Abstract
Objective
To compare AMH levels among three commercially available AMH immunoassays (AMH Gen II, Beckman Coulter; Ultrasensitive AMH, AnshLab; picoAMH, AnshLab)
Design
Cross-sectional
Setting
Academic reproductive endocrinology program
Patients
90 newly diagnosed breast cancer patients prior to cancer treatment
Interventions
None
Outcome
1) proportion of detectable AMH levels by immunoassay, 2) comparability among assays
Results
At a mean age of 38.1, the median (interquartile range) for AMH levels for the cohort were 0.92 [1.35] ng/mL for the Gen II assay, 1.68 [2.30] ng/mL for the Ultrasensitive and 1.5 [2.41] ng/mL for the picoAMH assays. Significantly higher proportions of detectable AMH levels were observed with the picoAMH kit (97%) compared to both Gen II (84%) and Ultrasensitive (92%) assays. Although AMH results were highly correlated among assays (r=0.92–0.99), Gen II AMH levels were consistently lower than both Ultrasensitive and picoAMH levels. Moreover, as AMH levels increased, the magnitude of difference grew larger between Gen II and each of the other two assays.
Conclusions
Measurement of AMH levels with the picoAMH kit maximized detection at very low levels, particularly in contrast to the Gen II kit. Conversion of AMH levels from different immunoassays using regression equations is potentially highly inaccurate.
Key terms: AMH, immunoassay, breast cancer
Introduction
Anti-Mullerian hormone (AMH), which is also known as Mullerian inhibiting substance and Mullerian inhibiting factor, is a 140-kDa dimeric glycoprotein that belongs to the transforming growth factor-beta family of growth and differentiation factors. AMH is produced by ovarian granulosa cells, where it regulates germ cell development.(1) AMH expression begins in primary follicles and is maximal in the early antral stage.(2) Prior to secretion, monomers containing an N-terminal domain (the “pro” region) and a C-terminal domain (the “mature” region) dimerize. AMH expression and secretion cease when follicles reach a diameter of 8–10 mm.(2) Thus, AMH levels indicate the size of the cohort of small growing follicles.
In recent years, measurement of AMH has become increasingly important in clinical practice and epidemiologic research. AMH measurements are used to predict ovarian response in assisted reproduction, assess ovarian reserve, and monitor patients with a history of granulosa cell tumors.(3–5) In addition, research studies have investigated AMH as a marker of fecundability, polycystic ovarian syndrome, menopausal transition, and the effects of cancer treatment on ovarian reserve.(6–9) As a result of these associations with reproductive outcomes and the relative stability of levels across menstrual cycles in a given woman, measurement of AMH has gained widespread clinical and research application.(10, 11)
AMH assays have evolved over the same time span. Until one year ago, there were three commercial ELISA kits used to measure AMH that differed in antibody pairs, standard curve ranges and limits of detection (Table 1). The first kit was introduced in 1999 by Immunotech (IOT, Marseille, France). The IOT assay used a monoclonal antibody pair, one directed at the pro-region and the other at the mature region. The second AMH kit was launched in 2003 by Diagnostic Systems Laboratories (DSL, Webster, TX). In the DSL ELISA, both monoclonal antibodies were directed at the mature region to minimize proteolysis. In 1997, IOT became part of newly created Beckman-Coulter, Inc., and in 2005 this company acquired DSL. Both the IOT and DSL AMH kits continued to be available until 2010 when Beckman Coulter developed a second generation (Gen II) AMH ELISA kit, in which the antibodies from the DSL kit were used with the IOT standards.(12)
Table 1.
Overview of commercial AMH assays
| Assay | Manufacturer | Standard Curve Range | Level of Detection |
|---|---|---|---|
| IOT | Immunotech, Marseille, France | 0.1 – 24.5 ng/ml | 0.05 ng/ml |
| DSL | Diagnostic Systems Laboratories, Webster, TX | 0.05 – 15 ng/ml | 0.006 ng/ml |
| Gen II | Beckman Coulter, Brea, CA | 0.16 – 22.5 ng/ml | 0.08 ng/ml |
| Ultrasensitive | Ansh Labs, Webster, TX | 0.1 – 14 ng/ml | 0.07 ng/ml |
| picoAMH | Ansh Labs, Webster, TX4 | 6 – 746 pg/ml | 0.01 ng/ml |
Very recently, two commercially available AMH ELISA kits have been developed by Ansh Labs (Webster, TX) (Table 1). These two kits use the same monoclonal antibody pair directed against specific linear epitopes in the stable pro-region and mature region of the associated form of human recombinant AMH and appear to have high accuracy in initial testing(13). The Ultrasensitive AMH ELISA kit was released in 2012 and the picoAMH ELISA kit was released in 2013. To date, there are limited data on the performance of the Ultrasensitive and picoAMH assays.(14)
The availability of multiple commercial AMH assays as well as the prediction of clinical outcomes using data derived from different assays pose a challenge to researchers and clinicians alike, who need to understand how AMH levels measured by different assays can be combined for research or interpreted in the context of established clinical cut-points. In recent studies, some investigators have generated conversion factors for AMH levels between different assays by linear regression methods in order to combine data across study cohorts.(15–20) The validity of this analytic approach is not clear.
