Abstract
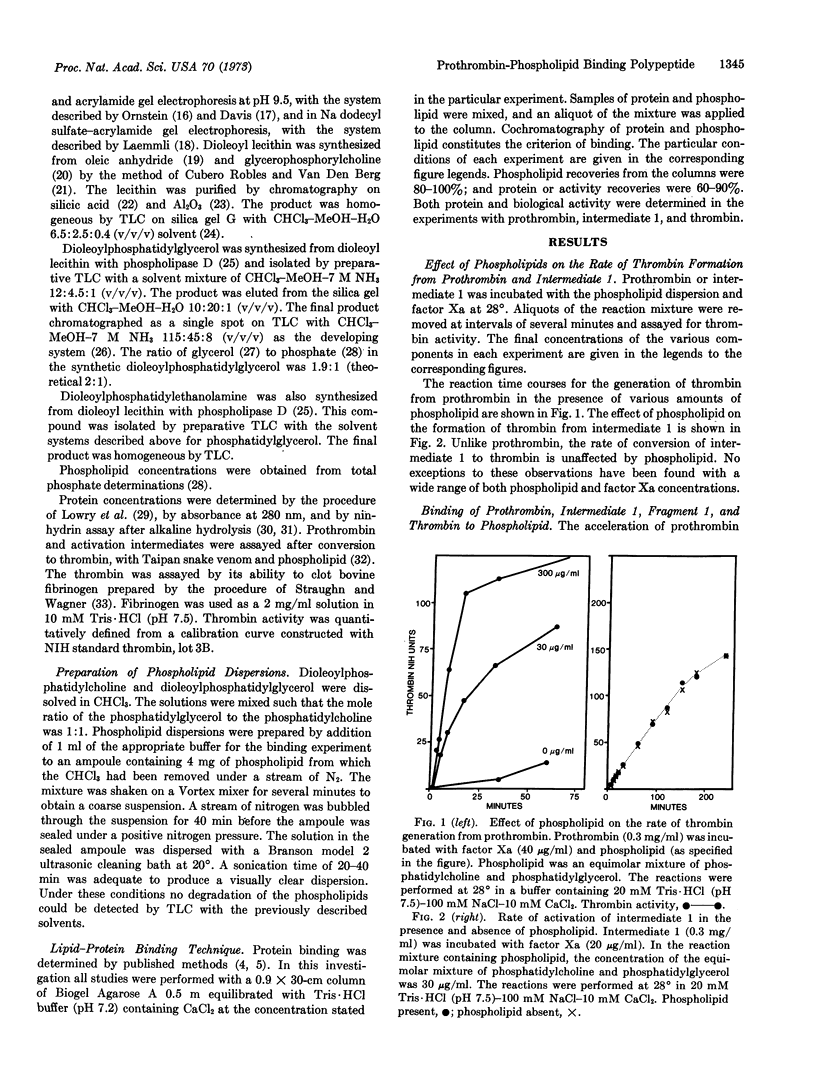
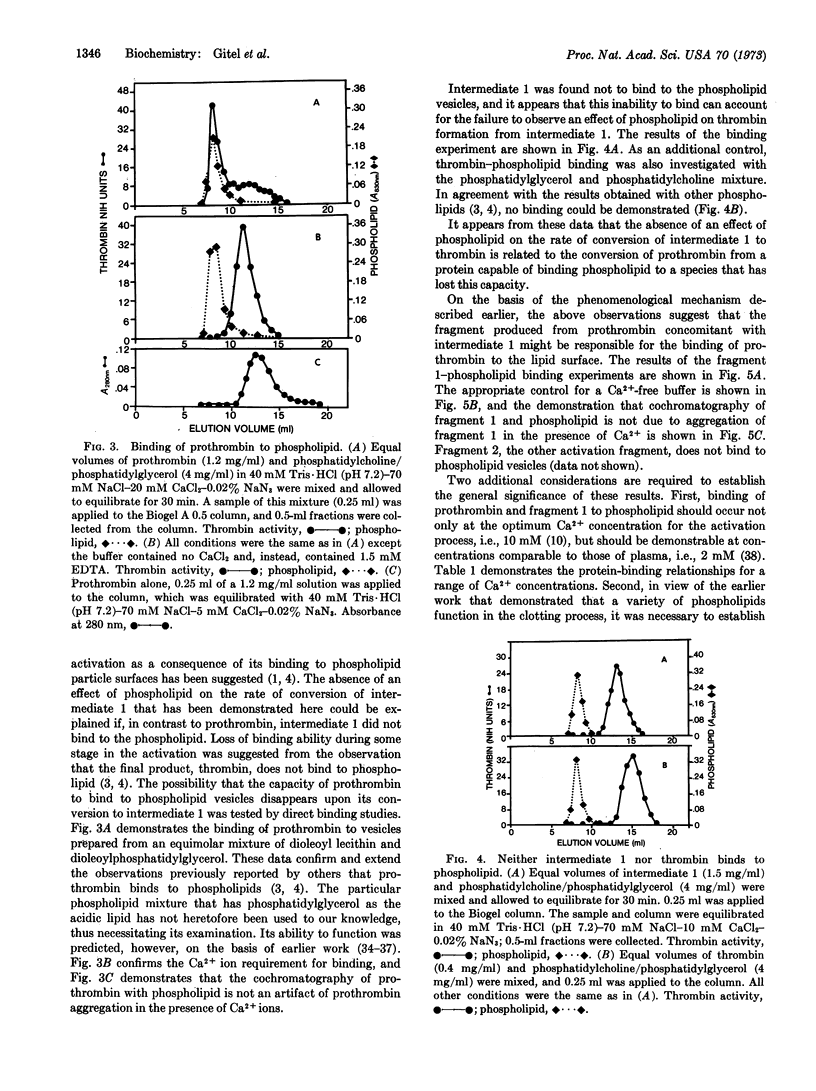
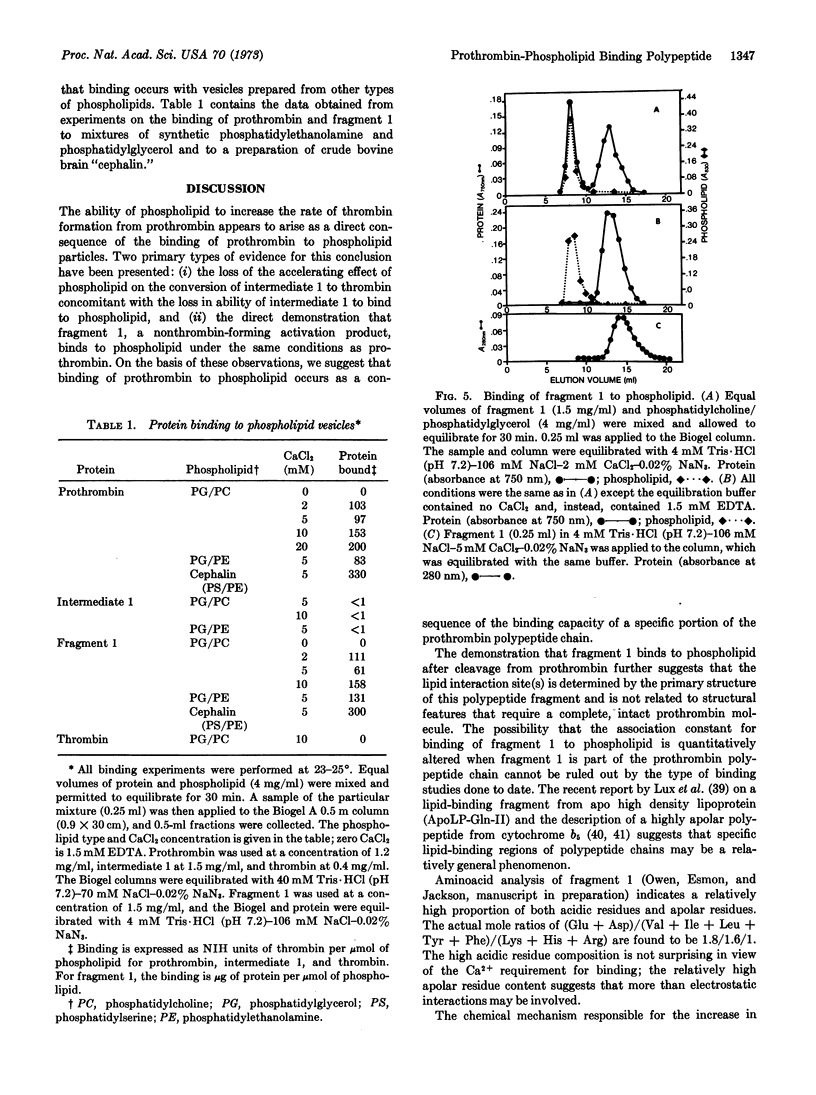
The blood-clotting protein prothrombin can be converted to thrombin in free solution by the proteolytic enzyme, activated factor X. When prothrombin is bound to the surface of phospholipid vesicles, the rate of thrombin generation is increased more than 30-fold over the rate of unbound prothrombin. If the prothrombin activation process is terminated after a time interval in which less than 10% of the expected thrombin has been produced, two major products are found in the activation mixture. These products have been termed intermediate 1, a precursor of thrombin, and fragment 1. The conversion of intermediate 1 to thrombin is not accelerated by phospholipid nor can binding of this intermediate to phospholipid particles be demonstrated. In contrast, fragment 1, the other activation product derived from prothrombin, binds to phospholipid particles under the same conditions as prothrombin. On the basis of these observations, we propose that the prothrombin molecule contains a specific region of polypeptide chain for binding to phospholipid particles. This specific polypeptide region or lipid interaction site is part of the nonthrombin-forming activation fragment.
