Abstract
Women have better verbal memory, and higher rates of resting regional cerebral blood flow (rCBF). This study examined whether there are also sex differences in the relationship between verbal episodic memory and resting rCBF. Twenty eight healthy right-handed volunteers (14 male, 14 female) underwent a neuropsychological evaluation and a Positron Emission Tomography (PET) 15O-water study. Immediate and delayed recall was measured on the logical memory subtest of the Wechsler Memory Scale — Revised (WMS-R), and on the California Verbal Learning Test (CVLT). Resting rCBF (ml/100 g/min) was calculated for four frontal, four temporal, and four limbic regions of interest (ROIs). Women had better immediate recall on both WMS-R and CVLT tasks. Sex differences in rCBF were found for temporal lobe regions. Women had greater bilateral blood flow in a mid-temporal brain region. There were also sex differences in rCBF correlations with performance. Women produced positive correlations with rCBF laterality in the temporal pole. Greater relative CBF in the left temporal pole was associated with better WMS-R immediate and delayed recall in women only. These results suggest that trait differences in temporal pole brain-behavior relationships may relate to sex differences in verbal episodic memory.
Keywords: Sex differences, episodic memory, cerebral blood flow, positron emission tomography, functional neuroimaging
1. Introduction
A consistent finding in the neuropsychological literature is that women are better on some verbal, and men are better on some spatial tasks [27,32]. In addition, women tend to perform better on verbal learning and recall tasks including the logical memory subtest of the Wechsler Memory Scale [24], and list learning on the California Verbal Learning Test [29], and the Rey Auditory Verbal Learning Test [5]. On list learning tasks women utilize better learning strategies, resulting in greater information retrieval across learning and delay trials [29]. This female superiority has been documented in children [30], and appears to increase with age [5]. The purpose of this study is to determine whether sex differences in verbal episodic memory are accompanied by different patterns of correlations between memory and resting regional cerebral blood flow (rCBF) in frontal, temporal, and limbic brain regions.
Most functional imaging studies employ activation paradigms in which a cognitive function is “mapped” by subtracting rCBF between baseline and task conditions (e.g., [42]). These activation studies have contributed to our understanding of brain-behavior relationships. However, resting rCBF also provides a valuable measure of how well brain regions are perfused with blood. Better cerebral perfusion translates to greater availability of oxygen and nutrients which, in turn, facilitates neural response to cognitive demands. Although a resting baseline condition is relatively unstructured, it yields reproducible data [63], that correlate with neuropsychological performance [21]. Whereas rCBF activation studies measure a state-dependent phenomenon, resting rCBF may be sensitive to trait variables such as sex differences [19]. With a few exceptions [34,35], investigators have documented higher rates of resting global CBF in women, using both 133Xenon-clearance [19,33,48], and positron emission tomography (PET) 15O-water methods [3,45]. A tendency for women to show less strongly lateralized rCBF in frontal lobe regions has also been documented [13,48].
Although sex differences in verbal memory and resting cerebral perfusion have been found, there have been few attempts to relate behavioral to physiologic data in healthy subjects. In a PET rCBF study of normal aging, Eustache and colleagues [14] found positive correlations between verbal paired associate learning and left hippocampal and thalamic resting blood flow. However, they did not examine the effect of gender. In a PET glucose metabolism study of patients with schizophrenia, Mozley et al. [38] found that increased left hemisphere metabolism in inferior frontal, inferior temporal, mid-temporal, and superior temporal regions in patients with schizophrenia predicted worse logical memory recall on the Wechsler Memory Scale. They did not study healthy participants or examine sex differences. The remaining studies that relate resting rCBF with neuropsychological performance have done so primarily for neurological populations including stroke [57], and progressive dementia [9,18], and have not investigated sex effects. Thus, there is preliminary evidence of positive relationships between resting pre-frontal, temporal, and limbic rCBF and verbal memory that may be reversed for glucose metabolism in disease states such as schizophrenia. However, these relationships have not been examined separately for healthy men and women.
The pre-frontal, temporal, and limbic regions of interest (ROIs) examined in the current study comprise components of an integrated fronto-temporal network [16,17] associated with working and episodic memory. This network has been shown to mediate perceptual and mnemonic processing of object identity in primates [12,36,67], and has been implicated in PET activation studies of working memory (e.g., [49,53]), and episodic memory (e.g., [1,40,45,61]). This network will be examined for sex differences in rCBF, and sex differences in rCBF correlations with verbal memory performance.
2. Method
2.1. Participants
The sample was obtained from a previous PET study [45] of 30 healthy participants (16 men, 14 women; 23 Caucasian, six African-American, and one Asian-American) classified as right-handed based on a standard behavioral and self-report inventory [44]. The previous study focused on activation effects rather than on resting rCBF, and did not examine sex differences or relationships with neuropsychological performance. For the current study, two male participants were eliminated because they did not have complete verbal memory data.
Participants were (mean ± SD) 26.0 ± 6.1 years old (range 18–43 yrs), with 15.3 ± 2 years of education. There was no difference in education between men (15.6 ± 2.0 yrs) and women (14.9 ± 1.9 yrs). However, males (mean age = 28.8 ± 6.5; range 22–42 yrs) were older than females (mean = 23.3 ± 4.5, range 18–33 yrs; t = 2.6, p = 0.02). Intellectual ability, estimated with the Vocabulary and Block Design Subtests of the Wechsler Adult Intelligence Scale — Revised (WAIS-R, [64]), was in the Average to High Average range for men and women (Vocabulary scaled score = 11.6 ± 8.0 vs 12.9 ± 10.0; Block Design = 13.2 ± 5.0 vs 12.6 ± 9.0). There were no sex differences, or any interactions with sex when these WAIS-R scores were examined using a multivariate analysis of variance (MANOVA; Proc GLM, general linear procedure [51]).
Participants responded to newspaper and community advertisements for the University of Pennsylvania Mental Health Clinical Research Center (MHCRC), and underwent a comprehensive evaluation [52] including medical, neurologic and structured psychiatric examinations [55], and laboratory testing. Participants were free of any present or past disorder or injury that might affect brain function, including substance abuse. Informed consent was obtained prior to participation in the study.
2.2. Neuropsychological assessment
A comprehensive neuropsychological battery was administered by trained examiners following standard test administration procedures [8,50]. The neuropsychological evaluation occurred an average of 15.9 days after the PET study (range 1–91 days). A second examiner independently re-scored neuropsychological data to eliminate errors. Raw test scores were standardized (z-scores) with a mean of zero, and a standard deviation (SD) of one, and then grouped into eight summary measures by averaging each subject’s z-scores on tests assessing the same functional domain [8,50]. For the purpose of this study, only test variables comprising the verbal memory (VMEM) function were examined. The four variables were immediate (LOGM_I) and delayed (LOGM_D) logical memory recall on the Wechsler Memory Scale — Revised (WMS-R, [65]), total immediate recall of trials 1–5 (MONTOT) of the California Verbal Learning Test (CVLT [11]), and long delay (LD) recall on the CVLT.
