Abstract
Clinically-important links have been established between mitochondrial function and cardiac physiology and disease in the context of signaling mechanisms, energy production, and muscle cell development. The proteins and processes that drive mitochondrial fusion and fission are now known to have emergent functions in intracellular calcium homeostasis, apoptosis, vascular smooth muscle cell proliferation, myofibril organization, and Notch-driven cell differentiation, all key issues in cardiac disease. Moreover, decreasing fission may confer protection against ischemic heart disease, particularly in the setting of obesity, diabetes, and heart failure. The importance of lipids in controlling mitochondrial fission and fusion is increasing becoming appreciated. Roles for the bulk and signaling lipids cardiolipin, phosphatidylethanolamine, phosphatidic acid, diacylglycerol, and lysophosphatidic acid and the enzymes that synthesize or metabolize them in the control of mitochondrial shape and function are reviewed here. A number of diseases have been linked to loss-of-function alleles for a subset of the enzymes, emphasizing the importance of the lipid environment in this context.
Keywords: mitochondria, fission, fusion, cardiolipin, phosphatidic acid
Mitochondria began by being defined by their role in cellular metabolism but now are known to undertake many other functions such as regulation of cell differentiation, motility, intracellular Ca2+ signaling and apoptosis [1, 2]. Initially thought to be morphologically inert, mitochondria are now viewed as dynamic organelles that fuse to generate larger mitochondria and subsequently undergo fission when demands change for energy production, when there are changes in cellular morphology, and when cell division occurs. Mitochondria are composed of two outer bilayer membranes, complicating the mechanisms required for fusion and fission relative to single-membrane organelles, and thus require specialized protein machinery. Fusion and fission of mitochondria are important in many larger events such as cell proliferation, spermatogenesis, and cardiac [3] and neuronal biology, and are connected to or altered in diseases such as Parkinson’s disease [4], Barth Syndrome [5–7], and Charcot-Marie-Tooth disease and autosomal Dominant Optic Atrophy [8].
Many reports link changes in mitochondrial morphology to metabolic and cardiac disease in the context of energetics. For example, patients with obesity, diabetes, or heart failure have elevated serum-free fatty acids [9] that promote lipotoxicity of cardiomyocytes [10]. The free fatty acids induce production of the lipid ceramide, which promotes apoptosis and mitochondrial fission through the activation of Drp1, the key protein mediator of fission [11]. Inhibition of ceramide synthesis or sequestration of the free fatty acids into lipid droplets prevents cardiomyocyte apoptosis [12], suggesting potential therapeutic cardioprotective approaches in these settings. Reciprocally, in addition to decreasing the efficiency of ATP production, loss of mitochondrial fusion triggers mitochondrial genome mutation and loss, resulting, for example, in lethal mitochondrial myopathy [13]. Presumably related in mechanism, cardiomyocyte differentiation requires mitochondrial fusion, which is essential for calcium homeostasis and thus Notch signaling [14]. Manipulation of mitochondrial morphology as a therapeutic approach for cardiac disease is complex, however. Although numerous reports have described clinical utility for inhibition of Drp1 and fission in the setting of ischemia / reperfusion to decrease mitochondrial metabolism and thus reduce cardiomyocyte apoptosis [15], the fission that is triggered by such cell stress may also be a critical part of the adaptive response, since it is required for cardiomyocyte hypertrophy [16]. Similarly, while fusion is important for myocyte biogenesis, muscle cells with reduced fusion capacity nonetheless undergo a metabolic remodeling that increases fatty acid utilization and results in increased endurance capacity at the physiological level [17]. Thus, pharmacological regulation of mitochondrial fission and fusion may be differentially beneficial in different settings, or inhibition useful in the short-term but counterproductive in long-term adaptation to sustained demands for increased cardiac performance.
Mitochondrial fission and fusion are tightly regulated events and the protein machinery that directly mediates them has been intensively studied, but other aspects of the mechanisms via which fusion and fission proceed are less well understood. The topic that will be reviewed here comprises the roles of mitochondrial membrane lipids including phosphatidylethanolamine (PE), cardiolipin (CL), diacylglycerol (DAG), and phosphatidic acid (PA) [5], which are generated and catabolized by phosphatases, phospholipases, and acyl-transferases.
There are many potential mechanisms through which lipids could influence mitochondrial morphology, including physical effects on membrane structure such as generating negative membrane curvature, which lowers the activation energy for fission and fusion of single membrane organelles such as exocytic vesicles fusing into the plasma membrane [18–21]. Many of these lipids have also been shown to recruit and or activate proteins that mediate the fission and fusion processes [22–25, 12]. Regardless of the means by which they function, these bulk and signaling lipid pathways undertake substantial roles in the control of mitochondrial morphology [26, 27, 22, 23, 28].
Cardiolipin roles in mitochondrial recruitment of proteins that mediate fission and fusion
Cardiolipin (CL), a negatively-charged lipid uniquely found in mitochondria and critical for mitochondrial functions, is found primarily in the inner membrane of the mitochondria where it makes up ~ 20% of the total lipids. Nonetheless, CL is also found in the outer membrane at levels as high as 25% at contact sites between the inner and outer membranes [29, 30], which is where both fusion [31] and fission [25, 32] events are thought to take place. CL is critical for fusion of the inner membrane via its interaction with the dynamin-related protein Opa1 [33, 31], a GTPase which mediates inner membrane fusion [34].