AMH is a marker of interest in young breast cancer survivors because pre-chemotherapy AMH levels may predict post-chemotherapy ovarian function, and a rise in levels after chemotherapy appears to reflect ovarian recovery.(21) In this setting, an assay with discrimination at low AMH levels would be ideal. Moreover, the ability to compare or combine AMH results across small, existing cohorts of breast cancer patients would facilitate research efforts. Therefore, the objectives of this study were two-fold. First, we tested if the Ultrasensitive and picoAMH assays were more sensitive than the Gen II assay in detecting low AMH levels in reproductive-aged women with a new diagnosis of breast cancer. Second, we analyzed the relationship among these three AMH assays to determine comparability.
Materials and Methods
A cross-sectional study was performed to compare AMH levels using three commercially available AMH immunoassays in serum samples from newly diagnosed breast cancer patients. STROBE guidelines for cross-sectional studies have been followed in the preparation of the manuscript. Participants were identified by systematic medical record screening of all new breast cancer patients at breast clinics at the University of California San Diego (UCSD) and University of Pennsylvania (Penn) between 2009 and 2012 and approached to enroll in a prospective cohort study of ovarian function and breast cancer. Eligibility criteria included age 18–45, early stage breast cancer (American Joint Committee on Cancer Stages I-III), presence of a uterus and at least one ovary, and premenopausal status, defined by at least one menses over the prior 12 months. Pregnancy, breastfeeding, use of psychotropic drugs known to impact ovulation, and history of prior cancer, chemotherapy or pelvic radiation were exclusion criteria. Institutional review board approval at the University of California San Diego, University of Pennsylvania and University of Southern California was obtained for the study.
Eligible patients were enrolled, completed a study questionnaire, and underwent a blood draw prior to chemotherapy. Due to urgency in starting chemotherapy, enrollment blood specimens were drawn across the menstrual cycle and not timed to the early follicular phase. Specimens were processed for serum and frozen in aliquots at −80°C until assayed for AMH. The study questionnaire obtained self-reported demographic, medical and reproductive health history. Cancer-related data were abstracted from medical records.
Assays
Study samples were assayed for AMH using each of three immunoassays (Gen II, Ultrasensitive and picoAMH) at the University of Southern California Reproductive Endocrine Research Laboratory. In general, the procedures for the three different assays were similar. All are two-site immunoassays carried out in wells of microtiter plates coated with anti-AMH antibody in 3 steps. In the first step, calibration, controls and samples were added to the wells and incubated. In the second step, anti-AMH antibody labeled with biotin was added to each well and incubated. The third step involved addition of the substrate, tetramethylbenzidine, followed by an acidic stopping solution. The degree of enzymatic turnover is determined by dual wavelength absorbance measurement at 450 nm (primary test filter) and 630 nm (reference filter). The absorbance measured was directly proportional to the AMH concentration.
There were important differences among the three assays. The Beckman-Coulter assay used a 20 µl aliquot of sample, which required dilution with assay buffer and incubation before adding it to the well; bovine serum AMH was used for the calibrators; the assay utilized a pair of monoclonal antibodies that bind to the mature region of AMH. In contrast, the Ansh Labs Ultrasensitive assay used a 25 µl of sample, with no prior dilution step, and recombinant human AMH was used for the calibrators. Monoclonal antibodies were also used, but one is specific for the mid pro-region of AMH, whereas the detection antibody was specific for the mature region of AMH. The picoAMH assay used the same antibodies as the Ultrasensitive assay and the same calibrator source, however, a 100 µl aliquot of sample was required and the standard curve range was considerably lower to accommodate a much lower AMH level of detection. The standard curve ranges and the levels of detection for the 3 assays are shown in Table 1. The inter-assay coefficients of variation are as follows: 5.6% and 4.5% at 4.42 and 14.0 ng/ml, respectively (Beckman-Coulter); 4.6%, 4.8%, 2.0% at 0.346, 0.715 and 1.85 ng/ml, respectively (Ultrasensitive); 4.5%, 2.2%, 3.8% at 22.6, 86.5 and 373 pg/ml, respectively (picoAMH).