2.3. PET procedures
For detailed information on imaging procedures please refer to Ragland et al., [45]. After placement of arterial and venous catheters, participants were positioned in a volume imaging PET camera (UGM Medical Systems, Philadelphia; [26]), in a supine position. After positioning, and after starting the 15O-water infusion, four 10 minute CBF determinations were made in counterbalanced order (Latin Square Design): resting baseline (eyes open, ears unoccluded; [19]), number matching, paired associate recognition (PART; [46]) and wisconsin card sorting (WCST; [23]). Infusion of 15O-water and simultaneous start of the first task began at time zero followed by four 10 min scans. The first scan started 8 min after infusion and subsequent scans were separated by 6 min to allow for re-equilibration [25]. For the current study only data from the resting baseline were utilized.
Radioactivity was localized by co-registering PET scans with magnetic resonance images (MRI) acquired using a standard MHCRC protocol [62]. For each subject a set of templates with multiple regions of interest (ROIs) were custom fit to each MRI slice by operators trained to an inter-rater reliability criterion of >0.85 [47]. ROI templates were developed on a digitized MRI image of the original Talairach brain using the Talairach Atlas [59] to determine standard anatomical boundaries. The ROI placement procedure was originally described in Resnick et al. [47], and has been utilized in subsequent publications (e.g., [20,38,45]). Each ROI was slightly smaller than the actual structure being sampled in order to minimize resolution induced problems with ill defined edges. To further reduce effects of volume averaging, regions were not placed at the extreme axial ends of a structure. The end result was that ROIs represented the central volume of each structure. The only exceptions were whole brain boundaries. These were placed to insure that the MRI templates fitted well on the PET scans when the edge of the functional images were determined by displaying the PET scans in a dichotomous scale that only gave color to a pixel if it contained 50% or more of the mean maximum count density in that image slice. After the MRIs were co-registered with the PET scans, the MRI-fitted ROIs were applied to all PET scans of each individual across conditions with global adjustment of whole brain boundaries. This adjustment of whole brain boundaries was performed while holding the relative position of individual ROIs constant such that the atlas of regions was adjusted as a whole [22].
Absolute CBF (in ml/100 g/min) was calculated using the equilibrium infusion technique [15,31,54]. rCBF for each region in each hemisphere was calculated by volume averaging over all slices in which that region could be identified. Of 36 available ROIs, four pre-frontal, four temporal, and four limbic regions were chosen. Placement of ROIs is illustrated in Fig. 1. These 12 ROIs were chosen a priori based on a previous review of the literature, and on PET activation data indicating that they comprise a fronto-temporal network sensitive to working and episodic memory task demands (see [45]). By reducing the number of ROIs studied, the increased chance of Type I error due to multiple comparisons is contained. Left and right hemisphere rCBF values for the 12 regions are presented for men and women in Table 1.
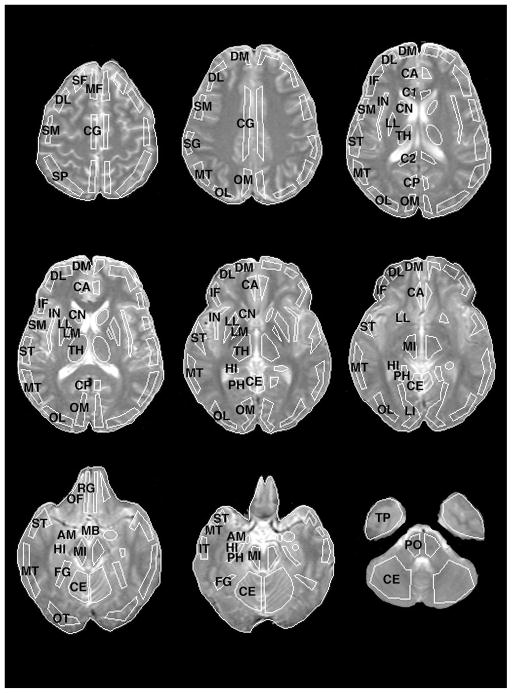
Fig. 1.
Placement of representative Regions of Interest (ROIs) on MRI images. ROI labels abbreviated as follows: SF = superior frontal, DL = dorsolateral prefrontal, DM = dorsomedial prefrontal, MF = mid-frontal, IF = inferior frontal, SM = sensorimotor, SP = superior parietal, SG = supramarginal gyrus, OM = occipital-medial, OL = occipital-lateral, LI = lingual gyrus, FG = fusiform gyrus, OT = occipitotemporal, ST = superior temporal, MT = mid-temporal, IT = inferior temporal, TP = temporal pole, PH = parahippocampal gyrus, HI = hippocampus, AM = amygdala, IN = insula, OF = orbital frontal, RG = rectal gyrus, CA = cingulate gyrus-anterior, CG = cingulate gyrus, CP = cingulate gyrus-posterior, C1 = corpus callosum-anterior, C2 = corpus callosum-posterior, CN = caudate nucleus, LM = lenticular-medial [globus pallidus], LL = lenticula-lateral [putamen], MB = mammillary body, TH = thalamus, MI = midbrain, PO = pons, CE = cerebellum. Regions included in the analysis are in bold.
Table 1.
