Abstract
Staphylococcus aureus is a major human pathogen and emergence of antibiotic resistance in clinical staphylococcal isolates raises concerns about our ability to control these infections. Cell wall-active antibiotics cause elevated synthesis of methionine sulfoxide reductases (Msrs: MsrA1 and MsrB) in S. aureus. MsrA and MsrB enzymes reduce S-epimers and R-epimers of methionine sulfoxide, respectively, that are generated under oxidative stress. In the S. aureus chromosome, there are three msrA genes (msrA1, msrA2 and msrA3) and one msrB gene. To understand the precise physiological roles of Msr proteins in S. aureus, mutations in msrA1, msrA2 and msrA3 and msrB genes were created by site-directed mutagenesis. These mutants were combined to create a triple msrA (msrA1, msrA2 and msrA3) and a quadruple msrAB (msrA1, msrA2, msrA3, msrB) mutant. These mutants were used to determine the roles of Msr proteins in staphylococcal growth, antibiotic resistance, adherence to human lung epithelial cells, pigment production, and survival in mice relative to the wild-type strains. MsrA1-deficient strains were sensitive to oxidative stress conditions, less pigmented and less adherent to human lung epithelial cells, and showed reduced survival in mouse tissues. In contrast, MsrB-deficient strains were resistant to oxidants and were highly pigmented. Lack of MsrA2 and MsrA3 caused no apparent growth defect in S. aureus. In complementation experiments with the triple and quadruple mutants, it was MsrA1 and not MsrB that was determined to be critical for adherence and phagocytic resistance of S. aureus. Overall, the data suggests that MsrA1 may be an important virulence factor and MsrB probably plays a balancing act to counter the effect of MsrA1 in S. aureus.
Introduction
Staphylococcus aureus is an aggressive and versatile pathogen that is responsible for a wide array of diseases ranging from pyogenic skin infections to complicated life-threatening diseases such as bacteremia, central nervous system infections, and endocarditis [1,2,3,4]. Treatment of S. aureus infections is a great challenge because of the ability of the organism to develop or acquire antibiotic resistance. A widespread use of methicillin and other semi-synthetic penicillins has led to the emergence of methicillin-resistant S. aureus (MRSA) strains that have become prevalent both in the hospitals and the community throughout the world [5,6]. Infections by MRSA strains cause higher mortality and require longer and more expensive medical care than infections caused by methicillin-sensitive S. aureus [5].
Host phagocytic cells play key roles in determining the extent of bacterial infections. The phagocytic cells induce a respiratory burst and produce superoxide anion that serves as a precursor to generate additional reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), hydroxyl radical, singlet oxygen, and hypochlorous acid. These highly reactive species lead to the oxidation of DNA, lipids and proteins. S. aureus produces antioxidant enzymes such as superoxide dismutases, catalase, alkyl hydroperoxide reductases, etc. to defend itself from the ROS [7]. However, the ROS and other oxidizing conditions still cause damage to cellular macromolecules. The ROS oxidize the sulfur atom of protein-bound methionine residues, resulting in methionine sulfoxide (MetO) that typically leads to loss of protein function. MetO are reduced back to methionine by methionine sulfoxide reductase (Msr) enzymes that restore normal protein functions [8]. Oxidation of methionine results in two diastereomeric forms of MetO, R-MetO and S-MetO, which are reduced by two different Msr enzymes. MsrB is specific for R-MetO whereas MsrA is specific for S-MetO [9,10].
Msr proteins have also been shown to contribute to the virulence of bacterial pathogens [11,12,13,14,15]. Absence of Msr enzymes reduces the ability of bacterial cells to adhere to eukaryotic cells that probably impacts colonization of the host [13,14,16,17]. In the absence of the Msr enzymes, the integrity of the bacterial surface proteins is compromised and this deficiency may contribute to the reduced bacterial adherence to eukaryotic cells [13,14,16,17]. In addition, reduced Msr activity impacts bacterial survival within phagocytic cells [12].
In S. aureus chromosome, there are three msrA genes (msrA1, msrA2 and msrA3) and one msrB gene [18]. The msrA1 and msrB genes are co-transcribed in S. aureus and their expression is induced specifically in response to cell wall-active antibiotics [19]. The expression of msrA1/msrB occurs at much higher levels in S. aureus relative to the expression levels of msrA2 or msrA3 genes [20].
In view of multiple msrA and msrB genes in S. aureus; with potential roles in virulence [12,21] and oxidative stress tolerance [18,22], mutations were generated in each of the msrA and msrB genes. Subsequently, three unique msr mutants were constructed by combining the individual mutants that included an msrB mutant (lacks ability to reduce R-MetO), a triple msrA mutant (msrA1, msrA2, msrA3; lacks ability to reduce S-MetO), and a quadruple msrAB mutant (msrA1, msrA2, msrA3, msrB; lacks ability to reduce either R- or S-MetO). These mutants were used to determine the precise roles of Msr proteins in survival of S. aureus under a variety of stress conditions. The presented data suggest that MsrA2 and MsrA3 play little or no role in staphylococcal protection from oxidative stress or in mice. However, the role of the msrA1/msrB locus is complex. While lack of MsrA1 increases the sensitivity of S. aureus to oxidative stress and host immune defense, the lack of MsrB, to some extent, is actually beneficial to the bacterial organism under these conditions.
Materials and Methods
Ethics statement
Animal studies were approved by the A.T. Still University-Kirksville College of Osteopathic Medicine’s Animal Care and Use Committee (IACUC protocol # 166).
Bacterial strains, plasmids, antibiotics and growth conditions
The bacterial strains and plasmids used in this study are shown in Table 1. S. aureus cells were grown in tryptic soy broth (TSB) or tryptic soy agar (TSA) and Escherichia coli cells were grown in Luria-Bertani broth or Luria-Bertani agar. Plasmids in E. coli cells were maintained by adding ampicillin at 100 μg ml-1, kanamycin at 20 μg ml-1, erythromycin at 15 μg ml-1 and tetracyclin at 10 μg ml-1, when required. S. aureus mutant strains were cultured with kanamycin at 100 μg ml-1, erythromycin at 15 μg ml-1 and tetracyclin at 10 μg ml-1, when required.
Table 1. Bacterial strains used in this study.
| Strains | Characteristics | Reference |
|---|---|---|
| S. aureus RN4220 | A restriction minus derivative of S. aureus strain 8325–4 | [54] |
| SH1000 | S. aureus strain 8325–4 with functional RsbU | [25] |
| SH1000:msrA1 | SH1000 with mutation in the msrA1 gene (KanR) | This study |
| SH1000:msrA2 | SH1000 with mutation in the msrA2 gene (TetR) | This study |
| SH1000:msrA3 | SH1000 with mutation in the msrA3 gene (ErmR) | This study |
| SH1000:msrA1-B | SH1000 with mutation in the msrA1-msrB genes (KanR) | This study |
| SH1000:msrB | SH1000 with mutation in the msrB gene (KanR) | This study |
| SH1000:msrA | SH1000 with mutation in the msrA1, msrA2 and msrA3 genes (KanR, TetR, ErmR) | This study |
| SH1000:msrAB | SH1000 with mutation in the msrA1, msrA2, msrA3, and msrB genes (KanR, TetR, ErmR) | This study |
| BB270 | A homogeneous methicillin resistant S. aureus | [26] |
| BB270:msrA1 | BB270 with mutation in the msrA1 gene (KanR) | This study |
| BB270:msrA2 | BB270 with mutation in the msrA2 gene (TetR) | This study |
| BB270:msrA3 | BB270 with mutation in the msrA3 gene (ErmR) | This study |
| BB270:msrA1-B | BB270 with mutation in the msrA1-msrB genes (KanR) | This study |
| BB270:msrB | BB270 with mutation in the msrB gene (KanR) | This study |
| BB270:msrA | BB270 with mutation in the msrA1, msrA2 and msrA3 genes (KanR, TetR, ErmR) | This study |
| BB270:msrAB | BB270 with mutation in the msrA1, msrA2, msrA3, and msrB genes (KanR, TetR, ErmR) | This study |
| SH1000+pCU1 | SH1000 with plasmid pCU1 (CamR) | This study |
| SH1000:msrA+pCU1 | SH1000:msrA with plasmid pCU1 (KanR, TetR, ErmR, CamR) | This study |
| SH1000:msrAB+pCU1 | SH1000:msrAB with pCU1 (KanR, TetR, ErmR, CamR) | This study |
| SH1000:msrA+msrA1 | SH1000:msrA with pCU1-msrA1P-msrA1 (KanR, TetR, ErmR, CamR) | This study |
| SH1000:msrAB+msrA1 | SH1000:msrAB with pCU1-msrA1P-msrA1 (KanR, TetR, ErmR, CamR) | This study |
| SH1000:msrAB+msrB | SH1000:msrAB with pCU1-msrA1P-msrB (KanR, TetR, ErmR, CamR) | This study |
ErmR, erythromycin resistant; KanR, kanamycin resistant; TetR, tetracycline resistant; CamR, chloramphenicol resistant
DNA manipulations
Plasmid DNA was isolated using the Qiaprep Miniprep kit (Qiagen Inc). Chromosomal DNA was isolated using a DNAzol kit (Molecular Research Center) from lysostaphin-treated S. aureus cells according to the manufacturer’s instructions. All restriction and modification enzymes were purchased from Promega. PCR was performed using a Peltier Thermal Cycler-200 system (MJ research). DNA manipulations were carried out using standard procedures. Oligonucleotide primers (Table 2) were obtained from Eurofins.