Statistical Methods
Analyses were performed using STATA (Release 12, Stata Corporation, College Station, TX). Baseline characteristics were summarized by frequencies and proportions, or means, medians, standard deviations and range, as appropriate. Values below detection thresholds were given half of the threshold value in analyses. AMH levels were analyzed first as untransformed values, then as natural log-transformed values in order to stabilize variance. Spearman correlation and linear regression were used to describe the relationship between each assay. Statistical tests of the regression coefficients were used to evaluate bias between the 2 assays. A fixed bias exists when the constant or intercept term in the model is significantly different form zero. Proportional bias exists when the slope of the regression equation is significantly different from one. Passing Bablok and Bland-Altman (BA) plots were used to compare assays graphically to assess bias and whether the variability in measures is homoscedastic, i.e., constant over the range of values.
Results
A total of 90 women with a mean (SD) age of 38.3 (5.1) years who were newly diagnosed with breast cancer were included.(22) Table 2 depicts their baseline clinical and reproductive characteristics. Median [IQR] AMH levels were 0.92 [1.35] ng/mL for the Gen II assay, 1.68 [2.30] ng/mL for the Ultrasensitive assay, and 1.52 [2.41] ng/mL for the picoAMH assay. The majority of samples required dilution prior to using the picoAMH assay. AMH levels were above the limit of detection for 97% of samples using the picoAMH assay, compared to 84% with the Gen II assay (p<0.001) and 92% with the Ultrasensitive assay (p=0.045).
Table 2.
Baseline cohort characteristics of 90 breast cancer patients
| Characteristic | |
|---|---|
| Age, mean (SD) | 38.3 (5.1) |
| Race, n(%) | |
| White | 65 (72) |
| Black | 15 (17) |
| Other | 10 (11) |
| BMI, mean (SD) | 25.1 (6.5) |
| Periods past year, n(%) | |
| >10 | 79 (87) |
| 4–9 | 4 (5) |
| 1–3 | 2 (2) |
| Current smoking, n(%) | 4 (4) |
| Cancer Stage, n(%) | |
| I | 24 (27) |
| II | 47 (52) |
| III | 19 (21) |
| Cancer histology, n(%) | |
| Ductal | 82 (91) |
| Lobular | 3 (3) |
| Mixed | 5 (5) |
| Gen II | |
| Median (IQR) | 0.92 (0.38–1.73) |
| Number detectable (>0.17 ng/mL), n(%) | 76 (84) |
| Ultrasensitive | |
| Median (IQR) | 1.68 (0.99–3.29) |
| Number detectable (>0.07 ng/mL), n(%) | 83 (92) |
| picoAMH | |
| Median (IQR) | 1.52 (0.80–3.22) |
| Number detectable (>0.01 ng/mL), n(%) | 87 (97) |
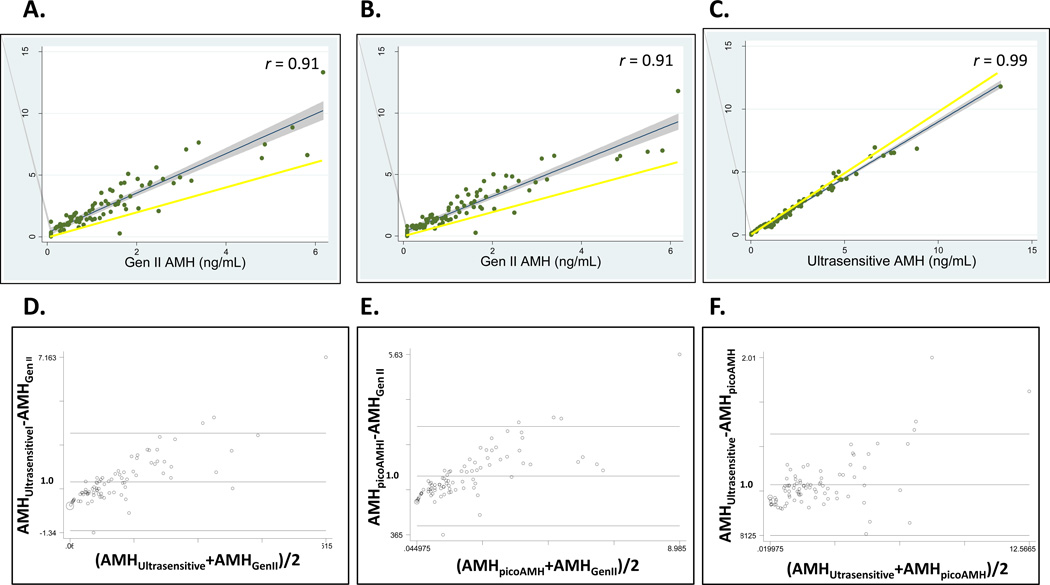
Using untransformed AMH levels, Gen II assay results were highly correlated with both Ultrasensitive and picoAMH assay results (Spearman rho=0.92, p<0.001, for both). The correlation coefficient between Ultrasensitive and picoAMH assay levels was 0.99 (p<0.001). Table 3 depicts equations from linear regression models that describe the relationship of untransformed AMH levels between pairs of assays. In each of the three comparisons, the slope is significantly different from 1 (all p<0.001), indicating the need to use a conversion factor to compare values from two different