engineered artery using decellularized scaffold and autologous ovine mesenchymal stem cells. Biomaterials 31,296, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Zhang L., Zhou J., Lu Q., Wei Y., and Hu S.A novel small-diameter vascular graft: in vivo behavior of biodegradable three‐layered tubular scaffolds. Biotechnol Bioeng 99,1007, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Nieponice A., Soletti L., Guan J., Hong Y., Gharaibeh B., Maul T.M., Huard J., Wagner W.R., and Vorp D.A.In vivo assessment of a tissue-engineered vascular graft combining a biodegradable elastomeric scaffold and muscle-derived stem cells in a rat model. Tissue Eng Part A 16,1215, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He W., Nieponice A., Soletti L., Hong Y., Gharaibeh B., Crisan M., Usas A., Peault B., Huard J., and Wagner W.R.Pericyte-based human tissue engineered vascular grafts. Biomaterials 31,8235, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He H., Shirota T., Yasui H., and Matsuda T.Canine endothelial progenitor cell-lined hybrid vascular graft with nonthrombogenic potential. J Thorac Cardiovasc Surg 126,455, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Kaushal S., Amiel G.E., Guleserian K.J., Shapira O.M., Perry T., Sutherland F.W., Rabkin E., Moran A.M., Schoen F.J., and Atala A.Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med 7,1035, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neff L.P., Tillman B.W., Yazdani S.K., Machingal M.A., Yoo J.J., Soker S., Bernish B.W., Geary R.L., and Christ G.J.Vascular smooth muscle enhances functionality of tissue-engineered blood vessels in vivo. J Vasc Surg 53,426, 2011 [DOI] [PubMed] [Google Scholar]
- 51.Zhu C., Ying D., Mi J., Li L., Zeng W., Hou C., Sun J., Yuan W., Wen C., and Zhang W.Development of anti-atherosclerotic tissue-engineered blood vessel by a 20-regulated endothelial progenitor cells seeding decellularized vascular matrix. Biomaterials 29,2628, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Quint C., Kondo Y., Manson R.J., Lawson J.H., Dardik A., and Niklason L.E.Decellularized tissue-engineered blood vessel as an arterial conduit. Proc Natl Acad Sci U S A 108,9214, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koch S., Flanagan T.C., Sachweh J.S., Tanios F., Schnoering H., Deichmann T., Ellä V., Kellomäki M., Gronloh N., and Gries T.Fibrin-polylactide-based tissue-engineered vascular graft in the arterial circulation. Biomaterials 31,4731, 2010 [DOI] [PubMed] [Google Scholar]
- 54.L'heureux N., Pâquet S., Labbé R., Germain L., and Auger F.A.A completely biological tissue-engineered human blood vessel. FASEB J 12,47, 1998 [DOI] [PubMed] [Google Scholar]
- 55.L'Heureux N., Dusserre N., Konig G., Victor B., Keire P., Wight T.N., Chronos N. A., Kyles A. E., Gregory C. R., and Hoyt G.Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med 12,361, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nelson G.N., Mirensky T., Brennan M.P., Roh J.D., Yi T., Wang Y., and Breuer C.K.Functional small-diameter human tissue-engineered arterial grafts in an immunodeficient mouse model: preliminary findings. Arch Surg 143,488, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He H., and Matsuda T.Newly designed compliant hierarchic hybrid vascular graft wrapped with microprocessed elastomeric film-ii: morphogenesis and compliance change upon implantation. Cell Transplant 11,75, 2002 [PubMed] [Google Scholar]