Table 2. Oligonucleotide primers used in this study.
| Oligo | Sequence (5’→3’) |
|---|---|
| P1 | ATCAATTACCTTGGCACCTACC |
| P2 | GGATCCTGACTTGATGCCTGGATATG |
| P3 | GGATCCAACTGAAGGAGAAGTTGTG |
| P4 | AAGCTTGGTCTTGATTGCTTGTTGC |
| P5 | GGATCCTGACACATTCAGCATAACCA |
| P6 | AAGCTTCAGATGCACATTCATGTGA |
| P7 | GCTGCTTACAAACATTTCGA |
| P8 | GGATCCGAACGACGTAAAGACAGAGA |
| P9 | GCTAACGTCATTGAATATG |
| P10 | GGAAGTAACCTCTGGATCA |
| P11 | ATCGTACTAAGGTCTAATG |
| P12 | CTTGGTGATAGTCTTCGGCT |
| P13 | ATGGTAGTTGTTTATGTAG |
| P14 | CTCCTCTGAAAATCACTTGT |
| P15 | GTTACACAAGAAAACGGCA |
| P16 | TCATCATCGTGTTTTGGG |
| P17 | AGGATGTTTCTGGTGCATGG |
| P18 | GACACAACTTCTCCTTCAGT |
Construction of msr mutants in S. aureus
Construction of the msrA1 [22], msrA2 [18], and msrB [23] mutants has been described previously.
To construct a mutation in msrA1 and msrB genes simultaneously, flanking regions (left of msrA1 and right of msrB) were PCR amplified and ligated. Briefly, primer pairs P1 and P2 were used to amplify a 1449 bp DNA fragment (starting 1364 nt upstream of the msrA1 start codon and going downstream). Another set of primers P3 and P4 were used to amplify an 841 bp DNA fragment (starting 156 nt downstream of the msrB stop codon and going further downstream). These two fragments were ligated in vector pTZ18R [24] which simultaneously engineered a unique BamHI site between the ligated fragments to which a 1.7 kb kanamycin-resistance cassette was cloned. This fragment was used to construct a deletion mutant (msrA1-msrB) in S. aureus utilizing the methodology described previously for the construction of individual msrA1 and msrA2 mutants [18,22].
To construct an msrA3 mutant, primers P5 and P6 were used to amplify a 1084 bp DNA fragment upstream of msrA3 (containing 151 nt of the 5’-end of the msrA3 gene and going upstream). Another set of primers, P7 and P8, were used to amplify a 1047 bp msrA3 downstream fragment (containing 153 nt of the 3’-end of the msrA3 gene and going downstream). These two fragments were ligated together in vector pTZ18R to generate a unique BamHI restriction site between the fragments (lacking a significant portion of the msrA3 gene, from nucleotide position 152–321 with respect to msrA3 start codon) to which a 1.4 kb erythromycin-resistance cassette was cloned. The above construct was used as a suicidal plasmid to construct a mutation in the msrA3 gene utilizing a method described previously [18,22].
For in vitro and in vivo studies, the S. aureus strain SH1000 [25], which is a sigB positive derivative of the S. aureus strain RN450, was used. Since most MRSA strains are naturally resistant to tetracycline and or erythromycin, a S. aureus MRSA strain BB270 [26] (sensitive to kanamycin, erythromycin and tetracycline) was used to combine msr mutations for antibiotic resistance studies. The individual msr mutants were combined in these two S. aureus strains to generate a triple (mutant of msrA1, msrA2, and msrA3 genes) and a quadruple mutant (mutant of msrA1, msrA2, msrA3, and msrB).
Determination of Msr activity
Msr activity in the cell free extract of the wild-type and the msr mutants of S. aureus was determined using 200 μM of Dabsyl-MetO and 20 mM DTT in 50 mM Tris-HCl (pH 7.5) and incubation at 37°C for 30 min, as previously described [18].
Growth kinetics of the wild-type S. aureus and its isogenic msr mutant under stress
Mid-exponential phase cultures (OD600 = 0.6) were diluted 50-fold in a nephelo culture flask (Wheaton) containing 50 ml fresh TSB with a flask-to-medium volume ratio of 6:1. Oxidative and antibiotic stress conditions were imposed by the addition of H2O2 and oxacillin in TSB to appropriate concentrations. Bacterial growth was subsequently monitored by incubating the flask in a shaking incubator (250 rpm) and measuring turbidity of the liquid culture.
Determination of the sensitivity of msrA mutants to oxidants and cell wall inhibitors
The minimum inhibitory concentrations (MICs) for the wild-type and different msr mutant strains of S. aureus were determined as previously described [27,28]. In addition to H2O2, the following oxidizing agents were used in MIC determination studies: cumene hydroperoxide that acts as an intracellular source of reactive oxygen species [29], N-ethylmaleimide that oxidizes thiols and increases disulfide bonds in proteins [30], sodium nitroprusside that serves as a nitric oxide donor [31]; methyl viologen (paraquat) that generates superoxide [31].
Determination of staphyloxanthin production in msr mutants
S. aureus wild-type and its isogenic msr mutant strains were grown at 37˚C for 18 h in TSB. Cells were harvested and washed twice with sterile water and the levels of staphyloxanthin in these cells were quantified as described previously [28,32].
Phagocytic killing of S. aureus msr mutant
The promyelocytic HL-60 cells (obtained from American Type Culture Collection) were grown in Iscove’s Modified Dulbecco’s Medium (IMDM) (ATCC) with 10% fetal bovine serum (Fisher) and treated with 1.3% dimethyl sulfoxide (Fisher) for 5 days to induce their differentiation into neutrophil-like cells. The differentiated neutrophils were used for phagocytic killing using a method described previously [33,34]. In brief, the neutrophils (1X106 cells) were added with S. aureus cells (2.5X106 CFUs) (MOI 1:2.5) in a 24-well plate. The plate was centrifuged at 4000 rpm for 10 min and incubated in a CO2 incubator at 37˚C for 1 h. The supernatant was gently aspirated and the neutrophils were lysed by the addition of IMDM containing 0.025% Titron X-100. The number of surviving bacteria was enumerated by making serial dilutions and plating of this lysate on TSA plate.
Adherence of msr mutant to A549 lung epithelial cells
Adherence of S. aureus SH1000 strain and its isogenic msr mutants was determined by infection of lung epithelial cells as described previously [35,36]. In these experiments, a mixture of msr mutant and wild-type (60:40 ratio) S. aureus was used to infect the monolayers of A549 cells. The ratio of the mutants cells adhered to the A549 cells after 1 h was enumerated and compared to the ratio of the mutants in the mixture used in these adherence assays.
Complementation of triple and quadruple mutants
For complementation studies, the triple mutant was complemented in trans with msrA1 and the quadruple mutant was complemented in trans with either msrA1 or msrB gene. The msrA1 and msrB coding regions were cloned immediately downstream of a previously described construct, pCU1-msrA1P [18]. The resulting constructs pCU1-msrA1P-msrA1 or pCU1-msrA1P-msrB was transferred into S. aureus RN4220 by electroporation and subsequently transduced into the triple or quadruple mutants. For comparative studies, wild-type SH1000, and the triple and quadruple mutants were also transformed with the empty plasmid pCU1 [37].
Levels of Protein A in S. aureus cells
Total protein was extracted from lysostaphin treated S. aureus cells, separated by SDS-PAGE, and transferred to a nitrocellulose membrane. The membrane was blocked with 5% skimmed milk and incubated with rabbit antibodies conjugated to horseradish peroxidase (Bio-rad). The membrane was visualized for Protein A using an Opti-4CN substrate kit (Bio-Rad).