assays. Second, the constants or intercepts are significantly different from 0 in the models relating Gen II results to either Ultrasensitive or picoAMH results. This finding indicates that a systematic bias is present between the two assays. This fixed bias should also be taken into account in converting AMH values between assays. Therefore, using these equations, a measurement of 1.0 ng/mL by the Gen II kit would “convert” to an Ultrasensitive kit value of 1.94 ng/mL or a picoAMH kit value of 1.77 ng/mL.
Table 3.
Linear regression equations that describe the relationship between AMH results from different immunoassays
| Comparison | Untransformed AMH levels | Log-transformed AMH levels | ||||
|---|---|---|---|---|---|---|
| Regression Equation | Slope p-valuea |
Constant p-value |
Regression Equation | Slope p-valuea |
Constant p-value |
|
| Gen II and Ultrasensitive | AMHUltrasensitive=(1.60*AMHGenII)+0.34 | <0.001 | 0.01 | AMHUltrasensitive=e([1.02* logAMHGenII]+0.60) | 0.61 | <0.001 |
| Gen II and picoAMH | AMHpicoAMH=(1.45*AMHGenII)+0.32 | <0.001 | 0.005 | AMHpicoAMH=e([1.05* logAMHGenII]+0.50) | 0.48 | <0.001 |
| Ultrasensitive and picoAMH | AMHpicoAMH=(0.89*AMHUltrasensitive)+0.05 | <0.001 | 0.26 | AMHpicoAMH=e([0.89* logAMHUltrasensitive]+0.13) | <0.001 | 0.003 |
testing the hypothesis that the slope is equal to or not equal to 1.
Passing Bablock and Bland-Altman (BA) plots graphically demonstrate the relationship between pairs of assays across the range of untransformed AMH values (Figure 1) allowing for an assessment of bias as well as the variability in measurements. Passing Bablock plots, which depict the linear regression line and 95% CI relative to a slope of 1, show that Gen II AMH values were consistently lower than those from the Ultrasensitive assay (Figure 1A) or picoAMH assay (Figure 1B). In contrast, Ultrasensitive and picoAMH assay values were more similar than the other 2 comparisons, with deviations primarily at higher levels (Figure 1C). Bland-Altman (BA) plots depict the difference in AMH values relative to the mean of the two assays to help determine systematic bias and whether it is uniform across the range of results. BA plots show that as mean AMH levels increase, the differences between the two values become larger (Figures 1D–F). Linear regression models of the difference in AMH values compared to the mean confirm proportional bias in all three comparisons (all p<0.001). In addition to fixed bias, the variability in measurements increased with the mean values, indicating that conversion at higher AMH levels would be less precise. For example, using the regression equation, a Gen II AMH level of 0.19 ng/mL would convert to an Ultrasensitive AMH level of 0.65 ng/mL with a 95% CI of 0.41–0.88, which is a difference of 0.47 ng/ml in the CI range. The measured Ultrasensitive AMH level in the same sample was 0.71 ng/mL. In contrast, a Gen II AMH level of 6.2 ng/mL would convert to an Ultrasensitive AMH level of 10.2 ng/mL (95% CI, 9.5–10.9), where the true level was 13.3 ng/mL. Here the 95% CI range difference is 1.4 ng/mL, which is much wider than at lower values.