Keywords: activation fragment, lipid-protein interaction, lipid-binding site, surface catalysis, factor X
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANGHAM A. D. A correlation between surface charge and coagulant action of phospholipids. Nature. 1961 Dec 23;192:1197–1198. doi: 10.1038/1921197a0. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Barthels M., Seegers W. H. Substitution of lipids with bile salts in the formation of thrombin. Thromb Diath Haemorrh. 1969 Aug 31;22(1):13–27. [PubMed] [Google Scholar]
- Barton P. G., Hanahan D. J. Some lipid-protein interactions involved in prothrombin activation. Biochim Biophys Acta. 1969 Oct 28;187(3):319–327. doi: 10.1016/0005-2760(69)90005-8. [DOI] [PubMed] [Google Scholar]
- Barton P. G., Jackson C. M., Hanahan D. J. Relationship between factor V and activated factor X in the generation of prothrombinase. Nature. 1967 May 27;214(5091):923–924. doi: 10.1038/214923a0. [DOI] [PubMed] [Google Scholar]
- Brockerhoff H., Yurkowski M. Simplified preparation of L-alpha-glyceryl phosphoryl choline. Can J Biochem. 1965 Oct;43(10):1777–1777. doi: 10.1139/o65-197. [DOI] [PubMed] [Google Scholar]
- Cox A. C., Hanahan D. J. The isolation of undegraded bovine prothrombin and its partial characterization. Biochim Biophys Acta. 1970 Apr 28;207(1):49–64. doi: 10.1016/0005-2795(70)90136-4. [DOI] [PubMed] [Google Scholar]
- Cubero Robles E., van den Berg D. Synthesis of lecithins by acylation of O-(sn-glycero-3-phosphoryl) choline with fatty acid anhydrides. Biochim Biophys Acta. 1969 Dec 17;187(4):520–526. doi: 10.1016/0005-2760(69)90049-6. [DOI] [PubMed] [Google Scholar]
- DAEMEN F. J., VAN ARKEL, HART H. C., VAN DER DRIFT C., VAN DEENEN L. ACTIVITY OF SYNTHETIC PHOSPHOLIPIDS IN BLOOD COAGULATION. Thromb Diath Haemorrh. 1965 Mar 15;13:194–217. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Denson K. W. Coagulant and anticoagulant action of snake venoms. Toxicon. 1969 Jun;7(1):5–11. doi: 10.1016/0041-0101(69)90154-8. [DOI] [PubMed] [Google Scholar]
- HANAHAN D. J., OLLEY J. N. Chemical nature of monophosphoinositides. J Biol Chem. 1958 Apr;231(2):813–828. [PubMed] [Google Scholar]
- HANAHAN D. J., TURNER M. B., JAYKO M. E. The isolation of egg phosphatidyl choline by an adsorption column technique. J Biol Chem. 1951 Oct;192(2):623–628. [PubMed] [Google Scholar]
- Hauser H. O. The effect of ultrasonic irradiation on the chemical structure of egg lecithin. Biochem Biophys Res Commun. 1971 Nov;45(4):1049–1055. doi: 10.1016/0006-291x(71)90443-8. [DOI] [PubMed] [Google Scholar]
- Hemker H. C., Muller A. D., Loeliger E. A. Two types of prothrombin in vitamin K deficiency. Thromb Diath Haemorrh. 1970 Jun 30;23(3):633–637. [PubMed] [Google Scholar]
- Jackson C. M. Characterization of two glycoprotein variants of bovine factor X and demonstration that the factor X zymogen contains two polypeptide chains. Biochemistry. 1972 Dec 19;11(26):4873–4882. doi: 10.1021/bi00776a001. [DOI] [PubMed] [Google Scholar]
- Jackson C. M., Hanahan D. J. Studies on bovine factor X. II. Characterization of purified factor X. Observations on some alterations in zone electrophoretic and chromatographic behavior occurring during purification. Biochemistry. 1968 Dec;7(12):4506–4517. doi: 10.1021/bi00852a047. [DOI] [PubMed] [Google Scholar]
- Jackson C. M., Johnson T. F., Hanahan D. J. Studies on bovine factor X. I. Large-sclae purification of the bovine plasma protein possessing factor X activity. Biochemistry. 1968 Dec;7(12):4492–4505. doi: 10.1021/bi00852a046. [DOI] [PubMed] [Google Scholar]