Left and Right Hemisphere rCBF (ml/100 g/min) for men and women during resting baseline. rCBF_Bilat can be calculated by averaging left and right regional values. rCBF_Lat can be calculated by subtracting right from left regional values, dividing this di3erence by the average of left and right values, and multiplying by 100. rCBF = regional cerebral blood flow; Pre-frontal regions include SF = superior frontal, DL = dorsolateral prefrontal, DM = dorsome-dial prefrontal, and IF = inferior frontal; Temporal Lobe regions include OT = occipitotemporal, MT = middle temporal, IT = inferior temporal, and TP = temporal pole; Limbic regions include PH = parahippocampal gyrus, HI = hippocampus, AM = amygdala, and OF = orbital frontal
| Region | Male Left
|
Female Left
|
Male Right
|
Female Right
|
||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | |
| Pre-frontal | ||||||||
| SF | 30.6 | 2.6 | 30.8 | 1.4 | 26.1 | 1.8 | 26.4 | 1.3 |
| DL | 34.9 | 2.3 | 33.8 | 1.5 | 27.3 | 1.7 | 27.2 | 1.0 |
| DM | 32.9 | 2.3 | 32.1 | 1.7 | 28.7 | 1.8 | 28.5 | 1.1 |
| IF | 35.6 | 2.4 | 37.2 | 2.0 | 28.2 | 1.6 | 29.4 | 1.2 |
| Temporal | ||||||||
| OT | 30.7 | 2.6 | 32.2 | 2.0 | 30.3 | 2.6 | 29.4 | 1.6 |
| MT | 28.4 | 1.7 | 33.9 | 1.4 | 24.7 | 1.5 | 28.8 | 1.2 |
| IT | 30.3 | 2.5 | 36.2 | 1.9 | 25.2 | 1.7 | 27.9 | 1.3 |
| TP | 26.5 | 2.2 | 30.7 | 1.7 | 24.7 | 1.9 | 28.0 | 1.5 |
| Limbic | ||||||||
| PH | 40.0 | 2.9 | 42.3 | 2.8 | 38.5 | 2.9 | 41.2 | 2.0 |
| HI | 41.6 | 2.7 | 42.8 | 2.6 | 35.4 | 1.9 | 41.8 | 2.5 |
| AM | 46.1 | 3.7 | 49.6 | 4.2 | 45.6 | 3.6 | 48.9 | 2.8 |
| OF | 39.9 | 3.4 | 38.9 | 2.7 | 31.8 | 2.1 | 31.8 | 1.8 |
Anatomical boundaries for the 12 ROIs were as follows: The region for the superior frontal (SF) gyrus was taken from its medial aspect, and extended to its most lateral aspect. The dorsolateral prefrontal cortex (DL) was defined as the lateral most region of the frontal lobe beginning 4 mm above the uppermost aspect of the body of the caudate extending from the middle frontal gyrus posteriorly to precentral sulcus. The dorsomedial frontal lobe region (DM) included portions of the superior and middle frontal gyri as they dived side by side in the axial direction down the anterior most and medial most aspect of the frontal pole. The inferior frontal (IF) region corresponded to the lateral aspect of the IF gyrus from its most superior to most inferior extent. The region for the occipitotemporal (OT) cortex corresponded to the lateral visual association cortex. A mid-temporal region (MT) was centered over the fusiform gyrus, which excluded the inferior most aspects of hippocampus and amygdala medially. The inferior temporal (IT) region included the posterior aspect of the IT gyrus. It was placed below the level at which the sylvian fissure first became visible, and ended above the petrous ridge. The temporal pole (TP) region included the contents of the middle temporal fossa below the petrous ridge. The parahippocampal gyrus (PH) was represented by a thin strip just medial to the hippocampus on the external surface of the medial temporal lobe. The region for the hippocampus (HI) extended posteriorly from the rami of the posterior ventricles anteriorly to the anteromedial most walls of the temporal horns. In the plane, the amygdala (AM) was defined as that region of the temporal lobe anterior to the anterior wall of the temporal horns of the lateral ventricle, dorsal to the middle cerebral artery, and just lateral to the uncus. Axially, the amygdala was defined on cuts below the anterior commissure and white matter tracts running ventrally to the globus pallidus, and above the petrous ridge of the middle temporal fossa. The region for the orbital frontal (OF) cortex was placed adjacent to the sulcus separating the infra-orbital from the rectal gyrus, and continued to the anterior aspect of the OF gyrus. The region was placed when the rectal gyrus first became visible, and continued to the base of the brain. Two rCBF indices were calculated for each ROI: rCBF_Bilat was calculated by averaging across hemispheres for each region; rCBF_Lat was calculated by subtracting right from left hemisphere rCBF, dividing this difference by the average of left and right hemispheres, and multiplying by 100. Dividing by the average of both hemispheres accounts for initial values, thereby reducing possible regression to the mean effects.
2.4. Data analysis
Statistical analysis proceeded in three stages. (1) Sex differences in verbal memory were examined by entering the four memory variables into a two (male, female), by two (WMS-R, CVLT), by two (immediate, delay) MANOVA with repeated measures for the second two factors. Post-hoc analysis of variance (ANOVA) was used to decompose any significant interactions. Because men were older than women, a multivariate analysis of covariance (MANCOVA) was also performed with age as a co-variate. (2) Sex differences in rCBF were examined by entering the hemispheric rCBF data in Table 1 into a two (sex), by two (hemisphere), by three (lobe), by four (regions of interest within each lobe) MANOVA with repeated-measures for the last three factors. This overall test was also performed as a MANCOVA with age co-varied. Post-hoc multivariate analyses were used to decompose any significant interactions. Results of these posthoc analyses were used to guide subsequent t-test and correlational analyses of regional differences. A significant two-way or higher order interaction between hemisphere and sex was required for analysis of both rCBF_Lat and rCBF_Bilat indices. If no interaction with hemisphere was found, only rCBF_Bilat was examined. Likewise, within a given lobe, a main effect of, or interaction with sex was required for subsequent analysis of regional effects. These rCBF differences between men and women were assessed by entering appropriate rCBF indices into between-group t-tests. The significance criterion for all statistical analyses in stages 1 and 2 was set at an alpha level of 0.05, two-tailed. (3) Sex differences in brain-behavior relationships between resting rCBF and memory were tested by calculating Pearson correlation coefficients between the four memory variables and rCBF indices separately for men and women. Correlational analyses were restricted to lobar regions that had shown significant sex effects in the previous MANOVA analyses. These correlations were tested for sex differences after normalization using a Fisher z-transformation. To further reduce the likelihood of Type 1 error a Bonferroni correction was performed by dividing an uncorrected p-value of 0.05 by the number of regions within each lobe. Thus, a corrected alpha level of 0.0125, two tailed, was used to establish significance for the Fisher z test analysis.
3. Results
3.1. Verbal memory function
Male and female immediate and delayed recall of WMS-R stories and CVLT word lists is illustrated in Fig. 2. A multivariate analysis revealed significant interactions between sex and delay, F(1,26) = 5.6, p < 0.05, and between sex, delay and task, F(1,26) = 5.4, p < 0.05. There was no effect of age when it was added as a covariate. The interactions between sex and delay, F(1,25) = 13.4, p < 0.001, and between sex and delay and task, F(1,25) = 9.7, p < 0.005 became stronger when age was co-varied.
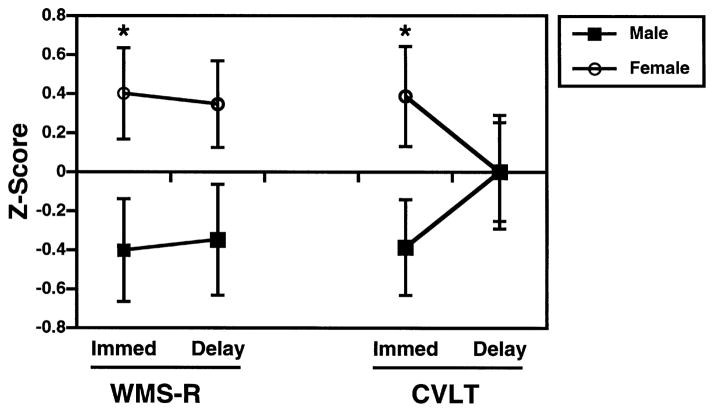
Fig. 2.