- 58.Quint C., Arief M., Muto A., Dardik A., and Niklason L.E.Allogeneic human tissue-engineered blood vessel. J Vasc Surg 55,790, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Niklason L., Gao J., Abbott W., Hirschi K., Houser S., Marini R., and Langer R.Functional arteries grown in vitro. Science 284,489, 1999 [DOI] [PubMed] [Google Scholar]
- 60.Zhao J., Liu L., Wei J., Ma D., Geng W., Yan X., Zhu J., Du H., Liu Y., and Li L.A novel strategy to engineer small‐diameter vascular grafts from marrow-derived mesenchymal stem cells. Artif Organs 36,93, 2012 [DOI] [PubMed] [Google Scholar]
- 61.He W., Nieponice A., Hong Y., Wagner W.R., and Vorp D.A.Rapid engineered small diameter vascular grafts from smooth muscle cells. Cardiovasc Eng Technol 2,149, 2011 [Google Scholar]
- 62.Hoerstrup S.P., Cummings I., Lachat M., Schoen F.J., Jenni R., Leschka S., Neuenschwander S., Schmidt D., Mol A., and Günter C.Functional growth in tissue-engineered living, vascular grafts follow-up at 100 weeks in a large animal model. Circulation 114,1159, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Watanabe M., Shin'oka T., Tohyama S., Hibino N., Konuma T., Matsumura G., Kosaka Y., Ishida T., Imai Y., and Yamakawa M.Tissue-engineered vascular autograft: inferior vena cava replacement in a dog model. Tissue Eng 7,429, 2001 [DOI] [PubMed] [Google Scholar]
- 64.Dokken B.B.The pathophysiology of cardiovascular disease and diabetes: beyond blood pressure and lipids. Diabetes Spectr 21,160, 2008 [Google Scholar]
- 65.Imazu M., Sumii K., Yamamoto H., Toyofuku M., Tadehara F., Okubo M., Yamakido M., Kohno N., and Onaka A.T.Influence of type 2 diabetes mellitus on cardiovascular disease mortality: findings from the hawaii-los angeles-hiroshima study. Diabetes Res Clin Pract 57,61, 2002 [DOI] [PubMed] [Google Scholar]
- 66.Fuster J.J., and Andrés V.Telomere biology and cardiovascular disease. Circ Res 99,1167, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Conroy R., Pyörälä K., Fitzgerald A.E., Sans S., Menotti A., De Backer G., De Bacquer D., Ducimetiere P., Jousilahti P., and Keil U.Estimation of ten-year risk of fatal cardiovascular disease in Europe: the score project. Eur Heart J 24,987, 2003 [DOI] [PubMed] [Google Scholar]
- 68.Saliques S., Zeller M., Lorin J., Lorgis L., Teyssier J.-R., Cottin Y., Rochette L., and Vergely C.Telomere length and cardiovascular disease. Arch Cardiovasc Dis 103,454, 2010 [DOI] [PubMed] [Google Scholar]
- 69.Keats E.C., and Khan Z.A.Vascular stem cells in diabetic complications: evidence for a role in the pathogenesis and the therapeutic promise. Cardiovasc Diabetol 11,1, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stolzing A., Sellers D., Llewelyn O., and Scutt A.Diabetes induced changes in rat mesenchymal stem cells. Cells Tissues Organs 191,453, 2010 [DOI] [PubMed] [Google Scholar]
- 71.Cianfarani F., Toietta G., Di Rocco G., Cesareo E., Zambruno G., and Odorisio T.Diabetes impairs adipose tissue-derived stem cell function and efficiency in promoting wound healing. Wound Repair Regen 21,545, 2013 [DOI] [PubMed] [Google Scholar]
- 72.Kume S., Kato S., Yamagishi S.-C., Inagaki Y., Ueda S., Arima N., Okawa T., Kojiro M., and Nagata K.Advanced glycation end-products attenuate human mesenchymal stem cells and prevent cognate differentiation into adipose tissue, cartilage, and bone. J Bone Miner Res 20,1647, 2005 [DOI] [PubMed] [Google Scholar]
- 73.Hiyama E., and Hiyama K.Telomere and telomerase in stem cells. Br J Cancer 96,1020, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Madonna R., Renna F.V., Cellini C., Cotellese R., Picardi N., Francomano F., Innocenti P., and De Caterina R.Age-dependent impairment of number and angiogenic potential of adipose tissue‐derived progenitor cells. Eur J Clin Invest 41,126, 2011 [DOI] [PubMed] [Google Scholar]
