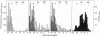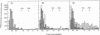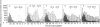Abstract
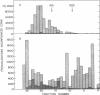
The product of transcription of rat-liver chromatin with homologous rat-liver Form-B polymerase in vitro is high molecular weight RNA with sedimentation coefficients principally in the range of 18-45 S. The average size is somewhat smaller than that of the heterogeneous high molecular weight RNA synthesized by the endogenous enzyme in vivo (or in isolated nuclei). We have excluded the possibility that this difference can be attributed to degradation of the nascent RNA occurring under the conditions of our experiments. The size of the RNA produced by the homologous enzyme nevertheless approaches that of the natural transcripts much more closely than does that of the RNA produced by bacterial RNA polymerases, which we, in addition to other authors, have found to sediment around 10 S.
Keywords: RNA polymerase, α-amanitin
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi G., Parnas H., Hwang M. I., Attardi B. Giant-size rapidly labeled nuclear ribonucleic acid and cytoplasmic messenger ribonucleic acid in immature duck erythrocytes. J Mol Biol. 1966 Sep;20(1):145–182. doi: 10.1016/0022-2836(66)90123-9. [DOI] [PubMed] [Google Scholar]
- Bekhor I., Kung G. M., Bonner J. Sequence-specific interaction of DNA and chromosomal protein. J Mol Biol. 1969 Jan;39(2):351–364. doi: 10.1016/0022-2836(69)90322-2. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C. Optimal conditions for counting of precipitated 3H-RNA on glass-fiber filters. Anal Biochem. 1970 Sep;37(1):178–182. doi: 10.1016/0003-2697(70)90275-7. [DOI] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Butterworth P. H., Cox R. F., Chesterton C. J. Transcription of mammalian chromatin by mammalian DNA-dependent RNA polymerases. Eur J Biochem. 1971 Nov 11;23(2):229–241. doi: 10.1111/j.1432-1033.1971.tb01613.x. [DOI] [PubMed] [Google Scholar]
- Chesterton C. J., Butterworth P. H. Selective extraction of form I DNA dependent RNA polymerase from rat liver nuclei and its separation into two species. Eur J Biochem. 1971 Mar 11;19(2):232–241. doi: 10.1111/j.1432-1033.1971.tb01309.x. [DOI] [PubMed] [Google Scholar]
- MacGillivray A. J., Carroll Dana, Paul J. The heterogeneity of the non-histone chromatin proteins from mouse tissues. FEBS Lett. 1971 Mar 16;13(4):204–208. doi: 10.1016/0014-5793(71)80536-7. [DOI] [PubMed] [Google Scholar]
- Paul J., Gilmour R. S. Organ-specific restriction of transcription in mammalian chromatin. J Mol Biol. 1968 Jul 14;34(2):305–316. doi: 10.1016/0022-2836(68)90255-6. [DOI] [PubMed] [Google Scholar]
- Paul J., Gilmour R. S. Template activity of DNA is restricted in chromatin. J Mol Biol. 1966 Mar;16(1):242–244. doi: 10.1016/s0022-2836(66)80276-0. [DOI] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Penman S., Vesco C., Penman M. Localization and kinetics of formation of nuclear heterodisperse RNA, cytoplasmic heterodisperse RNA and polyribosome-associated messenger RNA in HeLa cells. J Mol Biol. 1968 May 28;34(1):49–60. doi: 10.1016/0022-2836(68)90234-9. [DOI] [PubMed] [Google Scholar]
- Read R. S., Mauritzen C. M. Isolation and preservation of cell nuclei for studies on RNA polymerase activity. Can J Biochem. 1970 May;48(5):559–565. doi: 10.1139/o70-092. [DOI] [PubMed] [Google Scholar]
- Roeder R. G., Rutter W. J. Multiple forms of DNA-dependent RNA polymerase in eukaryotic organisms. Nature. 1969 Oct 18;224(5216):234–237. doi: 10.1038/224234a0. [DOI] [PubMed] [Google Scholar]
- Scherrer K., Marcaud L., Zajdela F., London I. M., Gros F. Patterns of RNA metabolism in a differentiated cell: a rapidly labeled, unstable 60S RNA with messenger properties in duck erythroblasts. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1571–1578. doi: 10.1073/pnas.56.5.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. D., Church R. B., McCarthy B. J. Template specificity of isolated chromatin. Biochemistry. 1969 Nov;8(11):4271–4277. doi: 10.1021/bi00839a007. [DOI] [PubMed] [Google Scholar]
- Spelsberg T. C., Hnilica L. S. Proteins of chromatin in template restriction. I. RNA synthesis in vitro. Biochim Biophys Acta. 1971 Jan 1;228(1):202–211. doi: 10.1016/0005-2787(71)90560-0. [DOI] [PubMed] [Google Scholar]
- Spelsberg T. C., Hnilica L. S. Proteins of chromatin in template restriction. II. Specificity of RNA synthesis. Biochim Biophys Acta. 1971 Jan 1;228(1):212–222. doi: 10.1016/0005-2787(71)90561-2. [DOI] [PubMed] [Google Scholar]
- Warner J. R., Soeiro R., Birnboim H. C., Girard M., Darnell J. E. Rapidly labeled HeLa cell nuclear RNA. I. Identification by zone sedimentation of a heterogeneous fraction separate from ribosomal precursor RNA. J Mol Biol. 1966 Aug;19(2):349–361. doi: 10.1016/s0022-2836(66)80009-8. [DOI] [PubMed] [Google Scholar]
- Zylber E. A., Penman S. Products of RNA polymerases in HeLa cell nuclei. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2861–2865. doi: 10.1073/pnas.68.11.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]