Mean (±SEM) Immediate and Delayed Wechsler Memory Scale-Revised Logical Memory (WMS-R) and California Verbal Learning Test (CVLT) Performance for men and women. Raw variables have been transformed into z-scores (mean = 0, SD = 1) to provide a common metric. *p<0.05, two-tailed.
As can be seen in Fig. 2, females had superior immediate recall on both tasks, F(1,26) = 6.1, p < 0.05. Although there was a trend for women to have better delayed recall on the WMS-R, t(26) = −1.9, p = 0.06, there was no difference between male and female delayed recall on the CVLT. An examination of CVLT individual learning trials (Fig. 3), illustrates that the lack of sex differences at long delay were because both groups had learned most words by trial 5, and showed little forgetting between trial 5 and the long delay (%savings: male = 97.3 ± 8.3, female = 94.3 ± 8.8). Both groups also forgot little of the WMS-R stories (%savings: male = 89.3 ± 11.4, female = 89.3 ± 7.6). As in previous studies [29], there was a trend for women to do a better job at semantic organization, which facilitated encoding and retrieval on the CVLT (semantic cluster ratio: male = 2.3 ± 0.8, female = 2.9 ± 0.8; t = −1.8, p = 0.08).
Fig. 3.
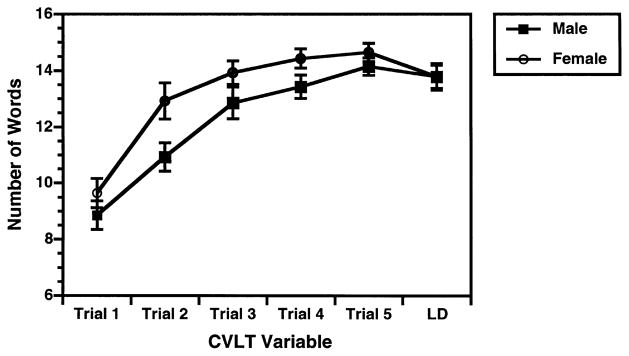
Mean (±SEM) CVLT Recall on Learning Trials 1–5 and Long Delay Trial for men and women.
3.2. Resting rCBF
The MANOVA analysis of rCBF values in Table 1 revealed main effects of hemisphere, F(1,24) = 6.8, p < 0.05; and lobe, F(2,23) = 12.2, p = 0.0005. As can be appreciated from Table 1, there was higher left hemisphere rCBF across groups and regions. The lobe effect was due to higher rCBF for regions located in limbic than in temporal, F(1,24) = 128.4, p = 0.0001, or frontal lobes, F(1,24) = 76.6, p = 0.0001; and greater rCBF in frontal than in temporal lobe structures, F(1,24) = 8.6, p<0.05.
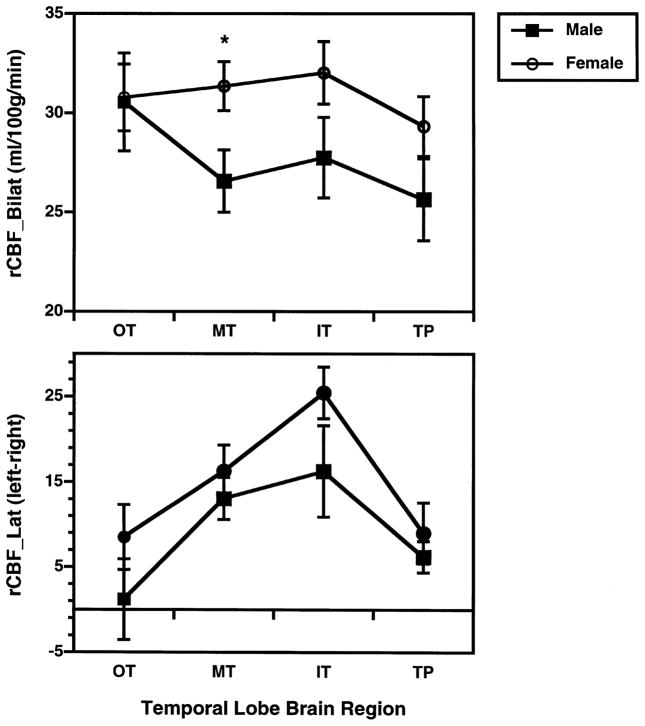
Although there was no main effect of sex, there was a significant interaction between sex and lobe, F(2,23) = 3.7, p < 0.05. There was also a hemisphere by lobe by sex interaction, F(2,23) = 4.1, p < 0.05. When age was co-varied, the three-way interaction between sex, lobe, and hemisphere remained significant, F(2,22) = 4.4, p < 0.05. However, the interaction between sex and lobe was no longer significant. To further examine these interactions, post-hoc MANOVAs were performed separately for each lobe. Post-hoc analysis of frontal and limbic areas did not reveal any sex differences or any interactions with sex. However, the analysis of temporal lobe regions revealed a significant sex by hemisphere interaction, F(1,26) = 4.3, p < 0.05. As can be seen in Table 1, this interaction was due to sex differences being relatively greater in the left hemisphere across the four brain regions. A final set of post-hoc MANOVAs were performed separately for each of the four temporal lobe ROIs. These analyses revealed a main effect of sex for the mid-temporal region only, F(1,26) = 5.73, p < 0.05. There were no significant sex by hemisphere interactions for this region. Women had higher rCBF in both left, t = −2.49, p<0.05, and right hemispheres, t = −2.11, p < 0.05. The top graph of Fig. 4 illustrates the greater bilateral mid-temporal rCBF in women. As can be seen in the bottom graph of Fig. 4, blood flow in temporal lobe regions was lateralized to the left hemisphere for both men and women, with no evidence of sex differences in rCBF_LAT.
Fig. 4.
Mean (±SEM) Bilateral rCBF (average of left and right hemisphere; top graph) and rCBF Laterality (100*left-right/left+right; bottom graph), for men and women in four pre-frontal, four temporal and four limbic regions. Regions abbreviated as in Fig. 1. *p< 0.05, two-tailed.