Hemolysis by msr deficient S. aureus
To visualize the hemolysis, 5.0 μl of the overnight cultures of the wild-type S. aureus SH1000 and the msr mutants were spotted on TSA plates with 5% sheep blood agar and the plate was incubated at 37°C for 48 h.
Survival of wild-type and msr mutants in a murine systemic infection model
Wild-type and msr mutants were mixed together and then tested in a murine systemic infection model to determine if these mutations had an effect on the ability of the organism to survive in vivo as described previously [28,36]. A 0.5 ml mixture of the wild-type and msr mutant cells (~1X108 CFU, approximately 40:60 ratio of the wild-type and mutant) was injected into the peritoneal cavity of Swiss white Hla (ICR)CVF female mice (16–20 g) (Hilltop Lab Animals, Inc.) and the fraction of mutants surviving in the spleen and liver of infected mice was enumerated relative to wild-type S. aureus as described previously [28,36].
Localization of S. aureus MsrA1 and MsrB
To determine the localization of MsrA1 and MsrB in S. aureus, wild type S. aureus SH1000 culture was grown in TSB to an OD600 = 0.3 and treated with 1.2 μg ml-1 oxacillin for 2.5 h to induce the synthesis of these proteins as described previously [19,22]. Bacterial cells were harvested and the cytosolic and the cytoplasmic membrane fractions were prepared as described previously [38], separated by 15% SDS-PAGE and subjected to western blot analysis for the presence of MsrA1 and MsrB.
Statistical analysis
Data were analyzed with a paired t-test using a statistical analysis computer program (R for Windows, version 3.0.2, The R Foundation for Statistical Computing). Statistical significance was set at p≤.05.
Results
Construction of the msr mutants
We previously reported the construction and findings of the msrA1, msrA2, and msrB mutants where the phenotypes of the mutant strains were restored by complementation of the mutated genes in trans [18,22,23]. The msrA1 and msrB genes in S. aureus are the first and second of a four-gene operon [18,22]. Also, the msrA1 mutant produced a significantly higher level of MsrB relative to wild-type S. aureus [18]. In this study, a mutant was created where the entire msrA1 and msrB gene segments were deleted from the bacterial chromosome and replaced with a kanamycin-resistance cassette to generate an msrA1-msrB null mutant. An msrA3 deletion mutant was also constructed. Subsequently, the three msrA (msrA1, msrA2, msrA3) mutants were combined to generate an MsrA-deficient triple mutant. In addition, the msrA2 and msrA3 individual mutants were combined with an msrA1-msrB mutant to generate a quadruple mutant. These mutations were verified by PCR using primer pairs flanking the region that had been deleted in the mutants and replaced by larger antibiotic resistance cassettes (Fig. 1).
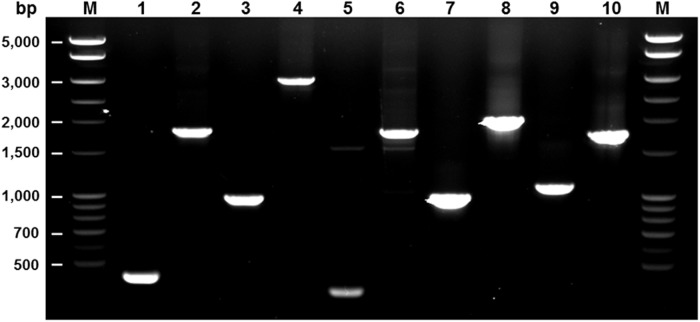
Fig 1. Confirmation of mutations in msr genes.
Primers from the regions flanking the site of the antibiotic-resistance cassette were used in the PCR. A larger PCR product was observed when genomic DNA from the mutant (even-numbered lanes) was used compared to when wild-type genomic DNA was used (odd-numbered lanes) as a template because of the insertion of a larger antibiotic-resistance cassette. Primers P9 and P10 were used to verify mutation in msrA1 (Lanes 1 & 2), P11 and P12 for mutation in msrA2 (Lanes 3 & 4), P13 and P14 for mutation in msrA3 (Lanes 5 & 6), P15 and P16 for mutation in msrB (Lanes 7 & 8), and P17 and P18 for mutation in msrA1-B (Lanes 9 & 10). Lane M—DNA ladder.
Msr activity in wild-type and msr mutants of S. aureus
Cell-free protein extracts from the wild-type and msr mutant cultures were used to determine Msr activity using dabsyl-MetO as a substrate. The Msr activity in various mutants was normalized against the enzymatic activity in the wild-type S. aureus SH1000 and the data are shown in Table 3. The data demonstrate that the MsrA2 and MsrA3 contribute little to the enzymatic activity in S. aureus cells (Table 3). An increase in Msr activity in the msrA1 mutant is because of a higher production of MsrB in this mutant [18]. Further, MsrB is responsible for most of the enzymatic activity (~83%) in wild-type S. aureus SH1000 (Table 3). There was no enzymatic activity noted in the quadruple msrAB mutant (Table 3).
Table 3. Methionine sulfoxide reductase activity levels in different msr mutants relative to wild-type S. aureus strain SH1000.
| Strain | Percent total activity |
|---|---|
| Wild-type SH1000 | 100 |
| SH1000:msrA1 | 218 |
| SH1000:msrA2 | 106 |
| SH1000:msrA3 | 93 |
| SH1000:msrB | 17 |
| SH1000:msrA1-B | 19 |
| SH1000:msrA | 123 |
| SH1000:msrAB | 0 |
Oxidative and antibiotic stress tolerance of the msr mutants
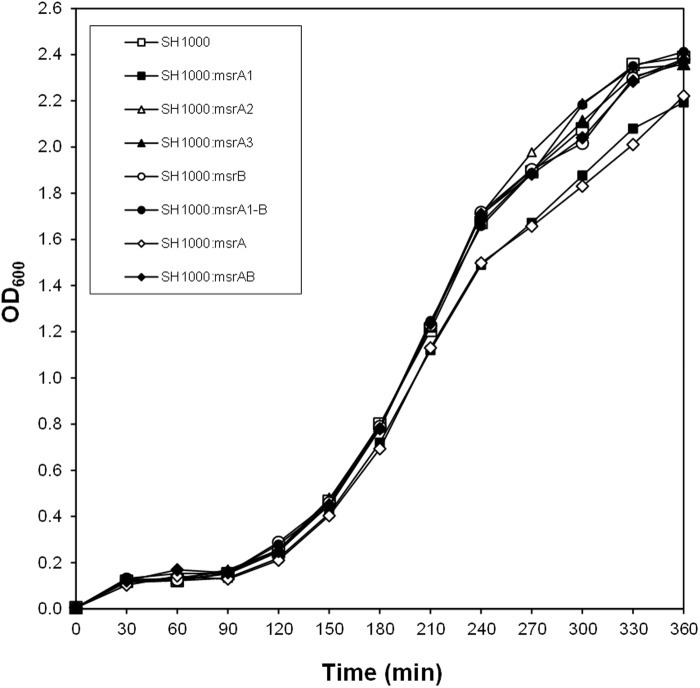
In growth kinetic experiments, the mutants specifically lacking MsrA1 or all three MsrA proteins showed slightly slower growth rate in TSB at 37°C (Fig. 2). Deletion of msrA2, msrA3, msrB, or msrA1-msrB had no apparent effect on the growth of the mutant cell compared to the growth of the wild-type S. aureus SH1000 (Fig. 2). When the Msr deficient mutants were cultured in TSB supplemented with 4.4 mM H2O2, the S. aureus strains lacking MsrA1 failed to grow (Fig. 3A). In the case of the combinatorial mutants, no growth was recorded for the triple msrA mutant even after 16 h in TSB with 8.8 mM H2O2 (Fig. 3B). The amount of H2O2 was raised to 8.8 mM in growth studies utilizing the combinatorial mutants to assess the resistance of MsrB-deficient S. aureus relative to other strains (Fig. 3B). The S. aureus strains that lacked MsrB (msrB, msrA1-msrB and the quadruple msrAB mutants) were moderately resistant to the presence of H2O2 in these growth experiments (Fig. 3B). The MsrB-deficient strains of methicillin-resistant S. aureus BB270 demonstrated better growth even in the presence of a cell wall-active antibiotic, oxacillin (Fig. 3C). In the MIC studies, the S. aureus strains deficient in MsrB were more resistant to H2O2 (Table 4). A similar increase in resistance to oxacillin and other cell wall-active antibiotics was observed in the case of MsrB-deficient S. aureus (Table 5). The strains that lacked MsrA1 were susceptible to oxidative stress conditions and the S. aureus strain that lacked all three MsrA proteins (the triple msrA mutant) showed most sensitivity to oxidants (Table 4). No such increase in sensitivity was noted in MsrA-deficient S. aureus to cell-wall active antibiotics (Table 5).