- 75.Zhu M., Kohan E., Bradley J., Hedrick M., Benhaim P., and Zuk P.The effect of age on osteogenic, adipogenic and proliferative potential of female adipose‐derived stem cells. J Tissue Eng Regen Med 3,290, 2009 [DOI] [PubMed] [Google Scholar]
- 76.Schipper B.M., Marra K.G., Zhang W., Donnenberg A.D., and Rubin J.P.Regional anatomic and age effects on cell function of human adipose-derived stem cells. Ann Plast Surg 60,538, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lavasani M., Robinson A.R., Lu A., Song M., Feduska J.M., Ahani B., Tilstra J.S., Feldman C.H., Robbins P.D., and Niedernhofer L.J.Muscle-derived stem/progenitor cell dysfunction limits healthspan and lifespan in a murine progeria model. Nat Commun 3,1, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang S.-C., Wu T.-C., Yu H.-C., Chen M.-R., Liu C.-M., Chiang W.-S., and Lin K.M.Mechanical strain modulates age-related changes in the proliferation and differentiation of mouse adipose-derived stromal cells. BMC Cell Biol 11,18, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen H.T., Lee M.J., Chen C.H., Chuang S.C., Chang L.F., Ho M.L., Hung S.H., Fu Y.C., Wang Y.H., and Wang H.I.Proliferation and differentiation potential of human adipose‐derived mesenchymal stem cells isolated from elderly patients with osteoporotic fractures. J Cell Mol Med 16,582, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cheleuitte D., Mizuno S., and Glowacki J.In vitro secretion of cytokines by human bone marrow: effects of age and estrogen status. J Clin Endocrinol Metab 83,2043, 1998 [DOI] [PubMed] [Google Scholar]
- 81.Dimmeler S., and Leri A.Aging and disease as modifiers of efficacy of cell therapy. Circ Res 102,1319, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sowa Y., Imura T., Numajiri T., Nishino K., and Fushiki S.Adipose-derived stem cells produce factors enhancing peripheral nerve regeneration: influence of age and anatomic site of origin. Stem Cells Dev 21,1852, 2011 [DOI] [PubMed] [Google Scholar]
- 83.Wu W., Niklason L., and Steinbacher D. M.The effect of age on human adipose-derived stem cells. Plast Reconstr Surg 131,27, 2013 [DOI] [PubMed] [Google Scholar]
- 84.Choudhery M.S., Badowski M., Muise A., Pierce J., and Harris D.T.Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J Transl Med 12,8, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Efimenko A., Starostina E., Kalinina N., and Stolzing A.Angiogenic properties of aged adipose derived mesenchymal stem cells after hypoxic conditioning. J Transl Med 9,1, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim W.-S., Park B.-S., and Sung J.-H.The wound-healing and antioxidant effects of adipose-derived stem cells. Expert Opin Biol Ther 9,879, 2009 [DOI] [PubMed] [Google Scholar]
- 87.Kim W.-S., Park B.-S., Park S.-H., Kim H.-K., and Sung J.-H.Antiwrinkle effect of adipose-derived stem cell: activation of dermal fibroblast by secretory factors. J Dermatol Sci 53,96, 2009 [DOI] [PubMed] [Google Scholar]
- 88.Wei X., Du Z., Zhao L., Feng D., Wei G., He Y., Tan J., Lee W.H., Hampel H., and Dodel R.Ifats collection: the conditioned media of adipose stromal cells protect against hypoxia-ischemia-induced brain damage in neonatal rats. Stem Cells 27,478, 2009 [DOI] [PubMed] [Google Scholar]
- 89.Kim W.-S., Park B.-S., Sung J.-H., Yang J.-M., Park S.-B., Kwak S.-J., and Park J.-S.Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci 48,15, 2007 [DOI] [PubMed] [Google Scholar]