3.3. Brain-behavior correlations
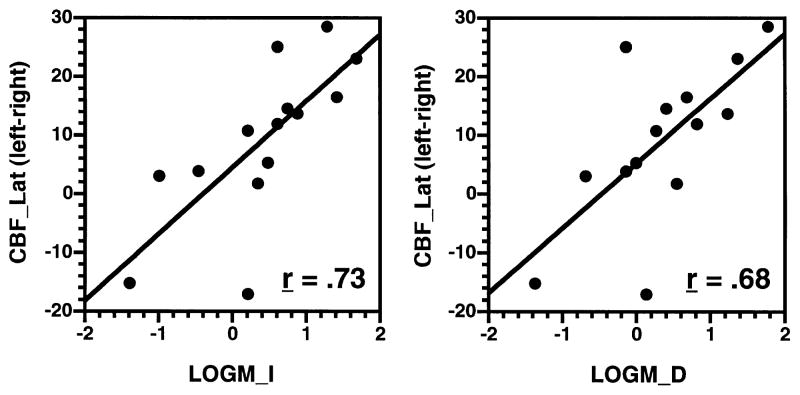
Correlational analyses were restricted to temporal lobe regions since they showed sex effects in previous multivariate analyses. Pearson correlation coefficients were calculated for rCBF_Bilat and rCBF_LAT with LOGM_I, LOGM_D, MONTOT, and LD for men and women. Fisher z analysis of these correlations did not reveal any sex differences in correlations with rCBF_Bilat for any test variable. However, the pattern of correlations between WMS-R story recall and rCBF_Lat differed between men and women for the temporal pole region (Table 2). More left lateralized CBF in the temporal pole was associated with better immediate (r = 0.73, p<0.005) and delayed (r = 0.68, p<0.05) logical memory recall in women only. Correlations in men were not significantly different than zero for any test variable. There were no significant correlations with CVLT performance for either group.
Table 2.
Fisher z comparison of male and female correlations between immediate (LOGM_I) and delayed (LOGM_D) logical memory and CBF laterality in temporal lobe regions. zr = Fisher z-transformation of r-value. Values in bold exceed Bonferroni corrected significance criterion of 0.0125
| Male (zr) | Female (zr) | z-score | p-value | |
|---|---|---|---|---|
| LOGM_I | ||||
| OT | −0.35 | 0.37 | −1.70 | 0.09 |
| MT | 0.14 | 0.78 | −1.49 | 0.14 |
| IT | −0.23 | 0.40 | −1.47 | 0.14 |
| TP | −0.18 | 0.94 | −2.64 | 0.008 |
| LOGM_D | ||||
| OT | −0.37 | 0.45 | −1.92 | 0.05 |
| MT | 0.06 | 0.73 | −1.58 | 0.11 |
| IT | −0.28 | 0.40 | −1.60 | 0.11 |
| TP | −0.20 | 0.83 | −2.42 | 0.01 |
Fig. 5 presents scatter plots of the correlations between rCBF_LAT in the temporal pole and LOGM_I and LOGM_D performance for females. These plots illustrate the consistency of the relationship across subjects, with the possible exception of two females who had negative laterality scores. However, if either or both of these subjects are removed, the overall correlation remains significant for both immediate (range r = 0.62–0.84) and delayed recall (range r = 0.53–0.77) conditions.
Fig. 5.
Scatter plots of significant correlations between WMS-R Logical Memory (x-axis) Immediate (LOGM_I, left graph) and Delayed Recall (LOGM_D, right graph) with rCBF Laterality (y-axis) for female subjects. Regions abbreviated as in Fig. 1.
4. Discussion
Women had better verbal memory than men. Better performance was characterized by higher rates of immediate acquisition and recall of WMS-R stories and CVLT word lists. Women also differed from men in their pattern of correlations between WMS-R performance and temporal lobe rCBF. Men had no significant correlations between rCBF and performance. In women, more left-lateralized blood flow in the temporal pole was associated with higher rates of immediate and delayed recall of WMS-R stories. Thus, appropriately lateralized rCBF in the temporal pole appeared to be more tightly coupled with episodic memory for prose passages in women than in men.
The female superiority in verbal memory was most striking for immediate stages of learning and recall. This result replicates the findings of Kramer and colleagues [29] who identified impaired learning rates in men, and high retention rates in both groups. However, in the Kramer et al. study men were impaired on all learning trials and at short and long delays. In the current study men equalled female performance by trial 5 and were not impaired at short and long delays. This difference in trial 5 and delayed recall performance is probably due to sample fluctuations, and due to the smaller size of the current sample which had less power to detect group differences. This explanation is supported by a post-hoc examination of a larger MHCRC normative sample (138 women, 143 men) which found lower male performance on all five learning trials and at both delay periods. As in the previous study, women did a better job of semantically organizing CVLT items. Both men and women showed little forgetting over delay. Thus, the overall pattern of test results characterize men as less efficient at encoding and retrieving information, but no different than women in their ability to maintain information over time.
Since it has been well documented that memory declines with age [28], a potential concern was that group differences might have been due to men being an average of 5 years older. However, when age effects were statistically controlled, the size of the sex differences in memory increased rather than decreased. This is consistent with previous findings that the female superiority in verbal memory increases with age [5]. Therefore, having slightly older males than females in the current sample more likely reduced than inflated sex differences.
The correlational finding that performance bears a closer relationship to rCBF laterality than to magnitude of bilateral flow has been found in previous activation studies [20,21]. This result suggests that although a sufficient level of blood flow in appropriate brain regions is probably necessary for task performance, better performance requires a gradiential shift of rCBF to the appropriate hemisphere for those same regions.
The region that showed the strongest correlation with memory performance in women was the temporal pole. The temporal pole has been characterized as a higher-order multimodal association cortex that has reciprocal connections with auditory, visual, olfactory and multimodal association cortices [2]. Atrophy of this region has been associated with both Pick’s and Alzheimer’s dementia [2], and left sided lesions have been linked to impaired recall of proper names [10]. As a higher order association area, it has been considered to have a role in semantic memory characterized as, “… retrieval of the multidimensional aspects of knowledge which are necessary and sufficient for the mental representation of a concept of a given entity”. ([60], p. 1324). The temporal pole and associated network is viewed as having an intermediary rather than explicit role in retrieving a concept. Therefore, the role of the temporal pole in episodic memory may best be viewed as providing knowledge about objects and their relations which allows a meaningful mental representation that can be more efficiently stored and retrieved by episodic memory networks in prefrontal and mesial temporal brain regions.
The finding that performance correlations in the temporal pole were specific to WMS-R story recall in women can also be understood within this framework. Successful storage of WMS-R stories requires greater knowledge of objects and their relationships than does storage of CVLT words which are not presented in a semantically related context. Correlations may, therefore, have been specific to the WMS-R because retrieval of story information was dependent upon having a gestalt-like mental representation of story concepts. The finding that temporal pole correlations were significant only for women supports the conclusion that women are more adept than men at forming these mental representations. It is potentially misleading to draw causal inferences from correlational findings, and these conclusions should be subjected to hypothesis testing in future studies.