Fig 2. Growth curve of the wild-type S. aureus strain and its derivative msr mutants in TSB.
Values indicate the average of two independent experiments.
Fig 3. Growth of the wild-type S. aureus strain and its derivative msr mutants in the presence of H2O2 or oxacillin.
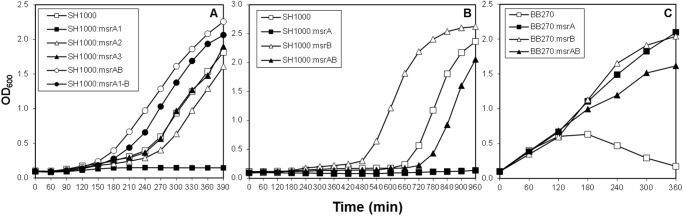
(A) growth in the presence of 4.4 mM H2O2, (B) growth in the presence of 8.8 mM H2O2 (C) growth in the presence of 400 μg ml-1 of oxacillin. Values indicate the average of two independent experiments.
Table 4. Susceptibilities of S. aureus parental strain SH1000 and its derivative msr mutants to oxidants. MIC values indicate average mM concentrations of three independent experiments.
| Strains | H2O2 | CHPO | NEM | SNP | Paraquat |
|---|---|---|---|---|---|
| Wild-type SH1000 | 1 | 9.5 | 0.625 | 250 | 125 |
| SH1000:msrA1 | 0.5 | 4.75 | 0.313 | 7.81 | 125 |
| SH1000:msrA2 | 1 | 9.5 | 0.625 | 250 | 125 |
| SH1000:msrA3 | 1 | 9.5 | 0.625 | 250 | 125 |
| SH1000:msrB | 2 | 9.5 | 0.625 | 250 | 125 |
| SH1000:msrA1-B | 0.5 | 9.5 | 0.625 | 125 | 125 |
| SH1000:msrA | 0.25 | 2.38 | 0.313 | 1.95 | 31.25 |
| SH1000:msrAB | 0.5 | 9.5 | 0.625 | 250 | 125 |
Abbreviations: H2O2, hydrogen peroxide; CHPO, cumene hydroperoxide; NEM, N-ethylmaleimide; SNP, sodium nitroprusside.
Table 5. Susceptibilities of S. aureus BB270 parental and msr mutant strains to cell wall-active antibiotics.
| Strains | Oxacillin | D-cycloserine | Bacitracin |
|---|---|---|---|
| Wild-type BB270 | 200 | 75 | 50 |
| BB270:msrA1 | 200 | 75 | 50 |
| BB270:msrA2 | 200 | 75 | 50 |
| BB270:msrA3 | 200 | 75 | 50 |
| BB270:msrB | 400 | 150 | 100 |
| BB270:msrA1-B | 300 | 100 | 75 |
| BB270:msrA | 200 | 75 | 50 |
| BB270:msrAB | 200 | 75 | 50 |
MIC values (μg ml-1) indicate average of three independent experiments.
Production of staphyloxanthin pigment in msr mutants
Of the seven msr mutants used in this study, production of staphyloxanthin pigment was highest in the msrB mutant strain (Fig. 3). The level of staphyloxanthin was lower in MsrA1-deficient strains (Fig. 3). The MsrA1-deficient S. aureus has been shown to produce a much higher level of MsrB [18]. Increased pigmentation in MsrB-deficient S. aureus and reduced pigmentation in cells producing a higher level of MsrB suggests that the MsrB protein suppresses the production of staphyloxanthin in S. aureus. Production of staphyloxanthin in msrA2 and msrA3 mutants was not affected relative to wild-type S. aureus (Fig. 4).
Fig 4. Production of staphyloxanthin in the wild-type S. aureus strain SH1000 and its derivative msr mutants.
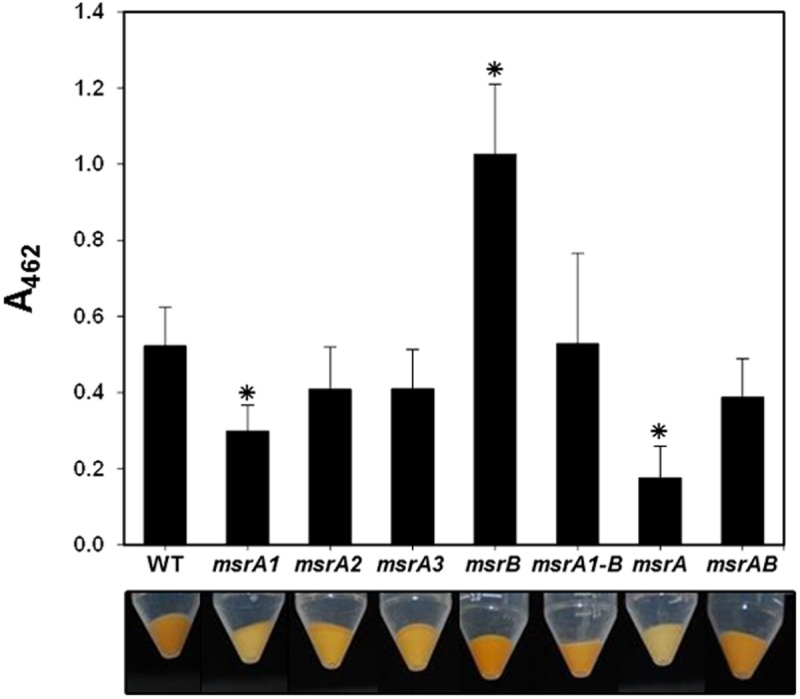
-The bottom panel shows the color of the bacterial cell pellet from 50 ml overnight grown cultures. The amount of the staphyloxanthin pigment produced by these cells was quantified and is shown as A462. Values indicate the average of three independent experiments ± standard deviation (* significant at p≤.05).
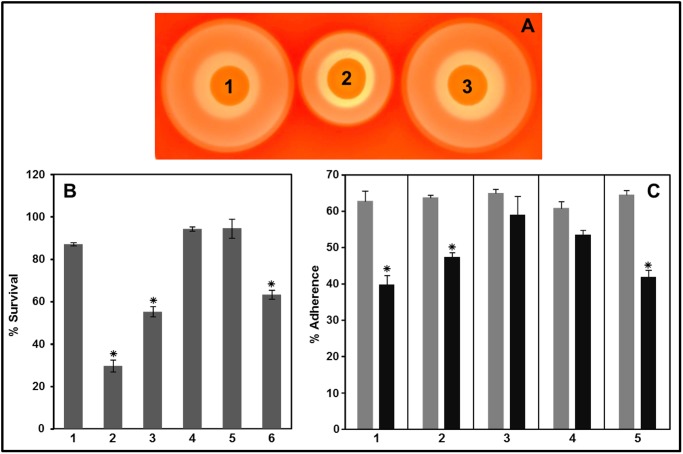
Phagocytic killing of the S. aureus msr mutant cells
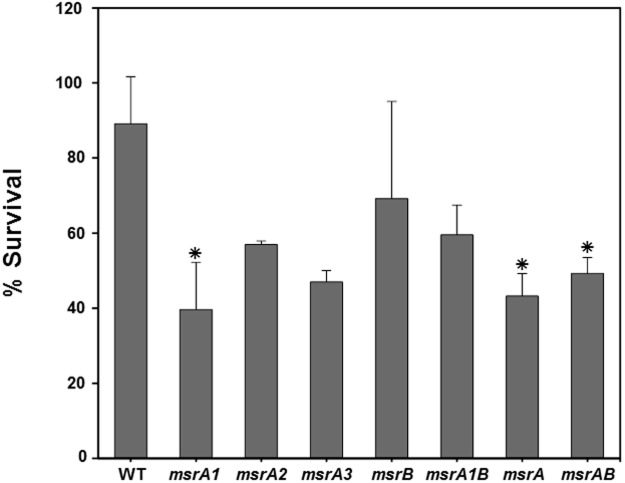
Polymorphonuclear cells utilize oxygen-dependent bactericidal pathways in the phagolysosomes. The impact of Msr deletion was investigated on staphylococcal survival in differentiated polymorphonuclear cells. In these studies, the S. aureus strains with a non-functional MsrA1 showed increased susceptibility to the polymorphonuclear cells (Fig. 5). The survival of the msrA2, msrA3, msrB or msrA1-B mutants of S. aureus was comparable to the wild-type S. aureus SH1000 in these assays (Fig. 5).