- 90.Zimmerlin L., Donnenberg V.S., Pfeifer M.E., Meyer E.M., Péault B., Rubin J.P., and Donnenberg A.D.Stromal vascular progenitors in adult human adipose tissue. Cytometry A 77,22, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim Y.M., Jeon E.S., Kim M.R., Jho S.K., Ryu S.W., and Kim J.H.Angiotensin ii-induced differentiation of adipose tissue-derived mesenchymal stem cells to smooth muscle-like cells. Int J Biochem Cell Biol 40,2482, 2008 [DOI] [PubMed] [Google Scholar]
- 92.van der Loop F.T., Gabbiani G., Kohnen G., Ramaekers F.C., and van Eys G.J.Differentiation of smooth muscle cells in human blood vessels as defined by smoothelin, a novel marker for the contractile phenotype. Arterioscler Thromb Vasc Biol 17,665, 1997 [DOI] [PubMed] [Google Scholar]
- 93.Beamish J.A., He P., Kottke-Marchant K., and Marchant R.E.Molecular regulation of contractile smooth muscle cell phenotype: implications for vascular tissue engineering. Tissue Eng Part B Rev 16,467, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rensen S., Doevendans P., and Van Eys G.Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J 15,100, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.El-Kurdi M.S., Hong Y., Stankus J.J., Soletti L., Wagner W.R., and Vorp D.A.Transient elastic support for vein grafts using a constricting microfibrillar polymer wrap. Biomaterials 29,3213, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zeng W., Wen C., Wu Y., Li L., Zhou Z., Mi J., Chen W., Yang M., Hou C., and Sun J.The use of bdnf to enhance the patency rate of small-diameter tissue-engineered blood vessels through stem cell homing mechanisms. Biomaterials 33,473, 2012 [DOI] [PubMed] [Google Scholar]
- 97.Soletti L., Hong Y., Guan J., Stankus J.J., El-Kurdi M.S., Wagner W.R., and Vorp D.A.A bilayered elastomeric scaffold for tissue engineering of small diameter vascular grafts. Acta Biomater 6,110, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ju Y.M., Choi J.S., Atala A., Yoo J.J., and Lee S.J.Bilayered scaffold for engineering cellularized blood vessels. Biomaterials 31,4313, 2010 [DOI] [PubMed] [Google Scholar]
- 99.Anderson L.A., McTernan P.G., Barnett A.H., and Kumar S.The effects of androgens and estrogens on preadipocyte proliferation in human adipose tissue: influence of gender and site. J Clin Endocrinol Metab 86,5045, 2001 [DOI] [PubMed] [Google Scholar]
- 100.van Harmelen V., Röhrig K., and Hauner H.Comparison of proliferation and differentiation capacity of human adipocyte precursor cells from the omental and subcutaneous adipose tissue depot of obese subjects. Metabolism 53,632, 2004 [DOI] [PubMed] [Google Scholar]
- 101.Van Harmelen V., Skurk T., Röhrig K., Lee Y., Halbleib M., Aprath-Husmann I., and Hauner H.Effect of BMI and age on adipose tissue cellularity and differentiation capacity in women. Int J Obes 27,889, 2003 [DOI] [PubMed] [Google Scholar]
- 102.Faries P.L., Rohan D.I., Takahara H., Wyers M.C., Contreras M.A., Quist W.C., King G.L., and LoGerfo F.W.Human vascular smooth muscle cells of diabetic origin exhibit increased proliferation, adhesion, and migration. J Vasc Surg 33,601, 2001 [DOI] [PubMed] [Google Scholar]
- 103.Ruiz-Torres A., Lozano R., Melón J., and Carraro R.Age-dependent decline of in vitro migration (basal and stimulated by IGF-1 or insulin) of human vascular smooth muscle cells. J Gerontol A Biol Sci Med Sci 58,B1074, 2003 [DOI] [PubMed] [Google Scholar]
- 104.USA Quickfacts from the US Census Bureau. 2013. Available at: http://quickfacts.census.gov/qfd/states/00000.html (Accessed December5, 2013)
- 105.Gerthoffer W.T.Mechanisms of vascular smooth muscle cell migration. Circ Res 100,607, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.