Correlations with performance were not found for pre-frontal or hippocampal regions for either group. The lack of frontal lobe findings is somewhat surprising given growing evidence that, although frontal lobe lesions might not produce amnesia [58], they can disrupt free and cued recall on episodic memory tasks [66]. Many functional imaging studies have found evidence of prefrontal activation during encoding and retrieval stages of episodic memory tasks (see [40] for review). However, there is a growing debate [7,37,41,43] about whether prefrontal activation primarily reflects retrieval effort (e.g., executive functions such as organization and integration of contextual cues), or retrieval success (i.e., actual recovery of stored information). Thus, the lack of correlations between resting rCBF in prefrontal regions and memory performance in the current study can have several interpretations. When rCBF was contrasted between resting baseline and paired associate recognition for this same sample in a PET activation study [45], there was evidence of both pre-frontal and temporal lobe activation. However, only rCBF change in temporal lobe regions correlated with task performance. This suggested that prefrontal activity was primarily related to retrieval effort rather than retrieval success. Given the rich reciprocal inter-connections between prefrontal and temporal-limbic regions [17], parsing out executive vs mnemonic components of memory task performance in healthy individuals remains a supreme challenge.
There were also no sex differences in correlations between performance and rCBF in the hippocampus. This is also somewhat surprising given focal lesion evidence that has unequivocally linked the hippocampus and underlying rhinal cortex with information storage and consolidation in primates [39,56]. However, sex differences in memory performance in the current study were most striking for initial encoding and retrieval. Both groups did a good job of maintaining information over delays, suggesting that information storage functions mediated by the hippocampus were similar between men and women. The absence of sex differences in correlations with hippocampal function parallel the results from a study of men and women undergoing left temporal lobectomy for intractable epilepsy [4]. In that study, investigators hypothesized that if better female CVLT performance was related to hippocampal function, then women would show less of a performance advantage after surgery. However, women performed better than men on the CVLT both before and after surgery, leading the investigators to conclude that CVLT sex differences were unrelated to group differences in hippocampal function. Thus, current results and previous findings support the conclusion that human sex differences in episodic memory are related to structures other than the hippocampal formation.
The lack of hippocampal results in the present study mirror the difficulties that cognitive activation studies have had in finding evidence of hippocampal activation in response to episodic memory tasks. There have been several explanations for this discrepancy. One attractive explanation may be that the process of consolidation and storage carried out by the hippocampus occurs gradually and over a relatively long time span that cannot be captured by functional imaging subtraction paradigms that examine acute changes over short time periods [6]. As pointed out by Wheeler and colleagues [66], a one-to-one correspondence between a lesion and a regional activation should also not always be expected. This is because interruption in any part of a broadly distributed network can often produce functional impairment.
There are several limitations that deserve mention. Although all participants were healthy young adults and had equivalent levels of education, men and women were not matched by age which, therefore, had to be statistically controlled. Because of the relatively small sample size it was decided to focus on only four memory variables, and only 12 brain regions during a resting state. With a sufficient sample size it would have been possible to examine other CVLT variables (e.g., learning slope, semantic and serial clustering, recognition) to explore why correlations with rCBF were not obtained for the CVLT. It would also be worth-while to include additional regions that have been associated with memory including the cerebellum, thalamus, and cingulate cortex. An investigation of sex differences in performance correlations with rCBF activation during a memory task would also be valuable in determining if current results extend to dynamic brain-behavior functions. Replicating the current results in a larger sample is necessary given potential pitfalls in interpreting brain-behavior interactions. Examining the relationship between rCBF and performance for cognitive tasks that males perform better such as spatial ability and fine motor speed will also be useful for establishing convergent results.
Acknowledgments
This research was supported by the EJLB Foundation, and by National Institutes of Health grants MH-19112, MH-00586, MH-48539, and MH-43880. We thank Abass Alavi, Robin Smith and the staff of the PET center for assistance in data collection; P. David Mozley for help with description of brain regions; and Warren Bilker for statistical consultation.
References
- 1.Andreasen NC, O’Leary DS, Arndt S, Cizadlo T, Hurtig R, Rezai K, Watkins GL, Ponto LLB, Hichwa RD. Short-term and long-term verbal memory: a positron emission tomography study. Proc Natl Acad Sci. 1995;92:5111–5. doi: 10.1073/pnas.92.11.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold SE, Hyman BT, Van Hoesen GW. Neuropathologic changes of the temporal pole in Alzheimer’s disease and Pick’s disease. Arch Neurol. 1994;51:145–50. doi: 10.1001/archneur.1994.00540140051014. [DOI] [PubMed] [Google Scholar]
- 3.Baxter LR, Mazziotta JC, Phelps ME, Selin CE, Guze BH, Fairbanks L. Cerebral glucose metabolic rates in normal human females versus normal males. Psychiatry Res. 1987;21:237–45. doi: 10.1016/0165-1781(87)90028-x. [DOI] [PubMed] [Google Scholar]
- 4.Berenbaum SA, Baxter L, Seidenberg M, Herman B. Role of the hippocampus in sex differences in verbal memory: Memory outcome following left anterior temporal lobectomy. Neuropsychology. 1997;11:585–91. doi: 10.1037//0894-4105.11.4.585. [DOI] [PubMed] [Google Scholar]