Fig 5. Survival of the wild-type S. aureus strain SH1000 and its derivative msr mutant cells exposed to polymorphonuclear (PMN) cells.
PMN cells were infected (MOI 1:2.5) with wild-type S. aureus SH1000 and its isogenic msr mutants for 1 h at 37˚C and then plated on TSA for enumeration. Values indicate the average of three independent experiments ± standard deviation (* significant at p≤.05).
Role of Msr proteins in adherence of S. aureus to lung epithelial cells
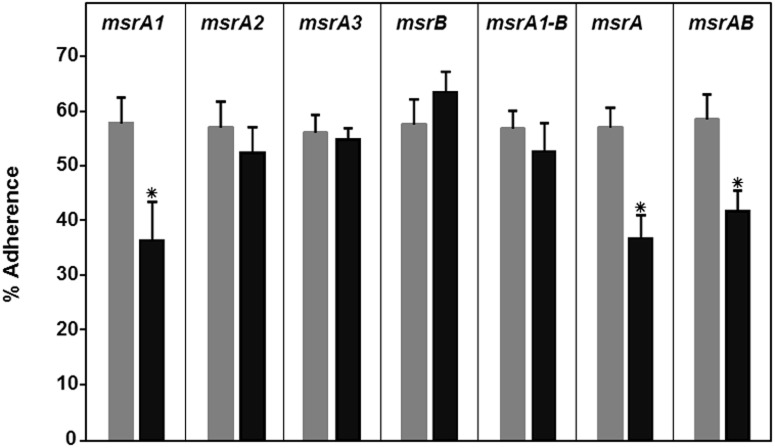
The mixture that was used in adherence assays was biased for an msr mutant (~60%) relative to the wild-type S. aureus SH1000 (~40%). In experiments investigating the adherence of this mixture to A549 cells, the MsrA1-deficient mutants (msrA1, msrA and msrAB) showed significantly reduced adherence (Fig. 6). Deficiency of MsrA2, MsrA3, or MsrB did not impact the adherence of the S. aureus cells to A549 cells (Fig. 6).
Fig 6. Adherence of the wild-type S. aureus strain SH1000 and its derivative msr mutant cells to A549 human lung epithelial cells.
A total of 5X105 bacterial cells were used in these assays. The left light bar in each panel represents the ratios of the msr mutant relative to wild-type SH1000 in the mixture used to infect the A549 cells. The right dark bar in each panel represents the ratios of the msr mutant in the mixture that adhered to the A549 cells after 1 h of incubation. Values indicate the average of three independent experiments ± standard deviation (* significant at p≤.05).
Protein A levels in msr mutants
Staphylococcal surface protein, Protein A, contributes to bacterial adhesion, virulence, and biofilm formation. In Western blot analysis involving total protein extract from wild-type S. aureus SH1000 and the derivative msr mutant cells, an apparent 55 kDa protein specific to Protein A was detected (Fig. 7). Individual msr gene deletions had no appreciable impact on the levels of Protein A in S. aureus (Fig. 7). However, the protein A-specific band was significantly lighter in the lane corresponding to the triple msrA mutant (Fig. 7, Lane 7).
Fig 7. Western analysis of the levels of staphylococcal Protein A in wild-type S. aureus strain SH1000 and its derivative msr mutants.
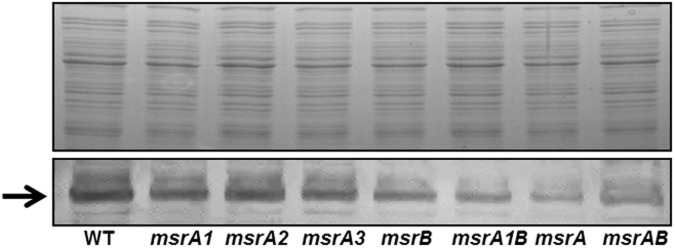
Top panel shows the total protein profile of wild-type and the msr mutant strains after SDS-PAGE suggesting that similar amounts of protein were used in the analysis of Protein A. The bottom panel shows the reactivity of Protein A (arrow) in each lane.
Hemolytic pattern of msr mutants
In qualitative assays, the S. aureus strain that lacked all three MsrA proteins showed a relatively smaller zone of beta-hemolysis relative to other strains (Fig. 8, Spot 7). Another interesting observation was the presence of a significantly reduced secondary zone of hemolysis for the triple mutant (Fig. 8, Spot 7).
Fig 8. Hemolytic pattern of the wild-type S. aureus strain SH1000 and its derivative msr mutants after 48 h of growth on 5% sheep blood agar plates.

(1) Wild-type SH1000, (2) SH1000:msrA1, (3) SH1000:msrA2, (4) SH1000:msrA3, (5) SH1000:msrB, (6) SH1000:msrA1-B, (7) SH1000:msrA, (8) SH1000:msrAB.
Hemolysis, phagocytic survival and adherence of complemented triple and quadruple mutants
The triple SH1000:msrA mutant showed a defective pattern in hemolysis but its complementation with the msrA1 gene in trans was shown to restore the level of hemolysis shown with the wild-type SH1000 (Fig. 9A, Spot 3). In phagocytic killing assays, the triple SH1000:msrA and the quadruple SH1000:msrAB mutants were more sensitive than the wild-type SH1000. In complementation experiments, when triple and quadruple mutants were complemented with the msrA1 gene in trans, these strains showed phagocytic resistance that was comparable to wild-type SH1000 (Fig. 9B). However, complementation of the quadruple mutant with the msrB gene in trans did not restore the phagocytic resistance in these strains (Fig. 9B). Similarly, in adherence experiments, complementation with msrA1 gene in trans, restored the defect in adherence that was initially seen in case of the triple or quadruple mutants (Fig. 9C). Complementation with msrB, on the other hand, had no appreciable effect on the adherence of the quadruple effect (Fig. 9C).
Fig 9. Hemolysis (A), phagocytic survival (B) and adherence (C) of complemented triple and quadruple mutants.
A. Hemolysis on 5% sheep blood agar plates. (1) SH1000+pCU1, (2) SH1000:msrA+pCU1, (3) SH1000:msrA+msrA1. B. PMN cells were infected (MOI 1:2.5) with wild-type, mutants, and the complemented strains for 1 h at 37˚C and then plated for enumeration. (1) SH1000+pCU1, (2) SH1000:msrA+pCU1, (3) SH1000:msrAB+pCU1, (4) SH1000:msrA+msrA1, (5) SH1000:msrAB+msrA1, (6) SH1000:msrAB+msrB. Values indicate the average of three independent experiments ± standard deviation (* significant at p≤.05). C. The left light bar in each panel represents the ratios of the mutant or the complemented strain relative to SH1000-pCU1 in the mixture used to infect the A549 cells. The right dark bar in each panel represents the ratios of the mutant or the complemented strain in the mixture that adhered after 1 h of incubation. (1) SH1000:msrA+pCU1, (2) SH1000:msrAB+pCU1, (3) SH1000:msrA+msrA1, (4) SH1000:msrAB+msrA1, (5) SH1000:msrAB+msrB. Values indicate the average of three independent experiments ± standard deviation (* significant at p≤.05).
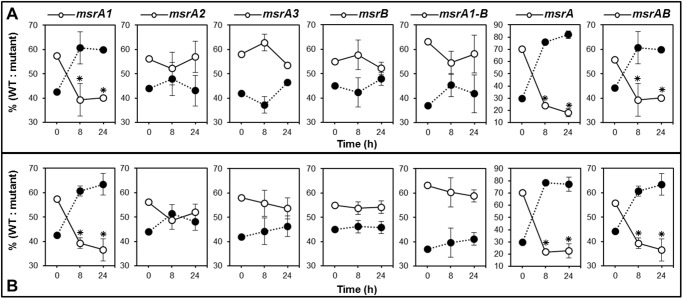
Survival of msr mutants in mice
To elucidate the role of Msr in virulence of S. aureus, Swiss white female mice were injected with a bacterial mixture of wild-type S. aureus SH1000 and its derivative seven msr mutants (40:60 ratio of wild-type to mutant). The data suggest that the msrA1 mutant of S. aureus had a lower survival rate in mice. Post infection, the fraction of msrA1 mutants in spleen and liver was lower at 8 h and declined even further at 24 h in these tissues relative to their fraction in the mixture that was injected into the mice (Fig. 10). Loss of MsrA2, MsrA3, or MsrB had little to no effect on the survival of S. aureus in mice (Fig. 10). The triple msrA mutant showed the highest decline in spleen and liver tissues with time suggesting some roles for MsrA2 and MsrA3 under MsrA1-deficient conditions (Fig. 10). Although, there is a slight growth defect in the msrA1 and triple msrA mutants as shown in Fig. 2, when cultured at 37°C in vitro, it is highly unlikely that there was much of a growth of the wild-type or the mutant bacteria in mice during our experiments that lasted only 24 h. Most of the bacteria that were injected were cleared in mice with time, as we recovered fewer bacteria after 8 h and far fewer bacteria after 24 h. It is indeed the lack of MsrA1 that significantly reduced the survival of S. aureus in mice.