Opa1 is found in long and short isoforms that are inactive when monomeric but exhibit GTPase activity as dimers and drive mitochondrial fusion [35]. The dimerization is dependent on the presence of CL and the GTPase activity is enhanced on liposomes composed of lipids matching that of the mitochondrial inner membrane, again in a manner dependent on CL [31, 33]. The yeast ortholog of Opa1, Mgm1, encodes a set of positively-charged lysine residues that are dispensable for oligomerization but are required for interaction with negatively-charged lipids like CL, indicating that the activation of the Opa1 GTPase is dependent on CL interaction [36].
Cardiolipin is transferred from the inner membrane to the outer membrane by a specific protein machinery [37], thus facilitating processes such as apoptosis [38, 39] and mitochondrial fission [24, 25]. The most obvious role for CL in the fission pathway involves Drp1, the protein that directly mediates the fission process [24, 25]. CL has been shown to mediate both Drp1 recruitment to membrane surfaces and to activate Drp1’s GTPase activity [24, 25, 32], similar to the role it undertakes for Opa1 [31, 33, 36].
CL may also stimulate activity of other fission-promoting proteins. α-Synuclein, which is linked to Parkinson’s Disease, drives mitochondrial fission [40] and fragments membrane tubules in a manner dependent on CL, again suggestive of a CL role in recruitment and possibly activation. Mitochondrial fragmentation potentially underlies or contributes to the neuronal degeneration and pathogenesis of Parkinson’s Disease.
Taken together, though, CL seems to predominantly function as a pro-fusion lipid, since CL deficiency leads to fragmented mitochondria [41]. Other proteins recruited to mitochondria via CL interaction include pro-apoptotic proteins that potentially interact with the CL present in outer membrane mitochondrial contact sites. For example, tBid encodes a three-helix domain that preferentially inserts into CL-enriched membranes [42], as does Bax [43], but Bax and tBid are not likely to influence fission or fusion pathways directly.
Roles for Phosphatidylethanolamine in mitochondrial fusion
Phosphatidylethanolamine (PE) is another mitochondrial membrane lipid that is a significant component of the outer [44] and inner [45] membranes. Most of the mitochondrial surface PE is synthesized by phosphatidylserine decarboxylase Pisd [46]. PE facilitates mitochondrial fusion [46]; yeast lacking Psd1, the homolog of mammalian Pisd, undergo extensive mitochondrial fragmentation [46, 45]. The mitochondria lacking PE which do undergo fusion are remarkable for incomplete mixing of the joined mitochondrial membranes, indicating effects on lipid transfer [46].
CL and PE may have connected roles in facilitating fusion since combining an inability to make CL with Psd1 deficiency in yeast (that is, in yeast lacking the CL synthase Crd1) drives mitochondrial fragmentation much more strongly [46]. Combined CL and PE deficiency lowers Opa1 protein levels, potentially offering a mechanism for the finding. Intriguingly, Barth Syndrome is caused by loss-of-function mutations in tafazzin, which remodels CL. Tafazzin dysfunction results in decreased PE and CL levels and extensively fragmented mitochondria, suggesting that the combined deficiency may be a component in the disease pathogenesis [47].
Phosphatidic acid drives mitochondrial aggregation and promotes fusion
CL also serves as substrate for phosphatidic acid (PA) production at the mitochondrial surface via the activity of MitoPLD, a mitochondrial-surface phospholipase D that produces PA by cleaving CL. MitoPLD overexpression reduces cardiolipin and increases PA on the mitochondrial surface [26, 27, 23]. MitoPLD also functions as an endonuclease during spermatogenesis, undertaking there a critical role in biogenesis of piRNAs [48]. MitoPLD is evolutionally most similar to the bacterial endonuclease and PLD superfamily member known as Nuc [27, 49], and almost as similar to the superfamily member yeast cardiolipin synthase [27].
PA can also be synthesized via distinct pathways such as through conversion of lyso-PA (LPA) to PA by LPA acetyltransferase (LPAAT), which takes place on peroxisome membranes in yeast during fission [22]. PA is a cone-shaped, negatively-charged phospholipid, which enables it to elicit negative membrane curvature, making it potentially key in morphology of membranes when concentrated at key sites [50]. MitoPLD-overexpressing cells exhibit enlarged and aggregated mitochondria [27], which is also observed with elevated levels of Mfn1, another protein critical for fusion [51]. Conversely, Drosophila [52] or mammalian cells [27] that express a dominant-negative, catalytically-inactive, allele or siRNA exhibit mitochondrial fragmentation and decreased mitochondrial fusion, demonstrating that it is the product PA rather than the MitoPLD protein that affects rates of fusion. In support of this, metabolism of mitochondrial surface PA by the PA-preferring phospholipase A1 (PA-PLA1) to generate LPA or by the Lipin 1b PA phosphatase to form diacylglycerol (DAG) opposes MitoPLD action and thus drives fragmentation of mitochondria [26, 23]. Conversely, reducing levels of PA-PLA1 or Lipin 1b causes mitochondrial elongation [26, 23]. Even though PA-PLA1 stimulates mitochondrial fission, mitochondrial glycerol-3-phosphate acyltransferase (Mt-GPAT), which synthesizes LPA through a separate pathway using a different substrate, is required for fusion of mitochondria in HeLa cells and in C. elegans [53], suggesting that LPA can sometimes be a pro-fusion lipid, perhaps through promoting the synthesis of PA via LPAATs. This result suggests that decreases in PA levels might be the driver in fission rather than synthesis of LPA. Alternately, Mt-GPAT localizes to the mitochondrial interior [54] instead of to the surface on which PA-PLA1 functions, and phospholipids like LPA and lysophosphatidylcholine that aid fission and fusion of membrane vesicles via effects on membrane curvature elicit opposite effects based on whether they are present on the inwardly or outwardly bending membrane surfaces [55]. Taken together, LPA may have a pro-fission function when elevated in concentration on the surface of the mitochondria while having a fusion-promoting function when increased on the interior of the mitochondrial membrane.