Figure 1.
Passing and Bablok plots and fit analysis, which depict the linear regression line and 95% CI relative to a slope of 1, compare untransformed Gen II and Ultrasensitive AMH assay results (A); Gen II AMH and picoAMH assay results (B); Ultrasensitive AMH and picoAMH assay results (C). Spearman correlation coefficients (r) are shown for comparisons. Bland-Altman plots, which depict the difference in AMH values relative to the mean of the two assays, compare Gen II and Ultrasensitive AMH assay results (D); Gen II AMH and picoAMH assay results (E); Ultrasensitive AMH and picoAMH assay results (F).
The same statistical approach was undertaken using log-transformed AMH levels to obtain variability that is more constant throughout the range of values. Linear regression equations that describe the relationship of log-transformed AMH levels between pairs of assays show a fixed bias in all three comparisons (constant p-values of 0.003 to <0.001) (Table 3). This indicates the need to account for the constant in converting values between assays. However, the slopes of comparisons with Gen II values were not significantly different from 1. Supplemental Table demonstrates that proportional bias may be corrected by log-transformed AMH levels. However, precision is lost with increasing AMH values. For example, a Gen II AMH level of 0.19 ng/mL would convert to an Ultrasensitive AMH level of 0.33 ng/mL (95% CI, 0.27–0.40), which is a difference of 0.13 ng/ml in the CI range when using the equation derived from log-transformed levels. A Gen II AMH level of 6.2 ng/mL would convert to an Ultrasensitive AMH level of 11.9 ng/mL (95% CI, 9.1–15.6), which is a CI difference of 6.5 ng/ml. This difference is significantly larger than with untransformed AMH levels.
Discussion
In reproductive-aged women who were newly diagnosed with breast cancer, the new picoAMH immunoassay maximized detection of AMH at low levels and was more sensitive than either the Ultrasensitive or Gen II immunoassays. Although correlations between assays were high and the relationships between kits were linear, the magnitude of the difference between the predicted value from linear regression and the actual AMH value grows significantly larger with higher AMH values, rendering conversions using the regression equations potentially highly inaccurate.
Several analytic approaches were used to compare AMH levels from three commercially available immunoassays. Although correlation coefficients between pairs of assays were high, by nature of the computation correlation coefficients should be high and published reports corroborate this.(16, 17) However reassuring, correlations do not directly address comparability of levels. Linear regression models were undertaken to estimate the relationship between pairs of assays. If the results of two assays were directly comparable, the slope of the regression line would be equal to 1, and the constant/error term would not differ from 0. Results using untransformed AMH levels showed that results from all three assay pairs (Gen II vs. Ultrasensitive, Gen II vs. picoAMH, and Ultrasensitive vs. picoAMH) were not directly comparable. Then, graphic depictions by Passing Blalock and Bland Altman plots were undertaken and showed proportional bias, i.e., loss of precision as values increased in addition to the systematic differences between assay values. Using log-transformed AMH levels did not improve precision. Taken together, because loss of precision occurs at higher AMH levels when using regression equations to compare values from two immunoassays, our results do not support using regression equations to convert AMH levels among Gen II, Ultrasensitive and picoAMH assays for research or clinical purposes.
Prior studies commonly report correlation coefficients and linear regression equations to compare AMH levels measured in the same serum sample by different immunoassays. Problematically, studies have reported widely different regression equations that describe AMH levels assayed by both DSL and IOT assays14,15,16–18. In those studies, both regression coefficients (range, 0.25–0.91) and error terms (range, 0.03–0.29 ng/mL) vary considerably. For example, if a DSL assay measured an AMH level of 1.0 ng/mL, this could “translate” to IOT values ranging from 0.78 ng/ml(18) to 4.98(16) ng/mL, or 22% lower to 498% higher. Most do not report the evaluation for fixed or proportional bias. Ignoring fixed bias by multiplying one assay value by the regression coefficient to convert it to the “new” metric without accounting for a significant constant term could result in inaccuracies.(19) Ideally, studies would have large enough sample sizes so that the precision (CI) is tight enough for clinical comparability.
