Key Points
A total of 47% of patients who achieved CR on brentuximab vedotin remain progression-free after being followed a median of 53 months.
Younger age, less functional impairment, and lower disease burden at baseline were associated with CR and prognostic for longer survival.
Abstract
We present response and survival outcomes of a pivotal phase 2 trial of the antibody-drug conjugate brentuximab vedotin in patients with relapsed/refractory Hodgkin lymphoma following autologous stem cell transplant (N = 102) after a median observation period of approximately 3 years. Median overall survival and progression-free survival were estimated at 40.5 months and 9.3 months, respectively. Improved outcomes were observed in patients who achieved a complete remission (CR) on brentuximab vedotin, with estimated 3-year overall survival and progression-free survival rates of 73% (95% confidence interval [CI]: 57%, 88%) and 58% (95% CI: 41%, 76%), respectively, in this group (medians not reached). Of the 34 patients who obtained CR, 16 (47%) remain progression-free after a median of 53.3 months (range, 29.0 to 56.2 months) of observation; 12 patients remain progression-free without a consolidative allogeneic stem cell transplant. Younger age, good performance status, and lower disease burden at baseline were characteristic of patients who achieved a CR and were favorable prognostic factors for overall survival. These results suggest that a significant proportion of patients who respond to brentuximab vedotin can achieve prolonged disease control. The trial was registered at www.clinicaltrials.gov as #NCT00848926.
Introduction
The standard of care for patients with relapsed or refractory Hodgkin lymphoma (HL) is salvage chemotherapy followed by autologous stem cell transplant (auto-SCT) in responding patients, which is curative in approximately half of those who undergo the procedure. The ability to achieve and maintain a complete remission (CR) prior to transplant has emerged as a factor important for a favorable progression-free and overall survival (OS) after transplant.1,2
Unfortunately, approximately 50% of patients will experience relapse or progression after auto-SCT. For this population, outcomes have historically been poor, with median OS rates from time of relapse ranging from 10.5 months to 27.6 months.3,4 Although reduced-intensity conditioning (RIC) allogeneic stem cell transplantation (allo-SCT) can induce long-term progression-free survival (PFS), and in some cases secondary cure, in a subset of patients who relapse following auto-SCT, its use is associated with high rates of progression and nonrelapse mortality.5
Brentuximab vedotin (ADCETRIS) is composed of an anti-CD30 antibody conjugated by a protease cleavable linker to monomethyl auristatin E, a microtubule-disrupting agent. In a pivotal phase 2 study of brentuximab vedotin in patients with relapsed or refractory HL after auto-SCT, 75% of patients achieved an objective response (95% confidence interval [CI]: 64.9%, 82.6%) and 34% of patients achieved CR (95% CI: 25.2%, 44.4%) per independent central review.6 The most common treatment-related adverse events were peripheral sensory neuropathy, nausea, fatigue, neutropenia, and diarrhea. Herein, we present response durability and survival in this trial population after a median follow-up period of approximately 3 years. Factors associated with durable remissions and favorable survival are explored.
Methods
Patient eligibility
Eligible patients were aged 12 years or older with relapsed or refractory HL after auto-SCT. Histologic confirmation of CD30-positive Hodgkin Reed-Sternberg cells by central pathology review was required, as well as fluorodeoxyglucose-avid disease by positron emission tomography (PET) and measurable disease of at least 1.5 cm by computed tomography (CT). Patients who had received a prior allo-SCT were ineligible. Additional eligibility criteria are reported by Younes et al.6
Study design and treatment
A complete description of this open-label, phase 2, single-arm study has been previously reported.6 Briefly, this clinical trial was conducted at 25 centers within the United States, Canada, and Europe and was approved by each investigational site’s institutional review board or ethics committee. Patients were recruited between February and August 2009, and all patients provided written informed consent.
Patients received brentuximab vedotin 1.8 mg/kg IV once every 3 weeks over 30 minutes on an outpatient basis for up to 16 infusions.
Study assessments
Clinical response was determined both by investigators and by an independent central review facility (Bioclinica, formerly known as CoreLab Partners and RadPharm; Princeton, NJ) according to the Revised Response Criteria for Malignant Lymphoma.7 Patients were assessed for response by CT at cycles 2, 4, 7, 10, 13, and 16 and by PET at cycles 4 and 7.
During the long-term follow-up period, all patients were followed for survival every 3 months during years 1 to 2, every 6 months during years 3 to 5, and annually thereafter. Patients who discontinued study treatment for any reason other than progressive disease or initiation of new anticancer therapy were also assessed on this schedule for radiographic progression. In October 2013, the protocol was amended to require a CT scan only if progression was suspected clinically. At the time of the amendment, 18 patients were still being assessed for progression; these patients had been in long-term follow-up for a median of over 30 months.