While the means by which PA supports fusion remains unsettled, it may involve functional interaction with Mfn1 and Mfn2, the GTPases that mediate fusion of the mitochondrial outer membrane [51]. Mfn is an outer membrane integral protein that trans-dimerizes to bring adjacent mitochondria to within 16nm of each other, driving fusion using its GTPase action after forming multimers. Overexpression of MitoPLD does not drive mitochondrial aggregation in Mfn1 and Mfn2-deficient cells, suggesting that Mfn action to draw mitochondria closely together is needed for MitoPLD to act in trans to hydrolyze CL and create PA. MitoPLD-overexpressing cells exhibit mitochondria apposed even closer, 9nm apart, indicating that generation of PA may help promote fusion by pulling the outer membranes nearer than Mfn can accomplish on its own [27]. Finally, conversion of CL to PA on the mitochondrial surface could also decrease the rate of fission, since CL is required for Drp1 recruitment and activation as discussed above.
PA is linked to separate types of membrane fusion events, for example SNARE-regulated exocytosis [50], which may have some commonalities with fusion of mitochondria. SNARE proteins are found on exocytic membrane vesicles and the plasma membrane and function to appose the membrane surfaces in a mechanism similar in some ways to the one employed by Mfn. PA, in exocytosis, promotes fusion of the juxtaposed membranes by increasing the fusogenic activity of the SNARE proteins and through eliciting membrane curvature to lower the activation energy barrier to fusion [18, 19].
PA also promotes mitochondrial and membrane vesicle fission by recruiting proteins that carry out vesicular membrane cleavage [56] or synthesize lipids like DAG which encourage fission. PA generated by MitoPLD recruits the Lipin 1b PA phosphatase to the surface of mitochondria to convert the PA to DAG, decreasing fusion by reducing PA and stimulating fission [23]. A Lipin C-terminal catalytic domain specifically translocates to preformed sites of mitochondrial fission, causing profound mitochondrial cleavage.
Roles for diacylglycerol in fission of mitochondria
Lipin 1b encodes a PA-binding motif in the center of the protein that recruits it to membrane surfaces enriched in PA, but additionally has a catalytic domain at its C-terminus that localizes to preformed sites of mitochondrial fission independent of PA [23], indicating that it binds there to a protein partner. Altogether, this and related results have suggested that the C-terminal fission-site interacting domain is sequestered by the N-terminal domain, which also suppresses catalytic activity, but when PA recruits Lipin 1b to the mitochondrial surface, this triggers a conformational change that provides access to the cryptic binding site and enables Lipin 1b to become focused onto fission sites to create DAG there. The means by which DAG drives fission is presently unsettled, but there are interesting leads for future exploration.
Drp1 drives fission by squeezing the mitochondrial membrane at future sites of cleavage [57]. Recruitment of Drp1 occurs after endoplasmic reticulum (ER) tubules wrap around and constrict the mitochondria at fission (and contact) sites [58]. Since oligomerized Drp1 creates a ring smaller than the width of standard mitochondrial tubules, ER tubule constriction of mitochondria is thought to be required to shrink mitochondria to a diameter that the Drp1 multimer can encircle and then further constrict and cleave. The mechanism via which ER constriction proceeds involves INF2, an actin polymerizing protein [59], and Myosin II [60]. Reorganization of actin [61, 62] and activation of myosin II [63, 64] are both regulated by production of PA and its metabolism, suggesting linkage between PA-PLA1, MitoPLD, and Lipin 1b action, and mitochondrial constriction mediated by actin/myosin II.
The actin cytoskeleton is also directly regulated by DAG. For example, DAG recruits DAG kinase ζ, which phosphorylates DAG to generate PA and functions as a protein scaffold to activate RhoA, which orchestrates reorganization of the actin cytoskeleton in many settings [65, 66]. Altogether, DAG’s function in fission of mitochondria may be to drive actin filament polymerization at sites of ER constriction in collaboration with Myosin II and INF2, thereby permitting the ER to squeeze the future sites of fission to a diameter that Drp1 activity can then proceed with. Intriguingly, the yeast actin patch protein App1p, which localizes to mitochondria, has recently been shown to be a PA phosphatase, i.e. to convert PA to DAG [67]. This reveals a second potential pathway to generate DAG from PA at fission sites that would be triggered by the prior initiation of actin-driven constriction of the mitochondrial tubules by INF2.