- Jobin F., Esnouf M. P. Studies on the formation of the prothrombin-converting complex. Biochem J. 1967 Mar;102(3):666–674. doi: 10.1042/bj1020666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOCHANG T. C., SWEELEY C. C. CHARACTERIZATION OF LIPIDS FROM CANINE ADRENAL GLANDS. Biochemistry. 1963 May-Jun;2:592–604. doi: 10.1021/bi00903a036. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanchantin G. F., Friedmann J. A., Hart D. W. On the occurrence of polymorphic human prothrombin. Electrophoretic and chromatographic alterations of the molecule due to the action of thrombin. J Biol Chem. 1968 Feb 10;243(3):476–486. [PubMed] [Google Scholar]
- Lux S. E., John K. M., Fleischer S., Jackson R. L., Gotto A. M., Jr Identification of the lipid-binding cyanogen bromide fragment from the cystine-containing high density apolipoprotein, APOLP-GLN-II. Biochem Biophys Res Commun. 1972 Oct 6;49(1):23–29. doi: 10.1016/0006-291x(72)90004-6. [DOI] [PubMed] [Google Scholar]
- Mann K. G., Heldebrant C. M., Fass D. N. Multiple active forms of thrombin. II. Mechanism of production from prothrombin. J Biol Chem. 1971 Oct 10;246(19):6106–6114. [PubMed] [Google Scholar]
- Nelsestuen G. L., Suttie J. W. Mode of action of vitamin K. Calcium binding properties of bovine prothrombin. Biochemistry. 1972 Dec 19;11(26):4961–4964. doi: 10.1021/bi00776a013. [DOI] [PubMed] [Google Scholar]
- Nelsestuen G. L., Suttie J. W. The purification and properties of an abnormal prothrombin protein produced by dicumarol-treated cows. A comparison to normal prothrombin. J Biol Chem. 1972 Dec 25;247(24):8176–8182. [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- PAPAHADJOPOULOS D., HANAHAN D. J. OBSERVATIONS ON THE INTERACTION OF PHOSPHOLIPIDS AND CERTAIN CLOTTING FACTORS IN PROTHROMBIN ACTIVATOR FORMATION. Biochim Biophys Acta. 1964 Aug 19;90:436–439. doi: 10.1016/0304-4165(64)90220-x. [DOI] [PubMed] [Google Scholar]
- Radcliffe R. D., Barton P. G. The purification and properties of activated factor X. Bovine factor X activated with Russell's viper venom. J Biol Chem. 1972 Dec 10;247(23):7735–7742. [PubMed] [Google Scholar]
- SEEGERS W. H., COLE E. R., AOKI N. FUNCTION OF AC-GLOBULIN AND LIPID IN BLOOD CLOTTING. Can J Biochem Physiol. 1963 Dec;41:2441–2461. [PubMed] [Google Scholar]
- Selinger Z., Lapidot Y. Synthesis of fatty acid anhydrides by reaction with dicyclohexylcarbodiimide. J Lipid Res. 1966 Jan;7(1):174–175. [PubMed] [Google Scholar]
- Spatz L., Strittmatter P. A form of cytochrome b5 that contains an additional hydrophobic sequence of 40 amino acid residues. Proc Natl Acad Sci U S A. 1971 May;68(5):1042–1046. doi: 10.1073/pnas.68.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenflo J., Ganrot P. O. Vitamin K and the biosynthesis of prothrombin. I. Identification and purification of a dicoumarol-induced abnormal prothrombin from bovine plasma. J Biol Chem. 1972 Dec 25;247(24):8160–8166. [PubMed] [Google Scholar]
- Stenn K. S., Blout E. R. Mechanism of bovine prothrombin activation by an insoluble preparation of bovine factor X a (thrombokinase). Biochemistry. 1972 Nov 21;11(24):4502–4515. doi: 10.1021/bi00774a012. [DOI] [PubMed] [Google Scholar]
- Straughn W., 3rd, Wagner R. H. A simple method for preparing fibrinogen. Thromb Diath Haemorrh. 1966 Jul 31;16(1):198–206. [PubMed] [Google Scholar]
- Strittmatter P., Rogers M. J., Spatz L. The binding of cytochrome b 5 to liver microsomes. J Biol Chem. 1972 Nov 25;247(22):7188–7194. [PubMed] [Google Scholar]
- TROLL W., CANNAN R. K. A modified photometric ninhydrin method for the analysis of amino and imino acids. J Biol Chem. 1953 Feb;200(2):803–811. [PubMed] [Google Scholar]
- WAGNER H., HOERHAMMER L., WOLFF P. [Thin layer chromatography of phosphatides and glycolipids]. Biochem Z. 1961;334:175–184. [PubMed] [Google Scholar]
- Yang S. F., Freer S., Benson A. A. Transphosphatidylation by phospholipase D. J Biol Chem. 1967 Feb 10;242(3):477–484. [PubMed] [Google Scholar]