Investigators were also asked to record whether patients had initiated new cancer-related therapy during the long-term follow-up period. Although investigators were asked to specify the type of therapy (eg, systemic chemotherapy vs allogeneic stem cell transplant), details of the therapy (eg, type of conditioning regimen for a transplant) were not prospectively collected.
An independent data monitoring committee assessed the safety of study participants during the trial and monitored the overall study conduct.
Statistical analysis
Data were analyzed by representatives of Seattle Genetics, Inc., and all authors had access to the primary clinical trial data.
The primary end point of the trial was objective response rate (ORR) per the independent review facility (IRF). Secondary end points included duration of response by IRF, CR rate by IRF, PFS by IRF, OS, and the incidence and severity of adverse events. These end points have been previously described and reported.6
The current paper, based on a March 2014 data cutoff, represents a median of approximately 3 years of observation time for all patients. In this paper, we present long-term OS results as well as investigator assessments of response duration and PFS, which were both prespecified additional analyses in the study’s statistical analysis plan. Response duration was calculated from the first objective tumor response (CR or partial remission [PR]) to the first documentation of progression or to death, and PFS was calculated from the start of study treatment to the first documentation of progression or to death. For these analyses, patients were censored at their last assessment (radiologic or clinical) that documented the absence of progressive disease if they were given another treatment before documentation of progression, with the exception of stem cell transplant as the first therapy after discontinuing brentuximab vedotin (“consolidative allo-SCT”).
OS was calculated from start of study treatment to the date of death due to any cause and was censored at the last date the patient was known to be alive. As a post hoc analysis, the Cox proportional hazard model with forward selection was used to assess the effect of multiple variables on OS.8 Significant variables at P = .05 in the univariate analyses were incorporated into the multivariate analyses. The hazard ratio with its 95% confidence interval and 2-sided P values are presented for the final selected factors.
An intrapatient PFS comparison (PFS achieved with the most recent prior systemic therapy before or after auto-SCT vs PFS per investigator with brentuximab vedotin) was performed as a prespecified exploratory analysis. Post hoc subgroup analyses of PFS and OS were performed for best response and for patients who achieved CR per the investigator and then either did or did not undergo allo-SCT.
Results
Patient demographics and characteristics prior to enrollment have been previously summarized.6 Briefly, 102 patients (53% female, 47% male) with a median age of 31 years (range, 15-77 years) were enrolled. The majority of enrolled patients were white (87%). Patients were heavily pretreated, and all had received at least 1 auto-SCT. The median time to relapse following the most recent auto-SCT was 6.7 months (range, 0-131 months; Table 1).
Table 1.
Patient demographics and characteristics at enrollment
| All patients (N = 102) | |
|---|---|
| Median time from initial HL diagnosis to first dose in months (range) | 39.9 (12-220) |
| Stage at initial diagnosis, n (%) | |
| Stage I/II | 51 (50) |
| Stage III | 27 (26) |
| Stage IV | 20 (20) |
| Unknown | 4 (4) |
| ECOG performance status, n (%) | |
| Grade 0 | 42 (41) |
| Grade 1 | 60 (59) |
| Patients with primary refractory disease,* n (%) | 72 (71) |
| Disease status relative to most recent prior therapy,† n (%) | |
| Relapse | 59 (58) |
| Refractory | 43 (42) |
| Median number of prior cancer-related systemic therapy regimens‡ (range) | 3.5 (1-13) |
| Median PFS for most recent regimen in months (95% CI) | 6.1 (4.4, 7.2) |
| Number of prior auto-SCTs, n (%) | |
| 1 | 91 (89) |
| 2 | 11 (11) |
| Median time from most recent auto-SCT to relapse after auto-SCT in months (range) | 6.7 (0-131) |
Absence of CR or relapse within 3 months of front-line therapy.
Relapse indicates the best response of CR or PR to most recent prior therapy, whereas refractory indicates the best response of stable or progressive disease to most recent prior therapy.
Includes chemotherapy given for stem cell mobilization.
By investigator assessment, the ORR to brentuximab vedotin was 72% (33% CR, 38% PR), which was nearly identical to the ORR per IRF that was previously reported.6 Median time to the first objective response per investigator was 5.7 weeks, or approximately the time of the first response assessment (CT) after the cycle 2 infusion. Median time to CR per investigator was 12.2 weeks, or approximately the time of the second response assessment (CT/PET) after the cycle 4 infusion.