In a related context in yeast, DAG drives fission of peroxisomes, which are double membrane-bound organelles similar to mitochondria. In this pathway, PA generated at the presumptive fission site is dephosphorylated to generate DAG, which causes a Drp1-related GTPase to translocate to the peroxisome to mediate fission [22]. It is not yet known whether this mechanism involving DAG is also relevant to peroxisome division in mammals. In a second example, DAG facilitates fission in the Golgi complex by recruiting Protein Kinase D (PKD) and thereby leading to increases in DAG, PA, and LPA, which then drive fission through creating membrane curvature [68]. In more detail [69], PKD is recruited to the trans-Golgi network (TGN) by DAG and ARF1, and, once activated by phosphorylation, stimulates PI4KIIIβ leading to production of PI4P in the cytoplasmic TGN leaflet. PI4P then recruits several proteins to the TGN membrane including CERT. CERT promotes ceramide transport to the TGN, which leads to the local generation of DAG, which in turn recruits more PKD leading to generation of more DAG. The local ARF1 activates PLD1 to generate PA from PC, and the PA is converted to LPA by the action of PLA2. Consumption of DAG via DAGK generates PA which subsequently is converted to LPA. The roles for DAG in promoting peroxisomal fission and budding from the Golgi complex suggest leads into the function of DAG in fission of mitochondria.
Other lipid-connected fission pathways
After infection, Listeria monocytogenes secrete the pore-forming toxin listeriolysin O (LLO) into mammalian cells, which triggers mitochondrial fission [28]. The LLO toxin multimerizes to form a pore and inserts into cholesterol-enriched membranes, which may include mitochondria as a target site. The ensuing fission requires ER-wrapping at fission sites and actin polymerization, but not Drp1, illustrating that there are ways to bypass the Drp1-mediated fission step. Drp1-indpendent fission is also observed upon silencing of the inner membrane protein LETM1 [70], as well as when α-Synuclein is overexpressed and directly interacts with CL in mitochondrial membranes [40], suggesting that a full understanding of the essential requirements for fission has not yet been generated.
Future Directions
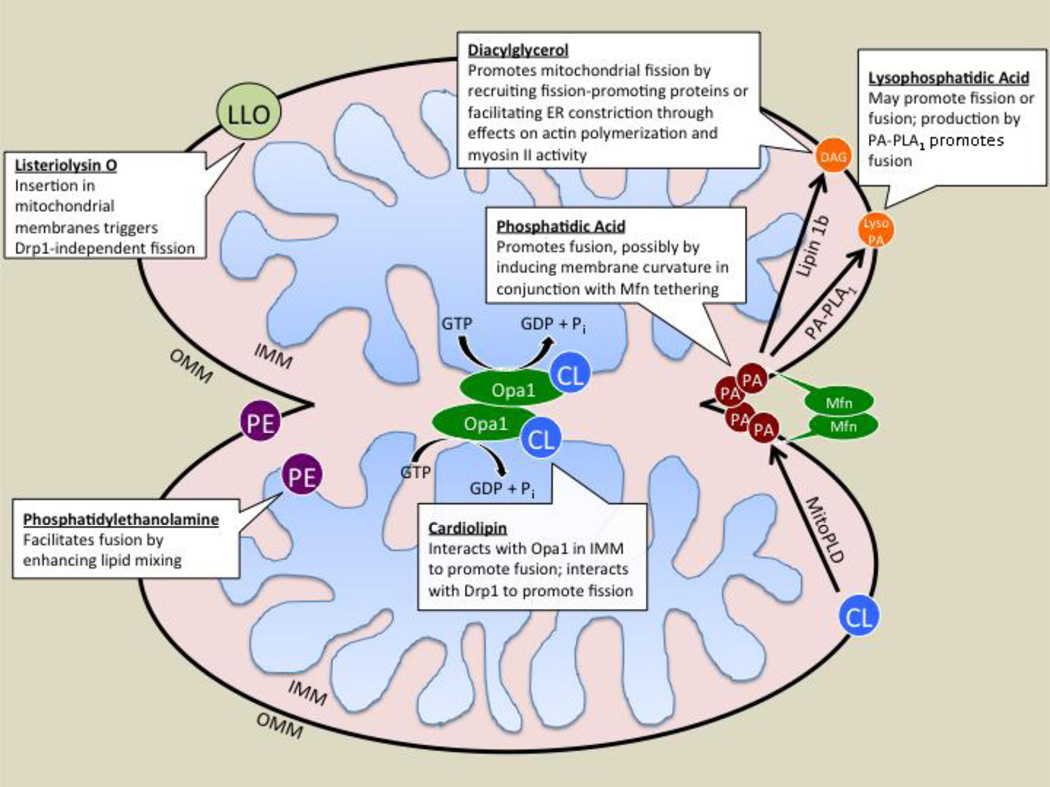
Lipids clearly play important roles in fission and fusion events (Fig. 1), although much remains unknown concerning their roles. The clinical relevance of this topic is increasingly apparent. Suppressing mitochondrial fission via inhibition of Drp1 prevents apoptosis in heart or brain ischemia / reperfusion and in kidney transplants [71–74]. On the other hand, inhibiting fusion through impeding Mfn1 function may increase cardiomyocyte tolerance in conditions of stress that result in dysfunctional mitochondria [75, 74]. Pursuing leads to explore how lipids function in combination with the mitochondrial surface proteins that drive fission and fusion will suggest new kinds of therapeutics, including for metabolic syndromes and bacterial infection.
Figure 1. A model for lipid roles in mitochondrial fusion and fission.