- 5.Bleecker ML, Bolla-Wilson K, Agnew J, Meyers DA. Age-related sex differences in verbal memory. J Clin Psychology. 1988;44:403–11. doi: 10.1002/1097-4679(198805)44:3<403::aid-jclp2270440315>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Buckner RL, Koutstaal W. Functional neuroimaging studies of encoding, priming, and explicit memory retrieval. Proc Natl Acad Sci. 1998;95:891–8. doi: 10.1073/pnas.95.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckner RL, Raichle ME, Miezin FM, Petersen SE. Functional anatomic studies of memory retrieval for auditory words and visual pictures. J Neurosci. 1996;16:6219–35. doi: 10.1523/JNEUROSCI.16-19-06219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Censits DM, Ragland JD, Gur RC, Gur RE. Neuropsychological evidence supporting a neurodevelopmental model of schizophrenia: a longitudinal study. Schiz Res. 1997;24:289–98. doi: 10.1016/s0920-9964(96)00091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen RM, Andreason PJ, Sunderland T. The ratio of mesial to neocortical temporal lobe blood flow as a predictor of dementia. J Am Geriatrics Soc. 1997;45:329–33. doi: 10.1111/j.1532-5415.1997.tb00948.x. [DOI] [PubMed] [Google Scholar]
- 10.Damiasio AR, Brandt JP, Tranel D, Damasio H. Name dropping: retrieval of proper or common nouns depends upon different systems in left temporal cortex. Soc Neurosci. 1991;17:4. [Google Scholar]
- 11.Delis DC, Kramer JH, Kaplan E, Ober BA. The California verbal learning test. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 12.Desimone R, Ungerleider LG. In: Handbook of neuropsychology. Boller F, Grafman J, editors. Amsterdam: Elsevier; 1990. pp. 267–99. [Google Scholar]
- 13.Esposito G, Vanhorn JD, Weinberger DR, Berman KF. Gender differences in cerebral blood flow as a function of cognitive state with PET. J Nuclear Med. 1995;37:559–64. [PubMed] [Google Scholar]
- 14.Eustache F, Rious P, Desgranges B, Marchal G, Petit-Taboue MC, Dary M, Lechevalier B, Baron JC. Healthy aging, memory subsystems and regional cerebral oxygen consumption. Neuropsychologia. 1995;33:867–87. doi: 10.1016/0028-3932(95)00021-t. [DOI] [PubMed] [Google Scholar]
- 15.Frackowiak RSJ, Lenzi GL, Jones T, Heather JD. Quantitative measurement of regional cerebral blood flow and oxygen metabolism in man using 15O and positron emission tomography: theory, procedure and normal values. J Comp Assisted Tomog. 1980;4:727–36. doi: 10.1097/00004728-198012000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Goldman-Rakic PS. Topography of cognition: parallel distributed networks in primate association cortex. Ann Rev Neurosci. 1988;11:137–56. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- 17.Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neurosci. 1984;12:719–43. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- 18.Greene JD, Miles K, Hodges JR. Neuropsychology of memory and SPECT in the diagnosis and staging of dementia of Alzheimer type. J Neurol. 1996;243:175–90. doi: 10.1007/BF02444012. [DOI] [PubMed] [Google Scholar]
- 19.Gur RC, Gur RE, Obrist WD, Hungerbuhler JP, Yoynkin D, Rosen AD, Skolnick BE, Reivich M. Sex and handedness differences in cerebral blood flow during rest and cognitive activity. Science. 1982;217:659–61. doi: 10.1126/science.7089587. [DOI] [PubMed] [Google Scholar]
- 20.Gur RC, Ragland JD, Mozley LH, Mozley PD, Smith R, Alavi A, Bilker W, Gur RE. Lateralized changes in regional cerebral blood flow during performance of verbal and facial recognition tasks: Correlations with performance and “effort”. Brain and Cog. 1997;33:388–414. doi: 10.1006/brcg.1997.0921. [DOI] [PubMed] [Google Scholar]
- 21.Gur RC, Ragland JD, Resnick SM, Skolnick BE, Jaggi J, Muenz L, Gur RE. Lateralized increases in cerebral blood flow during performance of verbal and spatial tasks: relationship with performance level. Brain and Cog. 1994;24:244–58. doi: 10.1006/brcg.1994.1013. [DOI] [PubMed] [Google Scholar]
- 22.Gur RE, Resnick SM, Alavi A, Gur RC, Caroff S, Dann R, Silver F, Saykin AJ, Chawluk JB, Kushner M, Reivich M. Regional brain function in schizophrenia: I. A positron emission tomography study. Arch Gen Psychiatry. 1987;44:119–25. doi: 10.1001/archpsyc.1987.01800140021003. [DOI] [PubMed] [Google Scholar]
- 23.Heaton RK. The Wisconsin card sorting test manual. Odessa, FL: Psychological Assessment Resources; 1981. [Google Scholar]
- 24.Ivison D. The Wechsler memory scale: preliminary findings toward an australian standardization. Australian Psychologist. 1977;12:303–12. [Google Scholar]
- 25.Jones SC, Greenberg JH, Dann R, Robinson GD, Jr, Kushner M, Alavi A, Reivich M. Cerebral blood flow with the continuous infusion of oxygen-15-labeled water. J Cereb Blood Flow and Metab. 1985;5:566–75. doi: 10.1038/jcbfm.1985.85. [DOI] [PubMed] [Google Scholar]
- 26.Karp JS, Kinahan PE, Muehllehner G. Effect of increased axial field of view on the performance of a volume PET scanner. IEEE Transact Med Imaging. 1993;12:299–306. doi: 10.1109/42.232259. [DOI] [PubMed] [Google Scholar]
- 27.Kimura D, Harshman RA. Sex differences in brain organization for verbal and non-verbal functions. Prog Brain Res. 1984;61:423–41. doi: 10.1016/S0079-6123(08)64452-0. [DOI] [PubMed] [Google Scholar]
- 28.Koss E, Haxby JV, DeCarli C, Schapiro MB, Friedland RP, Rapoport SI. Patterns of performance perseveration and loss in healthy aging. Dev Neuropsych. 1991;7:99–113. [Google Scholar]
- 29.Kramer JH, Delis DC, Daniel M. Sex differences in verbal learning. J Clin Psychol. 1988;44:907–15. [Google Scholar]
- 30.Kramer JH, Delis DC, Kaplan E, O’Donnell L, Prifitera A. Developmental sex differences in verbal learning. Neuropsychology. 1997;11:577–84. doi: 10.1037//0894-4105.11.4.577. [DOI] [PubMed] [Google Scholar]
- 31.Lammertsma AA, Frackowiak RSJ, Lenzi GL, Heather JD, Pozzilli C, Jones T. Accuracy of the oxygen-15 steady state technique for measuring rCBF and rCMR(O2): tracer modeling, statistics and spatial sampling. J Cereb Blood Flow Metab. 1981;1:S3–S4. [Google Scholar]
- 32.Maccoby EF, Jacklin CN. The psychology of sex differences. Stanford, CA: Stanford University Press; 1974. [Google Scholar]
- 33.Matthew RJ, Wilson WH, Tant SR. Determinants of resting regional cerebral blood flow in normal subjects. Biol Psychiat. 1986;21:907–14. doi: 10.1016/0006-3223(86)90264-7. [DOI] [PubMed] [Google Scholar]
- 34.Matsuda H, Maeda T, Yamada M, Gui LX, Hisada K. Age-matched normal values and topographic maps for regional cerebral blood flow measurements by 133Xe inhalation. Stroke. 1984;15:336–42. doi: 10.1161/01.str.15.2.336. [DOI] [PubMed] [Google Scholar]
- 35.Melamed E, Lavy S, Bentin S, Cooper G, Rinot Y. Reduction in regional cerebral blood flow during normal aging in man. Stroke. 1980;11:31–5. doi: 10.1161/01.str.11.1.31. [DOI] [PubMed] [Google Scholar]