After discontinuing treatment with brentuximab vedotin, patients could receive additional therapies at the discretion of the investigator. No specific salvage therapy was recommended per protocol; subsequent therapies were heterogeneous and included both single- and multiagent regimens. Eight patients received a consolidative allo-SCT (6 received the consolidative allo-SCT in CR and 2 received the consolidative allo-SCT in PR).
Long-term follow-up
Duration of response
Enrolled patients (N = 102) have been followed for a median of almost 3 years (median, 33.3 months; range, 1.8 to 57.3 months) from their first dose of brentuximab vedotin. The estimated median duration of response in the 73 patients who achieved at least a PR on treatment was 11.2 months (95% CI: 7.7, 18.7; Table 2). The median duration of response for the 34 patients who achieved a CR per the investigator has not been reached (95% CI: 20.5 months, —).
Table 2.
Duration of response per the investigator following treatment with brentuximab vedotin
| All patients (N = 102) | |
|---|---|
| Objective response according to the investigator, n (%) | 73 (72) |
| CR, n (%) | 34 (33) |
| PR, n (%) | 39 (38) |
| Duration of response for patients with CR, months | |
| Median | NE |
| 95% CI | 20.5, NE |
| Duration of objective response, months | |
| Median | 11.2 |
| 95% CI | 7.7, 18.7 |
Duration of response is calculated from the earliest occurrence of either CR or PR.
NE, not estimable.
As of last follow-up, a total of 18 patients, or 25% of patients with an objective response to brentuximab vedotin, are still on study and in remission without the start of new therapy, other than a consolidative allo-SCT (Figure 1). The 18 patients have been followed for a median of 53.3 months (range, 29.0 to 56.2 months) from their first dose of brentuximab vedotin. Sixteen of the 18 patients who are still in remission achieved a CR on brentuximab vedotin, which represents 47% (16/34) of all patients with an investigator assessment of CR.
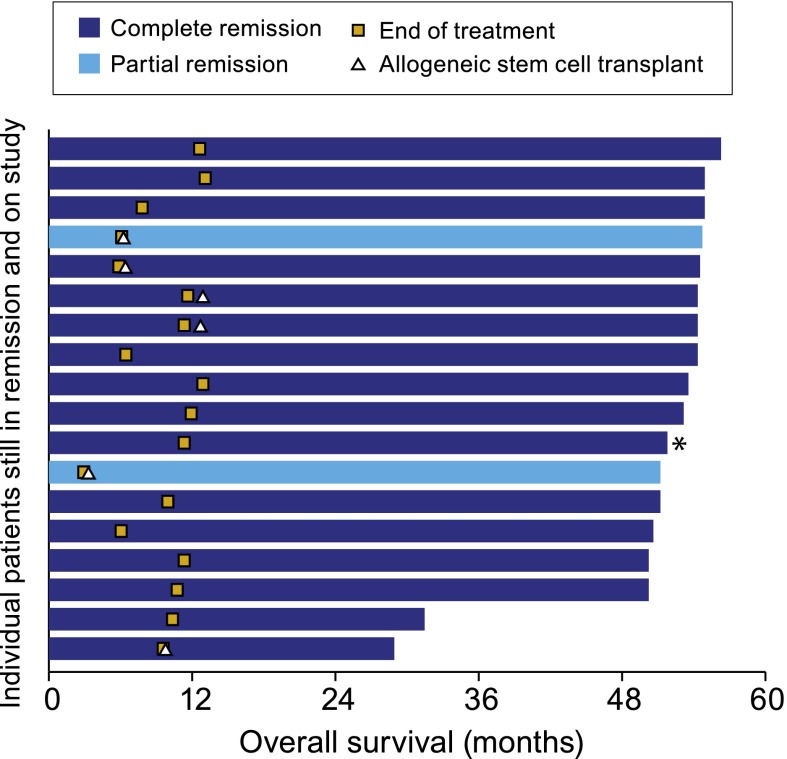
Figure 1.
Patients who remain in remission per the investigator following treatment with brentuximab vedotin. Includes patients who remain in remission according to the investigator, are still on study being followed for survival, and have not started new anticancer therapy (n = 18). Patients are shaded according to their best response on treatment with brentuximab vedotin. Six patients received an allogeneic stem cell transplant shortly after completing treatment with brentuximab vedotin. The 2 patients with a PR to brentuximab vedotin achieved a CR following transplant. Subsequent to end of treatment, 1 patient (indicated by an asterisk) received continued treatment with brentuximab vedotin as part of a separate treatment extension protocol.