Phosphatidic acid (PA) generated at the mitochondrial surface via cardiolipin (CL) cleavage by MitoPLD assists Mitofusin (Mfn)-mediated fusion of the outer mitochondrial membrane. Inner membrane fusion is mediated by Opa1, which needs CL for localization and activity. PA action is ended by its metabolism by Lipin 1b, which translocates to the mitochondrial surface through action of a PA-binding domain and metabolizes PA to yield diacylglycerol (DAG), or by PA-Phospholipase A1 (PA-PLA1), which hydrolyzes PA to yield LysoPA (LPA). LPA or DAG production from the substrate PA triggers mitochondrial fission; reducing Lipin 1b or PA-PLA1 activity suppresses fission, causing mitochondrial tubule elongation. Phosphatidylethanolamine (PE) is required for mitochondrial membrane lipid mixing after or late in fusion, but the mechanism through which this alters the efficiency of fusion is unknown. Listeriolysin O (LLO) inserts into cholesterol-rich membranes and triggers mitochondrial fission in a Drp1-independent manner that still requires enwrapping by the ER and actin polymerization. IMM, inner mitochondrial membrane; OMM, outer mitochondrial membrane. See text for additional details.
Acknowledgments
Supported by NIH GM084251 and GM100109 to MAF.
References
- 1.Braschi E, McBride HM. Mitochondria and the culture of the Borg: understanding the integration of mitochondrial function within the reticulum, the cell, and the organism. Bioessays. 2010;32(11):958–966. doi: 10.1002/bies.201000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kasahara A, Scorrano L. Mitochondria: from cell death executioners to regulators of cell differentiation. Trends Cell Biol. 2014 doi: 10.1016/j.tcb.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Hall AR, Burke N, Dongworth RK, Hausenloy DJ. Mitochondrial fusion and fission proteins: novel therapeutic targets for combating cardiovascular disease. Br J Pharmacol. 2014;171(8):1890–1906. doi: 10.1111/bph.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337(6098):1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osman C, Voelker DR, Langer T. Making heads or tails of phospholipids in mitochondria. J Cell Biol. 2011;192(1):7–16. doi: 10.1083/jcb.201006159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patil VA, Greenberg ML. Cardiolipin-mediated cellular signaling. Adv Exp Med Biol. 2013;991:195–213. doi: 10.1007/978-94-007-6331-9_11. [DOI] [PubMed] [Google Scholar]
- 7.Unsay JD, Cosentino K, Subburaj Y, Garcia-Saez AJ. Cardiolipin effects on membrane structure and dynamics. Langmuir. 2013;29(51):15878–15887. doi: 10.1021/la402669z. [DOI] [PubMed] [Google Scholar]
- 8.Palau F, Estela A, Pla-Martin D, Sanchez-Piris M. The role of mitochondrial network dynamics in the pathogenesis of Charcot-Marie-Tooth disease. Adv Exp Med Biol. 2009;652:129–137. doi: 10.1007/978-90-481-2813-6_9. [DOI] [PubMed] [Google Scholar]
- 9.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90(1):207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 10.Hickson-Bick DL, Sparagna GC, Buja LM, McMillin JB. Palmitate-induced apoptosis in neonatal cardiomyocytes is not dependent on the generation of ROS. Am J Physiol Heart Circ Physiol. 2002;282(2):H656–H664. doi: 10.1152/ajpheart.00726.2001. [DOI] [PubMed] [Google Scholar]
- 11.Parra V, Eisner V, Chiong M, Criollo A, Moraga F, Garcia A, Hartel S, Jaimovich E, Zorzano A, Hidalgo C, et al. Changes in mitochondrial dynamics during ceramide-induced cardiomyocyte early apoptosis. Cardiovasc Res. 2008;77(2):387–397. doi: 10.1093/cvr/cvm029. [DOI] [PubMed] [Google Scholar]
- 12.Kuzmicic J, Parra V, Verdejo HE, Lopez-Crisosto C, Chiong M, Garcia L, Jensen MD, Bernlohr DA, Castro PF, Lavandero S. Trimetazidine prevents palmitate-induced mitochondrial fission and dysfunction in cultured cardiomyocytes. Biochem Pharmacol. 2014;91(3):323–336. doi: 10.1016/j.bcp.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 13.Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 2010;141(2):280–289. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasahara A, Cipolat S, Chen Y, Dorn GW, 2nd, Scorrano L. Mitochondrial fusion directs cardiomyocyte differentiation via calcineurin and Notch signaling. Science. 2013;342(6159):734–737. doi: 10.1126/science.1241359. [DOI] [PubMed] [Google Scholar]
- 15.Zepeda R, Kuzmicic J, Parra V, Troncoso R, Pennanen C, Riquelme JA, Pedrozo Z, Chiong M, Sanchez G, Lavandero S. Drp1 loss-of-function reduces cardiomyocyte oxygen dependence protecting the heart from ischemia-reperfusion injury. J Cardiovasc Pharmacol. 2014;63(6):477–487. doi: 10.1097/FJC.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 16.Pennanen C, Parra V, Lopez-Crisosto C, Morales PE, Del Campo A, Gutierrez T, Rivera-Mejias P, Kuzmicic J, Chiong M, Zorzano A, et al. Mitochondrial fission is required for cardiomyocyte hypertrophy mediated by a Ca2+-calcineurin signaling pathway. J Cell Sci. 2014;127(Pt 12):2659–2671. doi: 10.1242/jcs.139394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caffin F, Prola A, Piquereau J, Novotova M, David DJ, Garnier A, Fortin D, Alavi MV, Veksler V, Ventura-Clapier R, et al. Altered skeletal muscle mitochondrial biogenesis but improved endurance capacity in trained OPA1-deficient mice. J Physiol. 