The lack of reproducibility of conversion factors between assays was also not surprising given that use of different antibody pairs may measure different populations of AMH molecules. For example, the regression equations comparing DSL to Gen II assays in two different studies appear more similar (20, 23). An AMH value of 1.0 ng/mL measured by the DSL assay would convert to Gen II assay AMH values of 0.79 to 1.05 ng/mL. This would be expected given the use of the same antibody pair in the two assays. Moreover, lack of an international AMH standard, heterogeneity of study populations, small sample sizes and potential matrix effects all contribute to the noted discrepancies. For these reasons, while internal assay validity has been demonstrated when samples are measured using the same commercial AMH ELISA kit, the challenge of converting AMH levels from one kit to another requires careful analysis that takes into account two components. The first is to test if the intercept of regression equation is significantly different from 0. If so, then there is a systematic or fixed difference between the two assays that is independent of the hormone level, and this difference needs to be taken into account in the mathematical conversions. Second, variation between the predicted and true AMH levels should be uniform across the range of values. If not, then the precision of the conversion will not be uniform across the range of observable values. Log-transformation of hormone levels prior to regression may stabilize this variability.(24, 25) Therefore, for investigators to convert AMH levels between assays, the statistical validation of the approach must be clear and based on a large enough sample to accurately estimate true levels with adequate precision. For clinicians who wish to interpret AMH cutpoints using results from a different assay, current data to support doing so are lacking. Another important consideration is the clinical relevance of reference ranges provided with AMH results from a clinical laboratory. Generally, reported reference ranges are based on small sample sizes and do not reflect validation against clinically relevant outcomes such as poor response to ovarian stimulation. Hence, larger scale clinical research studies are conducted to determine diagnostic levels and reported in scientific literature to guide clinical decisions. Based on validity and reliability of diagnostic levels reported across studies, clinical providers must then make decisions on how to interpret AMH results. The goal of this work is to show how diagnostic levels reported in the scientific literature will vary significantly by assay.
With regard to assay interference, an additional issue pertaining to the AMH Gen II ELISA kit arose when Beckman Coulter notified their customers in June and August, 2013 that AMH measured in undiluted serum samples with their kit may generate results that are lower than expected due to interference from complement and recommended using a premix procedure to evaluate potential matrix effects. A previous product notification by the manufacturer several months earlier indicated that dilution of samples prior to running the assay may exceed the expected values by 2-fold. In the present product notifications, the kit manufacturer points out that the magnitude of the decreased AMH levels in undiluted samples is dependent on the samples and sample storage conditions, and that freshly drawn or freshly frozen samples have a higher risk of complement interference.
Finally, while we demonstrate higher sensitivity using the Ultrasensitive and picoAMH assays, further studies are needed to determine clinical utility. In this dataset, there was a small subset of participants who underwent a pelvic ultrasound (n=22), in whom the relationship between antral follicle counts (AFC) and AMH levels from the three immunoassays was explored. Ultrasounds were performed with Voluson E6 or E8 (GE), and AFC (total follicles 2–10 mm in diameter) were obtained using 4Dview (GE). Images were analyzed by two readers. Mean AFC (SD) was 16.4 (12.8) for the 22 participants, mean age (SD) was 37.3 (4.0). By Spearman correlation, AFC was highly correlated with Gen II (r=0.69, p<0.001), Ultrasensitive (r=0.82, p<0.001), picoAMH (r=0.80, p<0.001). Dichotomizing AFC to ≤ 6 and >6 (common cutpoint for decreased ovarian reserve), 4 participants had low AFC. Using Student’s t-test to compare log-transformed AMH, AMH levels were significantly lower in participants with AFC ≤ 6 when measured with Ultrasensitive (p=0.03) and picoAMH (p=0.03) assays, but no different by AFC category with the Gen II assay (p=0.30). Compared to the group with AFC >6, the low follicle group had geometric mean AMH levels that were 77% lower using the Ultrasensitive assay and 82% lower using the picoAMH assay. Larger studies are required to confirm these pilot data. In addition, AMH levels from all three assays did not correlate with body size (data not shown), but the proportion of obese women in our population was small (20%). We speculate potential clinical relevance in settings where current assays cannot distinguish below relatively higher levels of detection, such as predicting low response to ovarian stimulation or delineating menopausal transition. Appropriately designed studies are needed to demonstrate clinical utility of these new assays.
