Abstract
The radiation leukemia virus (RadLV), a murine leukemia virus derived from thymic lymphomas induced by x-irradiation in strain C57BL/Ka mice, has been successfully propagated in sustained high titer in vitro in a newly established line, BL-5, of C57BL/Ka mouse-embryo fibroblasts. In addition, the production of endogenous virus, presumed to be RadLV, has been induced and sustained through multiple serial passages after treatment of BL-5 cell cultures with 5-bromodeoxyuridine. The chronically RadLV-infected subline, designated BL-5 (RadLV), sheds virus into the supernatant culture fluids that is biologically active in vitro in the XC cell plaque assay, in interference assays for focus-formation by murine sarcoma virus, and in the intracellular induction of group-specific antigens detectable by immunofluorescence, but is apparently devoid of leukemogenic activity after intrathymic inoculation into neonatal or immunosuppressed C57BL/Ka mice. Although BL-5 cells exhibited morphological alterations suggestive of transformation in vitro and gave rise to fibrosarcomatous ascites tumors after intraperitoneal inoculation with C57BL/Ka mice, the chronically infected BL-5(RadLV) cells remained normal in morphology and failed to yield fibrosarcomas in vivo.
Keywords: murine leukemia virus, thymic lymphomas, 5-bromodeoxyuridine
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Declève A., Lieberman M., Hahn G. M., Kaplan H. S. Focus formation by a murine sarcoma leukemia virus complex. II. Quantitative aspects of the interaction between radiation leukemia virus and its murine sarcoma virus pseudotype in strain C57BL mouse embryo cells. J Virol. 1970 Apr;5(4):437–445. doi: 10.1128/jvi.5.4.437-445.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEND C. Cell-free transmission in adult Swiss mice of a disease having the character of a leukemia. J Exp Med. 1957 Apr 1;105(4):307–318. doi: 10.1084/jem.105.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischinger P. J., O'Connor T. E. Radiation leukemia virus: quantitative tissue culture assay. Science. 1969 Jul 18;165(3890):306–309. doi: 10.1126/science.165.3890.306. [DOI] [PubMed] [Google Scholar]
- GINSBURG H., SACHS L. In vitro culture of a mammalian leukemia virus. Virology. 1961 Mar;13:380–382. doi: 10.1016/0042-6822(61)90165-9. [DOI] [PubMed] [Google Scholar]
- GROSS L. "Spontaneous" leukemia developing in C3H mice following inoculation in infancy, with AK-leukemic extracts, or AK-embrvos. Proc Soc Exp Biol Med. 1951 Jan;76(1):27–32. [PubMed] [Google Scholar]
- GROSS L. Attempt to recover filterable agent from x-ray induced leukemia. Acta Haematol. 1958 Jun;19(6):353–361. doi: 10.1159/000205455. [DOI] [PubMed] [Google Scholar]
- Gross L., Feldman D. G. Electron microscopic studies of radiation-induced leukemia in mice: virus release following total-body x-ray irradiation. Cancer Res. 1968 Sep;28(9):1677–1685. [PubMed] [Google Scholar]