Six of the 18 patients who remain in remission received a consolidative allo-SCT, including 4 of 6 patients who went to allo-SCT in CR and the 2 patients who went to allo-SCT in PR. The proportion of patients with a best response of CR who remain in remission without a consolidative allo-SCT is 43% (12/28); the median observation time in this group is 52.5 months (range, 31.5 to 56.2). The 2 patients who went to allo-SCT in PR subsequently converted to CR.
In an effort to identify characteristics associated with long-term remission, the 18 patients were compared with patients who had a CR or PR to brentuximab vedotin but who had subsequently progressed, started new therapy, or came off study (“all other responders”); as well as with patients who did not have at least a PR to brentuximab vedotin (“nonresponders”) (Table 3). Relative to other patients, those in remission tended to be female, younger, diagnosed with HL for a shorter period prior to receiving treatment with brentuximab vedotin, and have relapsed rather than refractory disease to the most recent prior therapy, a more favorable Eastern Cooperative Oncology Group (ECOG) performance score, and a smaller disease burden (ie, lower-stage disease and lower median sum of the products of diameters [SPD]) prior to enrollment. Relative to other patients, those still in remission also received more cycles of brentuximab vedotin, which is not unexpected given the protocol requirement that patients with progressive disease discontinue treatment. Notably, patients still in remission were comparable to other patients with respect to the incidence and severity of AEs, suggesting that their longer treatment duration relative to the other patients was not directly attributable to a lesser AE burden.
Table 3.
Characterization of patients who remain in remission per the investigator following treatment with brentuximab vedotin
| In remission* (n = 18) | All other responders† (n = 55) | Nonresponders (n = 29) | |
|---|---|---|---|
| Demographics and baseline disease characteristics | |||
| Median age in years (range) | 26.5 (15-63) | 32.0 (18-69) | 35.0 (18-77) |
| Female, n (%) | 12 (67) | 31 (56) | 11 (38) |
| ECOG performance status, n (%) | |||
| Grade 0 | 10 (56) | 22 (40) | 10 (34) |
| Grade 1 | 8 (44) | 33 (60) | 19 (66) |
| Relapsed relative to most recent therapy,‡ n (%) | 14 (78) | 29 (53) | 16 (55) |
| Primary refractory disease,§ n (%) | 13 (72) | 36 (65) | 23 (79) |
| Stage, n (%) | |||
| Stage I/II | 14 (78) | 27 (49) | 10 (34) |
| Stage III | 3 (17) | 14 (25) | 10 (34) |
| Stage IV | 1 (6) | 11 (20) | 8 (28) |
| Median time in months from initial diagnosis to first dose (range) | 36.5 (12-99) | 46.1 (14-219) | 38.1 (12-114) |
| Median time in months from last auto-SCT to relapse prior to b-v (range) | 7.8 (1-63) | 6.4 (1-131) | 6.5 (0-53) |
| Median SPD (cm2) per investigator (range) | 16.1 (2-55) | 24.5 (2-276) | 29.1 (3-157) |
| Exposure | |||
| Median duration of treatment in weeks (range) | 43.6 (13-56) | 30.1 (9-54) | 20.9 (3-52) |
| Median number of cycles (range) | 13.5 (4-16) | 10 (3-16) | 7 (1-16) |
| Median % relative dose intensity (range) | 94.5 (73-103) | 96.0 (69-107) | 98.7 (79-102) |
| Adverse events, n (%) | |||
| Serious adverse event | 3 (17) | 11 (20) | 11 (38) |
| Adverse event discontinuation | 5 (28) | 9 (16) | 6 (21) |
| ≥Grade 3 adverse event | 11 (61) | 28 (51) | 17 (59) |
| Peripheral neuropathy (standardized MedDRA query) | 13 (72) | 28 (51) | 15 (52) |
b-v, brentuximab vedotin.
Patients with a best response of CR (n = 16) or PR (n = 2) on treatment who are still on study and in remission without the start of new anticancer therapy, other than allogeneic stem cell transplant. The 2 patients with a PR to brentuximab vedotin achieved CR subsequent to transplant.
Patients with a best response of CR or PR on treatment who experienced progressive disease, initiated new therapy, or discontinued from the study for reasons including death, lost to follow-up, withdrawal of consent, and investigator decision.
Best response of CR or PR to most recent prior therapy.
No CR or relapse within 3 months of front-line therapy.
The demographics and baseline disease characteristics of the 16 patients who achieved CR on brentuximab vedotin and who remain in remission were compared with those of the other 18 patients who achieved CR but who subsequently progressed, started new therapy, or came off study (Table 4). Although patients with CR who remain in remission were younger and had been diagnosed with HL for a shorter period of time relative to other patients with a best response of CR, the 2 groups were generally comparable across variables.