2013;591(Pt 23):6017–6037. doi: 10.1113/jphysiol.2013.263079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang P, Altshuller YM, Chunqiu Hou J, Pessin JE, Frohman MA. Insulin-stimulated Plasma Membrane Fusion of Glut4 Glucose Transporter-containing Vesicles Is Regulated by Phospholipase D1. Mol Biol Cell. 2005;16:2614–2623. doi: 10.1091/mbc.E04-12-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vicogne J, Vollenweider D, Smith JR, Huang P, Frohman MA, Pessin JE. Asymmetric phospholipid distribution drives in vitro reconstituted SNARE-dependent membrane fusion. Proc Natl Acad Sci U S A. 2006;103(40):14761–14766. doi: 10.1073/pnas.0606881103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vitale N, Caumont AS, Chasserot-Golaz S, Du G, Wu S, Sciorra VA, Morris AJ, Frohman MA, Bader MF. Phospholipase D1: a key factor for the exocytotic machinery in neuroendocrine cells. Embo J. 2001;20(10):2424–2434. doi: 10.1093/emboj/20.10.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donaldson JG. Phospholipase D in endocytosis and endosomal recycling pathways. Biochim Biophys Acta. 2009;1791(9):845–849. doi: 10.1016/j.bbalip.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo T, Gregg C, Boukh-Viner T, Kyryakov P, Goldberg A, Bourque S, Banu F, Haile S, Milijevic S, San KH, et al. A signal from inside the peroxisome initiates its division by promoting the remodeling of the peroxisomal membrane. J Cell Biol. 2007;177(2):289–303. doi: 10.1083/jcb.200609072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang H, Gao Q, Peng XX, Choi S-Y, Sarma K, Ren H, Morris AJ, Frohman MA. piRNA-associated germline nuage formation and spermatogenesis require MitoPLD pro-fusogenic mitochondrial-surface lipid signaling. Dev Cell. 2011;20:376–387. doi: 10.1016/j.devcel.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montessuit S, Somasekharan SP, Terrones O, Lucken-Ardjomande S, Herzig S, Schwarzenbacher R, Manstein DJ, Bossy-Wetzel E, Basanez G, Meda P, et al. Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell. 2010;142(6):889–901. doi: 10.1016/j.cell.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macdonald PJ, Stepanyants N, Mehrotra N, Mears JA, Qi X, Sesaki H, Ramachandran R. A dimeric equilibrium intermediate nucleates Drp1 reassembly on mitochondrial membranes for fission. Mol Biol Cell. 2014;25(12):1905–1915. doi: 10.1091/mbc.E14-02-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baba T, Kashiwagi Y, Arimitsu N, Kogure T, Edo A, Maruyama T, Nakao K, Nakanishi H, Kinoshita M, Frohman MA, et al. Phosphatidic Acid (PA)-Preferring Phospholipase A1 Regulates Mitochondrial Dynamics. J Biol Chem. 2014 doi: 10.1074/jbc.M113.531921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi SY, Huang P, Jenkins GM, Chan DC, Schiller J, Frohman MA. A common lipid links Mfn-mediated mitochondrial fusion and SNARE-regulated exocytosis. Nat Cell Biol. 2006;8(11):1255–1262. doi: 10.1038/ncb1487. [DOI] [PubMed] [Google Scholar]
- 28.Stavru F, Palmer AE, Wang C, Youle RJ, Cossart P. Atypical mitochondrial fission upon bacterial infection. Proc Natl Acad Sci U S A. 2013;110(40):16003–16008. doi: 10.1073/pnas.1315784110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ardail D, Privat JP, Egret-Charlier M, Levrat C, Lerme F, Louisot P. Mitochondrial contact sites. Lipid composition and dynamics. J Biol Chem. 1990;265(31):18797–18802. [PubMed] [Google Scholar]
- 30.Schlattner U, Tokarska-Schlattner M, Rousseau D, Boissan M, Mannella C, Epand R, Lacombe ML. Mitochondrial cardiolipin/phospholipid trafficking: the role of membrane contact site complexes and lipid transfer proteins. Chem Phys Lipids. 2014;179:32–41. doi: 10.1016/j.chemphyslip.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 31.DeVay RM, Dominguez-Ramirez L, Lackner LL, Hoppins S, Stahlberg H, Nunnari J. Coassembly of Mgm1 isoforms requires cardiolipin and mediates mitochondrial inner membrane fusion. J Cell Biol. 2009;186(6):793–803. doi: 10.1083/jcb.200906098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ugarte-Uribe B, Mueller HM, Otsuki M, Nickel W, Garcia-Saez AJ. Dynamin-Related Protein 1 (Drp1) Promotes Structural Intermediates of Membrane Division. J Biol Chem. 2014 doi: 10.1074/jbc.M114.575779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ban T, Heymann JA, Song Z, Hinshaw JE, Chan DC. OPA1 disease alleles causing dominant optic atrophy have defects in cardiolipin-stimulated GTP hydrolysis and membrane tubulation. Hum Mol Genet. 2010;19(11):2113–2122. doi: 10.1093/hmg/ddq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mishra P, Carelli V, Manfredi G, Chan DC. Proteolytic cleavage of Opa1 stimulates mitochondrial inner membrane fusion and couples fusion to oxidative phosphorylation. Cell Metab. 2014;19(4):630–641. doi: 10.1016/j.cmet.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song Z, Chen H, Fiket M, Alexander C, Chan DC. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J Cell Biol. 2007;178(5):749–755. doi: 10.1083/jcb.200704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rujiviphat J, Meglei G, Rubinstein JL, McQuibban GA. Phospholipid association is essential for dynamin-related protein Mgm1 to function in mitochondrial membrane fusion. J Biol Chem. 2009;284(42):28682–28686. doi: 10.1074/jbc.M109.044933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlattner U, Tokarska-Schlattner M, Ramirez S, Tyurina YY, Amoscato AA, Mohammadyani D, Huang Z, Jiang J, Yanamala N, Seffouh A, et al. Dual function of mitochondrial Nm23-H4 protein in phosphotransfer and intermembrane lipid transfer: a cardiolipin-dependent switch. J Biol Chem. 2013;288(1):111–121. doi: 10.1074/jbc.M112.408633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Chen J, Dai Q, Lee RM. Phospholipid scramblase 3 is the mitochondrial target of protein kinase C delta-induced apoptosis. Cancer Res. 2003;63(6):1153–1156. [PubMed] [Google Scholar]
- 39.Liu J, Dai Q, Chen J, Durrant D, Freeman A, Liu T, Grossman D, Lee RM. Phospholipid scramblase 3 controls mitochondrial structure, function, and apoptotic response. Mol Cancer Res. 2003;1(12):892–902. [PubMed] [Google Scholar]
- 40.Nakamura K, Nemani VM, Azarbal F, Skibinski G, Levy JM, Egami K, Munishkina L, Zhang J, Gardner B, Wakabayashi J, et al. Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein alpha-synuclein. J Biol Chem. 2011;286(23):20710–20726. doi: 10.1074/jbc.M110.213538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joshi AS, Thompson MN, Fei N, Huttemann M, Greenberg ML. Cardiolipin and mitochondrial phosphatidylethanolamine have overlapping functions in mitochondrial fusion in Saccharomyces cerevisiae. J Biol Chem. 2012;287(21):17589–17597. doi: 10.1074/jbc.M111.330167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lutter M, Fang M, Luo X, Nishijima M, Xie X, Wang X. Cardiolipin provides specificity for targeting of tBid to mitochondria. Nat Cell Biol. 2000;2(10):754–761. doi: 10.1038/35036395. [DOI] [PubMed] [Google Scholar]
- 43.Lucken-Ardjomande S, Montessuit S, Martinou JC. Contributions to Bax insertion and oligomerization of lipids of the mitochondrial outer membrane. Cell Death Differ. 2008;15(5):929–937. doi: 10.1038/cdd.2008.9. [DOI] [PubMed] [Google Scholar]
- 44.Sperka-Gottlieb CD, Hermetter A, Paltauf F, Daum G. Lipid topology and physical properties of the outer mitochondrial membrane of the yeast, Saccharomyces cerevisiae. Biochim Biophys Acta. 1988;946(2):227–234. doi: 10.1016/0005-2736(88)90397-5. [DOI] [PubMed] [Google Scholar]
- 45.Tasseva G, Bai HD, Davidescu M, Haromy A, Michelakis E, Vance JE. Phosphatidylethanolamine deficiency in Mammalian mitochondria impairs oxidative phosphorylation and alters mitochondrial morphology. J Biol Chem. 2013;288(6):4158–4173. doi: 10.1074/jbc.M112.434183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan EY, McQuibban GA. Phosphatidylserine decarboxylase 1 (Psd1) promotes mitochondrial fusion by regulating the biophysical properties of the mitochondrial membrane and alternative topogenesis of mitochondrial genome maintenance protein 1 (Mgm1) J Biol Chem. 2012;287(48):40131–40139. doi: 10.1074/jbc.M112.399428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu Y, Sutachan JJ, Plesken H, Kelley RI, Schlame M. Characterization of lymphoblast mitochondria from patients with Barth syndrome. Lab Invest. 2005;85(6):823–830. doi: 10.1038/labinvest.3700274. [DOI] [PubMed] [Google Scholar]
- 48.Ipsaro JJ, Haase AD, Knott SR, Joshua-Tor L, Hannon GJ. The structural biochemistry of Zucchini implicates it as a nuclease in piRNA biogenesis. Nature. 2012;491(7423):279–283. doi: 10.1038/nature11502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stuckey JA, Dixon JE. Crystal structure of a phospholipase D family member. Nat Struct Biol. 1999;6(3):278–284. doi: 10.1038/6716. [DOI] [PubMed] [Google Scholar]
- 50.Ammar MR, Kassas N, Chasserot-Golaz S, Bader MF, Vitale N. Lipids in Regulated Exocytosis: What are They Doing? Front Endocrinol (Lausanne) 2013;4:125. doi: 10.3389/fendo.2013.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160(2):189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muliyil S, Krishnakumar P, Narasimha M. Spatial, temporal and molecular hierarchies in the link between death, delamination and dorsal closure. Development. 2011;138(14):3043–3054. doi: 10.1242/dev.060731. [DOI] [PubMed] [Google Scholar]
- 53.Ohba Y, Sakuragi T, Kage-Nakadai E, Tomioka NH, Kono N, Imae R, Inoue A, Aoki J, Ishihara N, Inoue T, et al. Mitochondria-type GPAT is required for mitochondrial fusion. Embo J. 2013;32(9):1265–1279. doi: 10.1038/emboj.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Igal RA, Wang S, Gonzalez-Baro M, Coleman RA. Mitochondrial glycerol phosphate acyltransferase directs the incorporation of exogenous fatty acids into triacylglycerol. J Biol Chem. 2001;276(45):42205–42212. doi: 10.1074/jbc.M103386200. [DOI] [PubMed] [Google Scholar]