Fig 10. Survival of the wild-type S. aureus strain SH1000 and its derivative msr mutant cells in mouse.
Approximately 1.0X108 CFUs (predominantly mutant cells) were injected intra-peritoneally into mice. Three mice were sacrificed at 8 and 24 h post-injection. Closed circles represent the fraction of wild-type SH1000 and the open circles represent the msr mutant bacteria recovered from murine spleens (A) and murine livers (B) at 8 and 24 h post infection. Values at 0 h indicate the fraction of msr mutants and isogenic wild-type cells in the injected inoculum. Values indicate the average of three independent experiments ± standard deviation (* significant at p≤.05).
Localization of msr protein
Localization was only investigated for MsrA1 and MsrB proteins because these two proteins have been shown to be expressed in S. aureus at a significantly higher level relative to MsrA2 and MsrA3 in S. aureus [20]. In addition, findings of this study suggest that the lack of MsrA1 or MsrB has a pleiotropic effect on S. aureus cells. Experiments utilizing anti-MsrA1 and anti-MsrB rabbit polyclonal antibodies demonstrated that the MsrA1 protein is distributed equally between the cytosolic and the membrane components in S. aureus (Fig. 11, Lanes 1 and 2). However, the MsrB protein appears to be predominantly a cytosolic protein and only a minor fraction of this protein is targeted into the bacterial membrane (Fig. 11, Lanes 3 and 4).
Fig 11. Distribution of MsrA1 and MsrB in the cytosolic and membrane fractions in S. aureus SH1000.
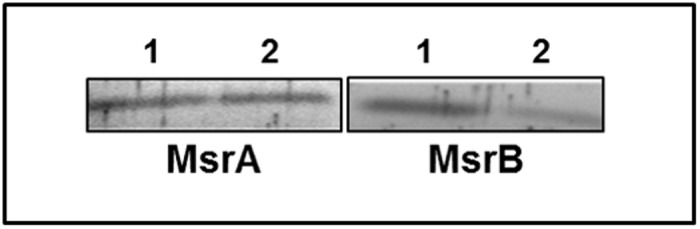
Lane 1 indicates the cytosolic fraction and Lane 2 indicates the membrane fraction.
Discussion
Numerous investigations in recent years have led to an increased interest and understanding of the biology of the methionine sulfoxide reductases. The reasons underlying this interest are because of a remarkable conservation across prokaryotes and eukaryotes, the importance in oxidative stress, and the novel protein repair functions of these enzymes. The two distinct Msr proteins, MsrA and MsrB, share no sequence homology. Orthologs of msrA and msrB show great variation in their genetic organization in bacterial chromosomes. In some bacterial species, the genes encoding MsrA and MsrB are located adjacent to each other and co-transcribed, and in others, the msrA and msrB genes are transcriptionally fused [17,39]. In addition, many bacterial species have multiple copies of these msrA and msrB genes distributed randomly in the bacterial chromosome and some are present even on plasmids [39].
S. aureus produces three different MsrA proteins (MsrA1, MsrA2 and MsrA3) and one MsrB protein. MsrA1 and MsrB production in S. aureus are induced by cell wall-active antibiotics. In the presence of these antibiotics, the cell wall is likely destabilized and the oxidizing agents have easy access to bacterial membrane and cytosolic compartments. In response, the staphylococcal cells produce a higher level of MsrA1 and MsrB; however, oxidative stress has not been shown to induce the synthesis of these proteins in S. aureus. In addition to these four Msr proteins, there is an additional gene (fRMsr) in S. aureus (SACOL1768 in S. aureus strain COL) that codes for a protein that reduces the free methionine sulfoxide. Although the structural and biochemical properties of this protein have been determined [40], its physiological relevance is unclear. The extent of expression of S. aureus fRMsr is also not clear. The fRMsr gene in S. aureus may be expressed at a very low level since there was no detectable Msr activity in the msrAB quadruple mutant (Table 3).
Studies with the individual msr gene mutants make it clear that the MsrA2 and MsrA3 contribute little to cellular Msr activities, play a little to no role in protecting S. aureus from oxidative stress and neutrophils, and have no impact on bacterial survival in mice. Using promoter fusion experiments, we have previously shown that msrA2 and msrA3 are expressed at significantly lower levels compared to the expression of the msrA1-msrB locus in S. aureus [20]. We also measured the relative transcript levels of the msrA2 and msrA3 relative to the transcript level of msrA1 in S. aureus. The expression level of msrA2 was 8–10 log lower compared to msrA1, and the msrA3-specific transcript was almost absent in a qRT-PCR assay (data not shown). In a previous study, with promoter reshuffling, we showed that the MsrA2 protein was as effective as MsrA1 in protection from oxidative stress when its expression level was raised in S. aureus [18]. The msrA3 gene may also be under the influence of a weaker promoter compared to the strength of the promoter that drives the transcription of the msrA1-msrB genes in S. aureus.
In contrast to MsrA2 and MsrA3, lack of either MsrA1 or MsrB showed pleiotropic effects in S. aureus. The lack of MsrA1 increased the sensitivity of S. aureus to oxidative stress. Studies with a triple msrA mutant, which lacked all three MsrA proteins and therefore had no apparent capability to reduce S-MetO, showed a further increase in bacterial sensitivity to oxidants compared to only MsrA1-deficient S. aureus. This phenomenon suggests that, even though MsrA2 and MsrA3 are present at very low levels in S. aureus, they may be somewhat relevant in protecting S. aureus under MsrA1-deficient conditions. The triple msrA mutant also showed reduced hemolysis and increased susceptibility to neutrophil-mediated killing. This observation was expected given that MsrA deficiency in several organisms leads to enhanced vulnerability to oxidative stress [18,41,42,43]. In addition, the msrA1 gene was up-regulated in neutrophils [12]. Within the neutrophils, the staphylococcal two-component regulatory system VraSR contributes to the msrA1 up-regulation [12].
The MsrA1-deficient strains showed reduced pigmentation compared to the wild-type S. aureus. It has been previously shown that the staphyloxanthin pigment plays an important role in the protection of S. aureus from oxidants and neutrophils and regulates bacterial membrane fluidity and virulence [44,45,46,47]. In this study, the MsrB-deficient S. aureus strains were more pigmented and more resistant to H2O2 and cell wall-active antibiotics. One possible explanation for this phenomenon is that an increased pigmentation in the MsrB-deficient S. aureus may contribute to an impermeable membrane that restricts the oxidants and antibiotics. In turn, this change may minimize damage to cellular components under these adverse conditions. We also noted that the MsrA1-deficient S. aureus or S. aureus that was deficient in all three MsrA proteins were less adherent to human lung epithelial cells and showed reduced survival in mouse spleen and liver. The quadruple msrAB mutant of S. aureus also showed reduced adherence to A549 cells and survival in mouse tissues. Furthermore, the complementation experiments with the triple and quadruple mutants provide evidence that it is the MsrA1 not MsrB that is critical for staphylococcal adherence to eukaryotic cells and its resistance to the killing by phagocytic cells.