- 36.Mishkin M, Ungerleider LG. Contribution of striate inputs to the visuospatial functions of parieto-preoccipital cortex in monkeys. Behav Brain Res. 1982;6:57–77. doi: 10.1016/0166-4328(82)90081-x. [DOI] [PubMed] [Google Scholar]
- 37.Moscovitch M. Memory and working-with-memory: a component process model based on modules and central systems. J Cog Neurosci. 1992;4:257–67. doi: 10.1162/jocn.1992.4.3.257. [DOI] [PubMed] [Google Scholar]
- 38.Mozley LH, Gur RC, Gur RE, Mozley PD, Alavi A. Relationships between verbal memory performance and the cerebral distribution of flurodeoxyglucose in patients with schizophrenia. Biolog Psychiat. 1996;40:443–51. doi: 10.1016/0006-3223(95)00421-1. [DOI] [PubMed] [Google Scholar]
- 39.Murray EA, Mishkin M. Visual recognition in monkeys following rhinal cortical ablations combined with either amygdalectomy or hippocampectomy. J Neurosci. 1986;6:1991–2003. doi: 10.1523/JNEUROSCI.06-07-01991.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nyberg L, Cabeza R, Tulving E. PET studies of encoding and retrieval: the HERA model. Psychonomic Bull Rev. 1996;2:134– 47. doi: 10.3758/BF03212412. [DOI] [PubMed] [Google Scholar]
- 41.Nyberg L, Tulving E, Habib R, Nilsson LG, Kapur S, Houle S, Cabeza R, McIntosh AR. Functional brain maps of retrieval mode and recovery of episodic information. 1995;7:249–252. [PubMed] [Google Scholar]
- 42.Posner MI, Petersen SE, Fox PT, Raichle ME. Localization of cognitive operations in the human brain. Science. 1988;240:1627–31. doi: 10.1126/science.3289116. [DOI] [PubMed] [Google Scholar]
- 43.Rugg MD, Fletcher PC, Frith CD, Frackowiak RSJ, Dolan RJ. Differential activation of the prefrontal cortex in successful and unsuccessful memory retrieval. Brain. 1996;119:2073–83. doi: 10.1093/brain/119.6.2073. [DOI] [PubMed] [Google Scholar]
- 44.Raczkowski D, Kalat J, Nebes R. Reliability and validity of some handedness questionnaire items. Neuropsychologia. 1974;12:43–8. doi: 10.1016/0028-3932(74)90025-6. [DOI] [PubMed] [Google Scholar]
- 45.Ragland JD, Glahn DC, Gur RC, Censits DM, Smith RJ, Mozley PD, Alavi A, Gur RE. PET regional cerebral blood flow change during working and declarative memory: relationship with task performance. Neuropsychology. 1997;11:222–31. doi: 10.1037//0894-4105.11.2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ragland JD, Gur RC, Deutsch GK, Censits DM, Gur RE. Reliability and construct validity of the paired-associate recognition test: A test of declarative memory using wisconsin card sorting stimuli. Psycholog Assess. 1995;7:25–32. [Google Scholar]
- 47.Resnick SM, Karp JS, Turetsky B, Gur RE. Comparison of anatomically-defined versus physiologically-based regional localization: effects on PET-FDG quantitation. J Nucl Med. 1994;34:2201–7. [PubMed] [Google Scholar]
- 48.Rodriguez G, Warkentin S, Risberg J, Rosadini G. Sex differences in regional cerebral blood flow. J Cereb Blood Flow Metab. 1988;8:783–9. doi: 10.1038/jcbfm.1988.133. [DOI] [PubMed] [Google Scholar]
- 49.Salmon E, Van der Linden M, Collette F, Delfiore G, Maquet P, Degueldre C, Luxen A, Franck G. Regional brain activity during working memory tasks. Brain. 1996;119:1617–25. doi: 10.1093/brain/119.5.1617. [DOI] [PubMed] [Google Scholar]
- 50.Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiat. 1994;51:124–31. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- 51.SAS Institute Inc SAS/STAT Software, Version 6.11. SAS Institute Inc; Cary, NC: 1996. [Google Scholar]
- 52.Shtasel DL, Gur RE, Mozley PD, Richards J, Taleff MM, Heimberg C, Gallacher F, Gur RC. Volunteers for biomedical research: recruitment and screening of normal controls. Arch Gen Psychiat. 1991;48:1022–5. doi: 10.1001/archpsyc.1991.01810350062010. [DOI] [PubMed] [Google Scholar]
- 53.Smith EE, Jonides J, Koeppe RA. Dissociating verbal and spatial working memory using PET. Cereb Cortex. 1996;6:11–20. doi: 10.1093/cercor/6.1.11. [DOI] [PubMed] [Google Scholar]
- 54.Smith RJ, Shao L, Freifelder R, Karp JS, Ragland JD. Quantitative measurement of cerebral blood flow in volume imaging PET scanners. IEEE Transact Nucl Sci. 1995;42:1018–23. [Google Scholar]
- 55.Spitzer RL, Williams JBW, Gibbon M, First MB. Structured clinical interview for DSM-III-R-non-patient version. New York: New York State Psychiatric Institute; 1989. (SCID-NP, 9/1/89) [Google Scholar]
- 56.Squire LR. Memory and the hippocampus: –a synthesis from findings with rats, monkeys, and humans. Psycholog Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 57.Starkstein SE, Sabe L, Vazquez S, Teson A, Petracca G, Chemerinski E, DiLorenzo G, Leiguarda R. Neuropsychological, psychiatric, and cerebral blood flow findings in vascular dementia and Alzheimer’s disease. Stroke. 1996;27:408–14. doi: 10.1161/01.str.27.3.408. [DOI] [PubMed] [Google Scholar]
- 58.Stuss DT, Benson DF. The frontal lobes. New York: Raven Press; 1997. pp. 180–93. [Google Scholar]
- 59.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- 60.Tranel D, Damasio H, Damasio AR. A neural basis for the retrieval of conceptual knowledge. Neuropsychologia. 1997;35:1319–27. doi: 10.1016/s0028-3932(97)00085-7. [DOI] [PubMed] [Google Scholar]
- 61.Tulving E, Kapur S, Craik FIM, Moscovitch M, Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc Natl Acad Sci. 1994;91:2016–20. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Turetsky BI, Cowell PE, Gur RC, Grossman RI, Shtasel DL, Gur RE. Frontal and temporal lobe brain volumes in schizophrenia: relationship to symptomatology and clinical subtype. Arch Gen Psychiat. 1995;52:1061–70. doi: 10.1001/archpsyc.1995.03950240079013. [DOI] [PubMed] [Google Scholar]
- 63.Warach S, Gur RC, Gur RE, Skolnick BE, Obrist WD, Reivich M. Decreases in frontal and parietal lobe regional cerebral blood flow related to habituation. J Cereb Blood Flow Metab. 1992;12:546–53. doi: 10.1038/jcbfm.1992.78. [DOI] [PubMed] [Google Scholar]
- 64.Wechsler D. Wechsler adult intelligence scale — revised, manual. Cleveland, Ohio: The Psychological Corporation; 1981. [Google Scholar]
- 65.Wechsler D. WMS-R manual: Wechsler memory scale, revised. New York: Psychological Corporation; 1987. [Google Scholar]
- 66.Wheeler MA, Stuss DT, Tulving E. Frontal lobe damage produces episodic memory impairment. J Int Neuropsychological Soc. 1995;1:525–36. doi: 10.1017/s1355617700000655. [DOI] [PubMed] [Google Scholar]
- 67.Wilson FAW, O’Scalaidhe SP, Goldman-Rakic PS. Dissociation of object and spatial processing domains in primate prefrontal cortex. Science. 1993;260:1955–8. doi: 10.1126/science.8316836. [DOI] [PubMed] [Google Scholar]