- Hall W. T., Andresen W. F., Sanford K. K., Evans V. J., Hartley J. W. Virus particles and murine leukemia virus complement-fixing antigen in neoplastic and nonneoplastic cell lines. Science. 1967 Apr 7;156(3771):85–88. doi: 10.1126/science.156.3771.85. [DOI] [PubMed] [Google Scholar]
- Haran-Ghera N. Leukemogenic activity of centrifugates from irradiated mouse thymus and bone marrow. Int J Cancer. 1966 Jan;1(1):81–87. doi: 10.1002/ijc.2910010111. [DOI] [PubMed] [Google Scholar]
- Huebner R. J., Hartley J. W., Rowe W. P., Lane W. T., Capps W. I. Rescue of the defective genome of Moloney sarcoma virus from a noninfectious hamster tumor and the production of pseudotype sarcoma viruses with various murine leukemia viruses. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1164–1169. doi: 10.1073/pnas.56.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igel H., Huebner R. J., Deppa B., Bumgarner S. Rescue of the defective murine sarcoma virus genome by radiation-induced leukemia virus from C57BL mice. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1870–1877. doi: 10.1073/pnas.58.5.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioachim H. L., Berwich L., Furth J. Replication of gross leukemia virus in long-term cultures of rat thymomas: bioassays and electron microscopy. Cancer Res. 1966 May;26(5):803–811. [PubMed] [Google Scholar]
- Ioachim H. L., Berwick L. Continuous viral replication and cellular neoplastic transformation in cultures of normal rat thymus infected with gross leukemia virus. Int J Cancer. 1968 Jan 15;3(1):61–73. doi: 10.1002/ijc.2910030109. [DOI] [PubMed] [Google Scholar]
- Jasmin C., Chermann J. C., Mathé G., Raynaud M. Evidence of an inhibitor of Friend leukaemia. Rev Eur Etud Clin Biol. 1970 Jan;15(1):56–60. [PubMed] [Google Scholar]
- Kaplan H. S. On the natural history of the murine leukemias: presidential address. Cancer Res. 1967 Aug;27(8):1325–1340. [PubMed] [Google Scholar]
- LIEBERMAN M., HARAN-GHERA N., KAPLAN H. S. POTENTIATION OF VIRUS LEUKAEMOGENESIS IN C57BL MICE BY X-IRRADIATION OR URETHANE. Nature. 1964 Jul 25;203:420–422. doi: 10.1038/203420b0. [DOI] [PubMed] [Google Scholar]
- LIEBERMAN M., KAPLAN H. S. Leukemogenic activity of filtrates from radiation-induced lymphoid tumors of mice. Science. 1959 Aug 14;130(3372):387–388. doi: 10.1126/science.130.3372.387. [DOI] [PubMed] [Google Scholar]
- Lasfargues E. Y., Pillsbury N., Lasfargues J. C., Moore D. H. Release of leukemia C particles from murine cell lines infected with Bittner virus-containing inocula. Cancer Res. 1970 Jan;30(1):167–178. [PubMed] [Google Scholar]
- Lowy D. R., Rowe W. P., Teich N., Hartley J. W. Murine leukemia virus: high-frequency activation in vitro by 5-iododeoxyuridine and 5-bromodeoxyuridine. Science. 1971 Oct 8;174(4005):155–156. doi: 10.1126/science.174.4005.155. [DOI] [PubMed] [Google Scholar]
- MANAKER R. A., JENSEN E. M., KOROL W. LONG-TERM PROPAGATION OF A MURINE LEUKEMIA VIRUS IN AN ESTABLISHED CELL LINE. J Natl Cancer Inst. 1964 Aug;33:363–371. [PubMed] [Google Scholar]