With respect to the role of the Msr proteins, it is well documented that these enzymes contribute to the ability of a pathogen to adhere to host tissue, evade immune system, form biofilms, survive inside macrophages, and resist oxidative killing [14].MsrA protein contributes to cell wall integrity and maintenance of adhesion properties in Streptococcus gordonii [48]. Msr proteins have also been shown to affect adherence properties of pathogenic Neisseria [17]. In S. gordonii, the MsrA enzyme was shown to maintain the integrity of bacterial adhesins during oxidative stress [49]. The current study confirms the role of Msr proteins, particularly the MsrAs in the adherence of S. aureus to human cells. The MsrA1-deficient S. aureus, the triple msrA and the quadruple msrAB null-mutants, all showed reduced adherence to lung epithelial cells. The role of Msr proteins in virulence of the bacterial pathogens is also well documented. Both MsrA and MsrB contributed to the enzymatic defenses of Mycobacterium tuberculosis from reactive oxygen species [50]. In Pseudomonas aeruginosa, inactivation of either msrA or msrB or both reduced virulence and increased its killing by oxidants [51]. In Campylobacter jejuni, the single msrA or msrB mutants showed no growth defect, but the msrA-msrB double mutant showed increased sensitivity to oxidative stress conditions [31]. Mutation in the msrA or msrB gene in Enterococcus faecalis resulted in increased sensitivity to H2O2. In addition, an msrA msrB double mutant showed further increase in sensitivity suggesting that the effect of mutations were additive [15]. In a later study, however, the msrA and msrB mutants were shown to behave differently; the msrA mutant was more sensitive to oxidative stress conditions whereas the msrB mutant showed stimulated growth under similar conditions [52]. In Salmonella Typhimurium, deletion of msrA increased bacterial susceptibility to H2O2 and reduced its virulence, but a mutation in msrB had no apparent phenotype [11]. In Mycobacterium smegmatis also, MsrB was shown to have a limited role in protection from oxidative stress conditions [53].
Thus, the role of MsrB protein in defense from oxidative stress is questionable in many bacterial species. It is possible that under oxidative stress the majority of the oxidized methionine is S-MetO and the MsrB protein has no activity against this epimer. This may be the reason why the MsrA-deficient bacteria showed a high sensitivity to conditions that impose oxidative stress. MsrB of S. aureus, seems to some extent, counterbalance the effect of MsrA1. For example, lack of MsrA1 reduces pigmentation and this may be due to previously shown higher level of MsrB in MsrA1-deficient S. aureus [18]. However, when MsrB is absent, the bacterium responds by increasing pigment production as a potential compensatory mechanism.
In summary, among the four Msr enzymes produced in S. aureus, MsrA2 and MsrA3 contribute little to the enzymatic activity and bacterial defense from oxidative stress. MsrA1 and MsrB have opposing roles in pigment production and resistance from oxidative stress. MsrA1 seems to be equally distributed between the cytosolic and membrane components but the MsrB appears to be predominantly cytosolic. Regulation of msrA1-msrB locus is currently under investigation because of its significant role in S. aureus physiology and virulence.
Acknowledgments
The authors thank Deborah Goggin for critical reading of this manuscript. This work was supported in part by a Warner/Fermaturo grant and A.T. Still University Board of Trustees Research Funds, by grant 1R15AI090680–01 from the National Institutes of Health to VKS, and grants from the Kirksville College of Osteopathic Medicine Biomedical Sciences Graduate Program to TRJ and KRB.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported in part by a Warner/Fermaturo grant and A.T. Still University Board of Trustees Research Funds, by grant 1R15AI090680-01 from the National Institutes of Health to VKS, and grants from the Kirksville College of Osteopathic Medicine Biomedical Sciences Graduate Program to TRJ and KRB. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Aguilar J, Urday-Cornejo V, Donabedian S, Perri M, Tibbetts R, et al. (2010) Staphylococcus aureus meningitis: case series and literature review. Medicine (Baltimore) 89: 117–125. 10.1097/MD.0b013e3181d5453d [DOI] [PubMed] [Google Scholar]
- 2. Gordon RJ, Lowy FD (2008) Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 46 Suppl 5: S350–359. 10.1086/533591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pierce D, Calkins BC, Thornton K (2012) Infectious endocarditis: diagnosis and treatment. Am Fam Physician 85: 981–986. [PubMed] [Google Scholar]
- 4. Singer AJ, Talan DA (2014) Management of skin abscesses in the era of methicillin-resistant Staphylococcus aureus. N Engl J Med 370: 1039–1047. 10.1056/NEJMra1212788 [DOI] [PubMed] [Google Scholar]
- 5. Kavanagh KT, Calderon LE, Saman DM, Abusalem SK (2014) The use of surveillance and preventative measures for methicillin-resistant infections in surgical patients. Antimicrob Resist Infect Control 3: 18 10.1186/2047-2994-3-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Watkins RR, David MZ, Salata RA (2012) Current concepts on the virulence mechanisms of meticillin-resistant Staphylococcus aureus. J Med Microbiol 61: 1179–1193. 10.1099/jmm.0.043513-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DeLeo FR, Diep BA, Otto M (2009) Host defense and pathogenesis in Staphylococcus aureus infections. Infect Dis Clin North Am 23: 17–34. 10.1016/j.idc.2008.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oien DB, Moskovitz J (2008) Substrates of the methionine sulfoxide reductase system and their physiological relevance. Curr Top Dev Biol 80: 93–133. [DOI] [PubMed] [Google Scholar]
- 9. Grimaud R, Ezraty B, Mitchell JK, Lafitte D, Briand C, et al. (2001) Repair of oxidized proteins. Identification of a new methionine sulfoxide reductase. J Biol Chem 276: 48915–48920. [DOI] [PubMed] [Google Scholar]
- 10. Moskovitz J, Poston JM, Berlett BS, Nosworthy NJ, Szczepanowski R, et al. (2000) Identification and characterization of a putative active site for peptide methionine sulfoxide reductase (MsrA) and its substrate stereospecificity. J Biol Chem 275: 14167–14172. [DOI] [PubMed] [Google Scholar]
- 11. Denkel LA, Horst SA, Rouf SF, Kitowski V, Bohm OM, et al. (2011) Methionine sulfoxide reductases are essential for virulence of Salmonella typhimurium. PLoS One 6: e26974 10.1371/journal.pone.0026974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pang YY, Schwartz J, Bloomberg S, Boyd JM, Horswill AR, et al. (2014) Methionine sulfoxide reductases protect against oxidative stress in Staphylococcus aureus encountering exogenous oxidants and human neutrophils. J Innate Immun 6: 353–364. 10.1159/000355915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saleh M, Bartual SG, Abdullah MR, Jensch I, Asmat TM, et al. (2013) Molecular architecture of Streptococcus pneumoniae surface thioredoxin-fold lipoproteins crucial for extracellular oxidative stress resistance and maintenance of virulence. EMBO Mol Med 5: 1852–1870. 10.1002/emmm.201202435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sasindran SJ, Saikolappan S, Dhandayuthapani S (2007) Methionine sulfoxide reductases and virulence of bacterial pathogens. Future Microbiol 2: 619–630. [DOI] [PubMed] [Google Scholar]
- 15. Zhao C, Hartke A, La Sorda M, Posteraro B, Laplace JM, et al. (2010) Role of methionine sulfoxide reductases A and B of Enterococcus faecalis in oxidative stress and virulence. Infect Immun 78: 3889–3897. 10.1128/IAI.00165-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dhandayuthapani S, Blaylock MW, Bebear CM, Rasmussen WG, Baseman JB (2001) Peptide methionine sulfoxide reductase (MsrA) is a virulence determinant in Mycoplasma genitalium. J Bacteriol 183: 5645–5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wizemann TM, Moskovitz J, Pearce BJ, Cundell D, Arvidson CG, et al. (1996) Peptide methionine sulfoxide reductase contributes to the maintenance of adhesins in three major pathogens. Proc Natl Acad Sci U S A 93: 7985–7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singh VK, Moskovitz J (2003) Multiple methionine sulfoxide reductase genes in Staphylococcus aureus: expression of activity and roles in tolerance of oxidative stress. Microbiology 149: 2739–2747. [DOI] [PubMed] [Google Scholar]
- 19. Singh VK, Jayaswal RK, Wilkinson BJ (2001) Cell wall-active antibiotic induced proteins of Staphylococcus aureus identified using a proteomic approach. FEMS Microbiol Lett 199: 79–84. [DOI] [PubMed] [Google Scholar]