In conclusion, in view of the importance of serum AMH measurements in clinical and research settings, there is a critical need for accurate and reproducible AMH assays, as well as development of an international AMH standard, to inform generalizability of findings. Use of published conversion factors to normalize AMH values previously obtained with the one kit so that they are consistent with values obtained using a different kit appear to be inappropriate for both routine clinical practice and research studies without appropriate statistical validation. In the clinical setting, use of such factors could result in patients being allocated to the wrong ovarian reserve group based on AMH cut-off levels and incurring false reassurance or concern. Gonadotropin dosing based on AMH levels in the setting of assisted reproduction may be misinformed. In addition, clinicians may encounter difficulties in interpreting changes in ovarian reserve over time if samples from the same individual are measured by different kits. Unfortunately there has been a great deal of uncertainty and confusion generated from the recent product notifications by Beckman Coulter regarding the AMH Gen II assay methodology. Thus, problems with the Gen II kit should be thoroughly investigated so that one can be confident in the AMH levels obtained with the kit. Furthermore, the newly developed Ansh ELISAs may provide the accuracy and sensitivity that is lacking in prior AMH assays, and their performance should be investigated in clinical and research settings.
Supplementary Material
Passing and Bablok plot and fit analysis, which depict the linear regression line and 95% CI relative to a slope of 1, compare log-transformed Gen II and Ultrasensitive AMH assay results (A). Spearman correlation coefficient (r) is shown in the comparison. Bland-Altman plot, which depicts the difference in AMH values relative to the mean of the two assays, compares Gen II and Ultrasensitive AMH assay results (B).
Acknowledgments
The authors wish to thank Ansh Labs for donating supplies and providing technical advice on the picoAMH and Ultrasensitive assays.
Support: HD058799 (HIS), American Cancer Society MRSG-08-110-01-CCE (HIS)
Footnotes
Conflicts of interest: Swiss Precision Diagnostics (MDS)
References
- 1.Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, et al. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology. 1999;140:5789–5796. doi: 10.1210/endo.140.12.7204. [DOI] [PubMed] [Google Scholar]
- 2.Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, et al. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 3.Fanchin R, Schonauer LM, Righini C, Frydman N, Frydman R, Taieb J. Serum anti-Mullerian hormone dynamics during controlled ovarian hyperstimulation. Hum Reprod. 2003;18:328–332. doi: 10.1093/humrep/deg043. [DOI] [PubMed] [Google Scholar]
- 4.Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12:685–718. doi: 10.1093/humupd/dml034. [DOI] [PubMed] [Google Scholar]
- 5.Rey R, Sabourin JC, Venara M, Long WQ, Jaubert F, Zeller WP, et al. Anti-Mullerian hormone is a specific marker of sertoli- and granulosa-cell origin in gonadal tumors. Human pathology. 2000;31:1202–1208. doi: 10.1053/hupa.2000.18498. [DOI] [PubMed] [Google Scholar]
- 6.Steiner AZ, Herring AH, Kesner JS, Meadows JW, Stanczyk FZ, Hoberman S, et al. Antimullerian hormone as a predictor of natural fecundability in women aged 30–42 years. Obstet Gynecol. 2011;117:798–804. doi: 10.1097/AOG.0b013e3182116bc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, et al. Executive summary of the stages of reproductive aging workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97:1159–1168. doi: 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su HI, Sammel MD, Green J, Velders L, Stankiewicz C, Matro J, et al. Antimullerian hormone and inhibin B are hormone measures of ovarian function in late reproductive-aged breast cancer survivors. Cancer. 2010;116:592–599. doi: 10.1002/cncr.24746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pigny P, Merlen E, Robert Y, Cortet-Rudelli C, Decanter C, Jonard S, et al. Elevated serum level of anti-mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88:5957–5962. doi: 10.1210/jc.2003-030727. [DOI] [PubMed] [Google Scholar]
- 10.Hehenkamp WJ, Looman CW, Themmen AP, de Jong FH, Te Velde ER, Broekmans FJ. Anti-Mullerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91:4057–4063. doi: 10.1210/jc.2006-0331. [DOI] [PubMed] [Google Scholar]