- MOLONEY J. B. Biological studies on a lymphoid-leukemia virus extracted from sarcoma 37. I. Origin and introductory investigations. J Natl Cancer Inst. 1960 Apr;24:933–951. [PubMed] [Google Scholar]
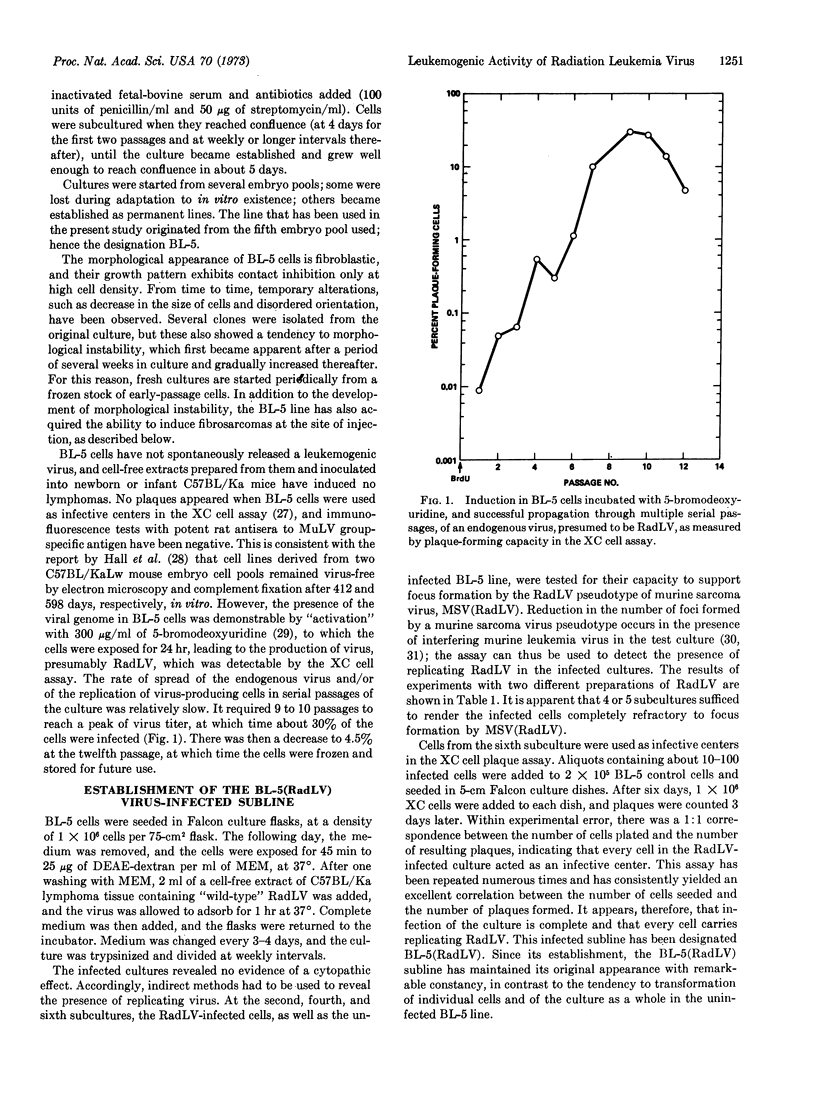
- Nomura S., Bassin R. H., Fischinger P. J. Replication of radiation-induced murine leukemia virus in normal and transformed mouse cells. J Virol. 1972 Mar;9(3):494–502. doi: 10.1128/jvi.9.3.494-502.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERIES J., LEVY J. P., BOIRON M., BERNARD J. MULTIPLICATION OF RAUSCHER VIRUS IN CULTURES OF MOUSE KIDNEY CELLS. Nature. 1964 Aug 8;203:672–673. doi: 10.1038/203672a0. [DOI] [PubMed] [Google Scholar]
- RAUSCHER F. J. A virus-induced disease of mice characterized by erythrocytopoiesis and lymphoid leukemia. J Natl Cancer Inst. 1962 Sep;29:515–543. [PubMed] [Google Scholar]
- Ribacchi R., Giraldo G. Leukemia virus release in chemically or physically induced lymphomas in BALB-c mice. Natl Cancer Inst Monogr. 1966 Sep;22:701–711. [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- SINKOVICS J. G., HOWE C. D. GROWTH CHARACTERISTICS, VIRUS YIELD, AND INTERFERON ASSAY OF LEUKEMIC MOUSE SPLEEN TISSUE CULTURES. J Infect Dis. 1964 Oct;114:359–372. doi: 10.1093/infdis/114.4.359. [DOI] [PubMed] [Google Scholar]
- Sarma P. S., Cheong M. P., Hartley J. W., Huebner R. J. A viral interference test for mouse leukemia viruses. Virology. 1967 Sep;33(1):180–184. doi: 10.1016/0042-6822(67)90111-0. [DOI] [PubMed] [Google Scholar]
- TENNANT J. R. Derivation of a murine lymphoid leukemia virus. J Natl Cancer Inst. 1962 Jun;28:1291–1303. [PubMed] [Google Scholar]
- Tennant J. R. Characterization of two BALB-c infant thymus cell lines infected with lympholeukemogenic virus. J Natl Cancer Inst. 1969 May;42(5):739–748. [PubMed] [Google Scholar]
- Witte O. N., Weissman I. L., Kaplan H. S. Structural characteristics of some murine RNA tumor viruses studied by lactoperoxidase iodination. Proc Natl Acad Sci U S A. 1973 Jan;70(1):36–40. doi: 10.1073/pnas.70.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods W. A., Wivel N. A., Massicot J. G., Chirigos M. A. Characterization of a rapidly growing AKR lymphoblastic cell line maintaining Gross antigens and viral replication. Cancer Res. 1970 Aug;30(8):2147–2155. [PubMed] [Google Scholar]
- Wright B. S., Lasfargues J. C. Attenuation of the Rauscher murine leukemia virus through serial passages in tissue culture. Natl Cancer Inst Monogr. 1966 Sep;22:685–700. [PubMed] [Google Scholar]
- Wright B. S., Lasfargues J. C. Long-term propagation of the Rauscher murine leukemia virus in tissue culture. J Natl Cancer Inst. 1965 Aug;35(2):319–327. [PubMed] [Google Scholar]
- Yoshikura H., Hirokawa Y., Ikawa Y., Sugano H. Infectious but non-leukemogenic Friend leukemia virus obtained after prolonged cultivation in vitro. Int J Cancer. 1969 Sep 15;4(5):636–640. doi: 10.1002/ijc.2910040508. [DOI] [PubMed] [Google Scholar]