- 20. Singh K, Singh VK (2012) Expression of Four Methionine Sulfoxide Reductases in Staphylococcus aureus. Int J Microbiol 2012: 719594 10.1155/2012/719594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mei JM, Nourbakhsh F, Ford CW, Holden DW (1997) Identification of Staphylococcus aureus virulence genes in a murine model of bacteraemia using signature-tagged mutagenesis. Mol Microbiol 26: 399–407. [DOI] [PubMed] [Google Scholar]
- 22. Singh VK, Moskovitz J, Wilkinson BJ, Jayaswal RK (2001) Molecular characterization of a chromosomal locus in Staphylococcus aureus that contributes to oxidative defence and is highly induced by the cell-wall-active antibiotic oxacillin. Microbiology 147: 3037–3045. [DOI] [PubMed] [Google Scholar]
- 23. Singh VK (2014) Lack of a functional methionine sulfoxide reductase (MsrB) increases antibiotic and oxidative stress tolerance and enhances pigmentation in Staphylococcus aureus. Can J Microbiol 60: 625–628. 10.1139/cjm-2014-0360 [DOI] [PubMed] [Google Scholar]
- 24. Mead DA, Szczesna-Skorupa E, Kemper B (1986) Single-stranded DNA ‘blue’ T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng 1: 67–74. [DOI] [PubMed] [Google Scholar]
- 25. Horsburgh MJ, Aish JL, White IJ, Shaw L, Lithgow JK, et al. (2002) sigmaB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325–4. J Bacteriology 184: 5457–5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ryffel C, Bucher R, Kayser FH, Berger-Bachi B (1991) The Staphylococcus aureus mec determinant comprises an unusual cluster of direct repeats and codes for a gene product similar to the Escherichia coli sn-glycerophosphoryl diester phosphodiesterase. J Bacteriol 173: 7416–7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Singh VK, Schmidt JL, Jayaswal RK, Wilkinson BJ (2003) Impact of sigB mutation on Staphylococcus aureus oxacillin and vancomycin resistance varies with parental background and method of assessment. Int J Antimicrob Agents 21: 256–261. [DOI] [PubMed] [Google Scholar]
- 28. Singh VK, Utaida S, Jackson LS, Jayaswal RK, Wilkinson BJ, et al. (2007) Role for dnaK locus in tolerance of multiple stresses in Staphylococcus aureus. Microbiology 153: 3162–3173. [DOI] [PubMed] [Google Scholar]
- 29. Ayala A, Parrado J, Bougria M, Machado A (1996) Effect of oxidative stress, produced by cumene hydroperoxide, on the various steps of protein synthesis. Modifications of elongation factor-2. J Biol Chem 271: 23105–23110. [DOI] [PubMed] [Google Scholar]
- 30. Smyth DG, Blumenfeld OO, Konigsberg W (1964) Reactions of N-ethylmaleimide with peptides and amino acids. Biochem J 91: 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Atack JM, Kelly DJ (2008) Contribution of the stereospecific methionine sulphoxide reductases MsrA and MsrB to oxidative and nitrosative stress resistance in the food-borne pathogen Campylobacter jejuni. Microbiology 154: 2219–2230. 10.1099/mic.0.2008/019711-0 [DOI] [PubMed] [Google Scholar]
- 32. Marshall JH, Wilmoth GJ (1981) Pigments of Staphylococcus aureus, a series of triterpenoid carotenoids. J Bacteriology 147: 900–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vaish M, Singh VK (2013) Antioxidant Functions of Nitric Oxide Synthase in a Methicillin Sensitive Staphylococcus aureus. Int J Microbiol 2013: 312146 10.1155/2013/312146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Voyich JM, Braughton KR, Sturdevant DE, Whitney AR, Said-Salim B, et al. (2005) Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J Immunology 175: 3907–3919. [DOI] [PubMed] [Google Scholar]
- 35. Liang X, Yu C, Sun J, Liu H, Landwehr C, et al. (2006) Inactivation of a two-component signal transduction system, SaeRS, eliminates adherence and attenuates virulence of Staphylococcus aureus. Infect Immun 74: 4655–4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Singh VK, Hattangady DS, Giotis ES, Singh AK, Chamberlain NR, et al. (2008) Insertional inactivation of branched-chain alpha-keto acid dehydrogenase in Staphylococcus aureus leads to decreased branched-chain membrane fatty acid content and increased susceptibility to certain stresses. Appl Environ Microbiol 74: 5882–5890. 10.1128/AEM.00882-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Augustin J, Rosenstein R, Wieland B, Schneider U, Schnell N, et al. (1992) Genetic analysis of epidermin biosynthetic genes and epidermin-negative mutants of Staphylococcus epidermidis. Eur J Biochem 204: 1149–1154. [DOI] [PubMed] [Google Scholar]
- 38. Brady RA, Leid JG, Camper AK, Costerton JW, Shirtliff ME (2006) Identification of Staphylococcus aureus proteins recognized by the antibody-mediated immune response to a biofilm infection. Infect Immun 74: 3415–3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ezraty B, Aussel L, Barras F (2005) Methionine sulfoxide reductases in prokaryotes. Biochim Biophys Acta 1703: 221–229. [DOI] [PubMed] [Google Scholar]
- 40. Bong SM, Kwak GH, Moon JH, Lee KS, Kim HS, et al. (2010) Structural and kinetic analysis of free methionine-R-sulfoxide reductase from Staphylococcus aureus: conformational changes during catalysis and implications for the catalytic and inhibitory mechanisms. J Biol Chem 285: 25044–25052. 10.1074/jbc.M110.103119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moskovitz J, Bar-Noy S, Williams WM, Requena J, Berlett BS, et al. (2001) Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Nat Acad Sci U S A 98: 12920–12925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moskovitz J, Berlett BS, Poston JM, Stadtman ER (1997) The yeast peptide-methionine sulfoxide reductase functions as an antioxidant in vivo. Proc Nat Acad Sci U S A 94: 9585–9589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Moskovitz J, Rahman MA, Strassman J, Yancey SO, Kushner SR, et al. (1995) Escherichia coli peptide methionine sulfoxide reductase gene: regulation of expression and role in protecting against oxidative damage. J Bacteriol 177: 502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Clauditz A, Resch A, Wieland KP, Peschel A, Gotz F (2006) Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect Immun 74: 4950–4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lan L, Cheng A, Dunman PM, Missiakas D, He C (2010) Golden pigment production and virulence gene expression are affected by metabolisms in Staphylococcus aureus. J Bacteriol 192: 3068–3077. 10.1128/JB.00928-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu GY, Essex A, Buchanan JT, Datta V, Hoffman HM, et al. (2005) Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J Exp Med 202: 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mishra NN, Liu GY, Yeaman MR, Nast CC, Proctor RA, et al. (2011) Carotenoid-related alteration of cell membrane fluidity impacts Staphylococcus aureus susceptibility to host defense peptides. Antimicrob Agents Chemother 55: 526–531. 10.1128/AAC.00680-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kuboniwa M, Tribble GD, James CE, Kilic AO, Tao L, et al. (2006) Streptococcus gordonii utilizes several distinct gene functions to recruit Porphyromonas gingivalis into a mixed community. Mol Microbiol 60: 121–139. [DOI] [PubMed] [Google Scholar]
- 49. Lei Y, Zhang Y, Guenther BD, Kreth J, Herzberg MC (2011) Mechanism of adhesion maintenance by methionine sulphoxide reductase in Streptococcus gordonii. Mol Microbiol 80: 726–738. 10.1111/j.1365-2958.2011.07603.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee WL, Gold B, Darby C, Brot N, Jiang X, et al. (2009) Mycobacterium tuberculosis expresses methionine sulphoxide reductases A and B that protect from killing by nitrite and hypochlorite. Mol Microbiol 71: 583–593. 10.1111/j.1365-2958.2008.06548.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Romsang A, Atichartpongkul S, Trinachartvanit W, Vattanaviboon P, Mongkolsuk S (2013) Gene expression and physiological role of Pseudomonas aeruginosa methionine sulfoxide reductases during oxidative stress. J Bacteriol 195: 3299–3308. 10.1128/JB.00167-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhao C, Bizzini A, Zhang X, Sauvageot N, Hartke A (2013) Mutations in msrA and msrB, encoding epimer-specific methionine sulfoxide reductases, affect expression of glycerol-catabolic operons in Enterococcus faecalis differently. Microbiology 159: 615–620. 10.1099/mic.0.065037-0 [DOI] [PubMed] [Google Scholar]
- 53. Dhandayuthapani S, Jagannath C, Nino C, Saikolappan S, Sasindran SJ (2009) Methionine sulfoxide reductase B (MsrB) of Mycobacterium smegmatis plays a limited role in resisting oxidative stress. Tuberculosis (Edinb) 89 Suppl 1: S26–32. 10.1016/S1472-9792(09)70008-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kreiswirth BN, Lofdahl S, Betley MJ, O’Reilly M, Schlievert PM, et al. (1983) The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305: 709–712. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.