- 55.Huang P, Altshuller YM, Hou JC, Pessin JE, Frohman MA. Insulin-stimulated plasma membrane fusion of Glut4 glucose transporter-containing vesicles is regulated by phospholipase D1. Mol Biol Cell. 2005;16(6):2614–2623. doi: 10.1091/mbc.E04-12-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang J-S, Gad H, Lee S, Mironov A, Zhang L, Beznoussenko GV, Valente C, Turacchio G, Bonsra AN, Du G, et al. COPI vesicle fission: a role for phosphatidic acid and insight into Golgi maintenance. Nat Cell Biol. 2008;10:1146–1153. doi: 10.1038/ncb1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mears JA, Lackner LL, Fang S, Ingerman E, Nunnari J, Hinshaw JE. Conformational changes in Dnm1 support a contractile mechanism for mitochondrial fission. Nat Struct Mol Biol. 2011;18(1):20–26. doi: 10.1038/nsmb.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK. ER tubules mark sites of mitochondrial division. Science. 2011;334(6054):358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Korobova F, Ramabhadran V, Higgs HN. An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2. Science. 2013;339(6118):464–467. doi: 10.1126/science.1228360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Korobova F, Gauvin TJ, Higgs HN. A Role for Myosin II in Mammalian Mitochondrial Fission. Curr Biol. 2014;24(4):409–414. doi: 10.1016/j.cub.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Itoh T, Hasegawa J, Tsujita K, Kanaho Y, Takenawa T. The tyrosine kinase Fer is a downstream target of the PLD-PA pathway that regulates cell migration. Sci Signal. 2009;2(87):ra52. doi: 10.1126/scisignal.2000393. [DOI] [PubMed] [Google Scholar]
- 62.Nishikimi A, Fukuhara H, Su W, Hongu T, Takasuga S, Mihara H, Cao Q, Sanematsu F, Kanai M, Hasegawa H, et al. Sequential regulation of DOCK2 dynamics by two phospholipids during neutrophil chemotaxis. Science. 2009;324(5925):384–387. doi: 10.1126/science.1170179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ito M, Feng J, Tsujino S, Inagaki N, Inagaki M, Tanaka J, Ichikawa K, Hartshorne DJ, Nakano T. Interaction of smooth muscle myosin phosphatase with phospholipids. Biochem. 1997;36(24):7607–7614. doi: 10.1021/bi9702647. [DOI] [PubMed] [Google Scholar]
- 64.Du G, Frohman MA. A lipid-signaled myosin phosphatase surge disperses cortical contractile force early in cell spreading. Mol Biol Cell. 2009;20:200–208. doi: 10.1091/mbc.E08-06-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abramovici H, Mojtabaie P, Parks RJ, Zhong XP, Koretzky GA, Topham MK, Gee SH. Diacylglycerol kinase zeta regulates actin cytoskeleton reorganization through dissociation of Rac1 from RhoGDI. Mol Biol Cell. 2009;20(7):2049–2059. doi: 10.1091/mbc.E07-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ard R, Mulatz K, Abramovici H, Maillet JC, Fottinger A, Foley T, Byham MR, Iqbal TA, Yoneda A, Couchman JR, et al. Diacylglycerol kinase zeta regulates RhoA activation via a kinase-independent scaffolding mechanism. Mol Biol Cell. 2012;23(20):4008–4019. doi: 10.1091/mbc.E12-01-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chae M, Carman GM. Characterization of the yeast actin patch protein App1p phosphatidate phosphatase. J Biol Chem. 2013;288(9):6427–6437. doi: 10.1074/jbc.M112.449629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bossard C, Bresson D, Polishchuk RS, Malhotra V. Dimeric PKD regulates membrane fission to form transport carriers at the TGN. J Cell Biol. 2007;179(6):1123–1131. doi: 10.1083/jcb.200703166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Malhotra V, Campelo F. PKD regulates membrane fission to generate TGN to cell surface transport carriers. Cold Spring Harb Perspect Biol. 2011;3(2) doi: 10.1101/cshperspect.a005280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dimmer KS, Navoni F, Casarin A, Trevisson E, Endele S, Winterpacht A, Salviati L, Scorrano L. LETM1, deleted in Wolf-Hirschhorn syndrome is required for normal mitochondrial morphology and cellular viability. Hum Mol Genet. 2008;17(2):201–214. doi: 10.1093/hmg/ddm297. [DOI] [PubMed] [Google Scholar]
- 71.Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009;119(5):1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grohm J, Kim SW, Mamrak U, Tobaben S, Cassidy-Stone A, Nunnari J, Plesnila N, Culmsee C. Inhibition of Drp1 provides neuroprotection in vitro and in vivo. Cell Death Differ. 2012;19(9):1446–1458. doi: 10.1038/cdd.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121(18):2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 74.Yang CY, Frohman MA. Mitochondria: signaling with phosphatidic acid. Int J Biochem Cell Biol. 2012;44(8):1346–1350. doi: 10.1016/j.biocel.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Papanicolaou KN, Ngoh GA, Dabkowski ER, O'Connell KA, Ribeiro RF, Jr, Stanley WC, Walsh K. Cardiomyocyte deletion of mitofusin-1 leads to mitochondrial fragmentation and improves tolerance to ROS-induced mitochondrial dysfunction and cell death. Am J Physiol Heart Circ Physiol. 2012;302(1):H167–H179. doi: 10.1152/ajpheart.00833.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]