- 11.Tsepelidis S, Devreker F, Demeestere I, Flahaut A, Gervy C, Englert Y. Stable serum levels of anti-Mullerian hormone during the menstrual cycle: a prospective study in normo-ovulatory women. Hum Reprod. 2007;22:1837–1840. doi: 10.1093/humrep/dem101. [DOI] [PubMed] [Google Scholar]
- 12.Kumar A, Kalra B, Patel A, McDavid L, Roudebush WE. Development of a second generation anti-Mullerian hormone (AMH) ELISA. J Immunol Methods. 2010;362:51–59. doi: 10.1016/j.jim.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Attaelmannan M, Pandian R, Sadril M, Thomassian K, Khachatryan S, Kumar A. Development of a broad-range, sensitive, and specific immunoassay for anti-mullerian hormone (AMH) with no dilution issues; American Association for Clinical Chemistry Annual Meeting; 2013. [Google Scholar]
- 14.Visser JA, Laven JS, McLuskey A, Louwers YV, van Dorp W, Themmen APN, et al. Development of a well characterized ultra-sensitive human anti-mullerian hormone chemiluminescence assay: evaluation of potential clinical applications; Endocrine Society annual meeting; 2013. [Google Scholar]
- 15.Bersinger NA, Wunder D, Birkhauser MH, Guibourdenche J. Measurement of anti-mullerian hormone by Beckman Coulter ELISA and DSL ELISA in assisted reproduction: differences between serum and follicular fluid. Clin Chim Acta. 2007;384:174–175. doi: 10.1016/j.cca.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Freour T, Mirallie S, Bach-Ngohou K, Denis M, Barriere P, Masson D. Measurement of serum anti-Mullerian hormone by Beckman Coulter ELISA and DSL ELISA: comparison and relevance in assisted reproduction technology (ART) Clin Chim Acta. 2007;375:162–164. doi: 10.1016/j.cca.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 17.Lee JR, Kim SH, Jee BC, Suh CS, Kim KC, Moon SY. Antimullerian hormone as a predictor of controlled ovarian hyperstimulation outcome: comparison of two commercial immunoassay kits. Fertil Steril. 2011;95:2602–2604. doi: 10.1016/j.fertnstert.2011.01.126. [DOI] [PubMed] [Google Scholar]
- 18.Streuli I, Fraisse T, Chapron C, Bijaoui G, Bischof P, de Ziegler D. Clinical uses of anti-Mullerian hormone assays: pitfalls and promises. Fertil Steril. 2009;91:226–230. doi: 10.1016/j.fertnstert.2007.10.067. [DOI] [PubMed] [Google Scholar]
- 19.Hehenkamp WJ, Volkers NA, Broekmans FJ, de Jong FH, Themmen AP, Birnie E, et al. Loss of ovarian reserve after uterine artery embolization: a randomized comparison with hysterectomy. Hum Reprod. 2007;22:1996–2005. doi: 10.1093/humrep/dem105. [DOI] [PubMed] [Google Scholar]
- 20.Wallace AM, Faye SA, Fleming R, Nelson SM. A multicentre evaluation of the new Beckman Coulter anti-Mullerian hormone immunoassay (AMH Gen II) Annals of clinical biochemistry. 2011;48:370–373. doi: 10.1258/acb.2011.010172. [DOI] [PubMed] [Google Scholar]
- 21.Dillon KE, Sammel MD, Prewitt M, Ginsberg JP, Walker D, Mersereau JE, et al. Pretreatment antimullerian hormone levels determine rate of posttherapy ovarian reserve recovery: acute changes in ovarian reserve during and after chemotherapy. Fertil Steril. 2013;99:477–483. doi: 10.1016/j.fertnstert.2012.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su HI, Flatt SW, Natarajan L, DeMichele A, Steiner AZ. Impact of breast cancer on anti-mullerian hormone levels in young women. Breast Cancer Res Treat. 2012;137:571–577. doi: 10.1007/s10549-012-2361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li HW, Ng EH, Wong BP, Anderson RA, Ho PC, Yeung WS. Correlation between three assay systems for anti-Mullerian hormone (AMH) determination. J Assist Reprod Genet. 2012;29:1443–1446. doi: 10.1007/s10815-012-9880-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludbrook J. Statistical techniques for comparing measurers and methods of measurement: a critical review. Clinical and experimental pharmacology & physiology. 2002;29:527–536. doi: 10.1046/j.1440-1681.2002.03686.x. [DOI] [PubMed] [Google Scholar]
- 25.Ludbrook J. Confidence in Altman-Bland plots: a critical review of the method of differences. Clinical and experimental pharmacology & physiology. 2010;37:143–149. doi: 10.1111/j.1440-1681.2009.05288.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Passing and Bablok plot and fit analysis, which depict the linear regression line and 95% CI relative to a slope of 1, compare log-transformed Gen II and Ultrasensitive AMH assay results (A). Spearman correlation coefficient (r) is shown in the comparison. Bland-Altman plot, which depicts the difference in AMH values relative to the mean of the two assays, compares Gen II and Ultrasensitive AMH assay results (B).