Table 4.
Demographics and baseline characteristics of patients with a best response of CR per the investigator by current status
| CR and in remission* (n = 16) | All other CR† (n = 18) | |
|---|---|---|
| Median age in years (range) | 26.5 (15-63) | 38.5 (21-51) |
| Female, n (%) | 10 (63) | 13 (72) |
| ECOG performance status, n (%) | ||
| Grade 0 | 9 (56) | 9 (50) |
| Grade 1 | 7 (44) | 9 (50) |
| Relapsed relative to most recent therapy,‡ n (%) | 12 (75) | 11 (61) |
| Primary refractory disease,§ n (%) | 12 (75) | 12 (67) |
| Stage, n (%) | ||
| Stage I/II | 12 (75) | 12 (67) |
| Stage III | 3 (19) | 2 (11) |
| Stage IV | 1 (6) | 3 (17) |
| Median time in months from initial diagnosis to first dose (range) | 36.5 (16-99) | 45.8 (14-185) |
| Median time in months from last auto-SCT to relapse prior to b-v (range) | 8.4 (1-63) | 8.9 (2-31) |
| Median SPD (cm2) per investigator (range) | 16.1 (2-55) | 17.3 (3-116) |
b-v, brentuximab vedotin.
Patients with a best response of CR on treatment who are still on study and in remission without the start of new anticancer therapy, other than allogeneic stem cell transplant.
Patients with a best response of CR on treatment who either: experienced progressive disease, initiated new therapy, or discontinued from the study for reasons including death, lost to follow-up, withdrawal of consent, and investigator decision.
Best response of CR or PR to most recent prior therapy.
No CR or relapse within 3 months of front-line therapy.
OS and PFS
After a median follow-up period of approximately 3 years for all enrolled patients, 47% of patients (48/102) were alive. The estimated median OS was 40.5 months (95% CI: 28.7, —; Figure 2). Patients who achieved a CR had a longer OS compared with those without a CR (CR: median not reached [95% CI: 48.1 months, —], PR: 39.4 months [95% CI: 22.9, —], and stable disease [SD]: 18.3 months [95% CI: 12.6, 36.8]). The estimated 3-year OS for the 34 patients with a CR to brentuximab vedotin was 73% (95% CI: 57%, 88%). The estimated 3-year OS was 80% (95% CI: 45%, 100%) for the subgroup of 6 patients with a CR who received a consolidative allo-SCT and 71% (95% CI: 54%, 88%) for the subgroup of 28 patients with a CR who did not receive a consolidative allo-SCT (Kaplan-Meier curves are shown in supplemental Figure 1, available on the Blood Web site).
Figure 2.
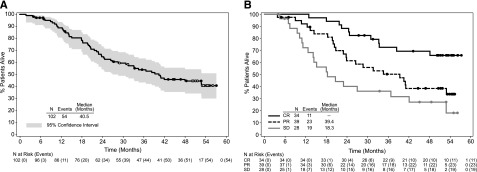
OS following treatment with brentuximab vedotin. OS was analyzed using Kaplan-Meier methodology and is shown overall (A) and by best response (B). All censored patients are indicated by dots on the Kaplan-Meier curve. Patients still on study and in remission without the start of new therapy are indicated by open dots on the Kaplan-Meier curve in panel A.
Univariate analyses were conducted to explore potential associations between patient characteristics and OS. Five characteristics emerged as significant prognostic factors in univariate analyses: age, number of systemic chemotherapy regimens prior to treatment with brentuximab vedotin, baseline ECOG performance status score, baseline SPD, and baseline serum concentration of soluble CD30 (Table 5). Multivariate analyses revealed age, baseline ECOG status, and baseline SPD as significant independent prognostic factors for OS (Table 6).
Table 5.
Patient characteristics and OS (univariate analysis)
| Characteristics | No. of patients (N = 102) | Hazard ratio | 95% CI | P |
|---|---|---|---|---|
| Age* | — | 1.33† | 1.07, 1.65 | .010 |
| Gender | .505 | |||
| Female‡ | 54 | |||
| Male | 48 | 1.20 | 0.70, 2.05 | |
| Total number of prior systemic therapy regimens* | — | 1.12 | 1.01, 1.24 | .026 |
| Best response achieved with most recent regimen | .537 | |||
| Objective response‡ | 47 | |||
| Stable disease | 23 | 0.86 | 0.43, 1.71 | |
| Progressive disease | 26 | 1.34 | 0.7, 2.58 | |
| Unknown/other | 6 | 0.55 | 0.13, 2.31 | |
| Any prior cancer-related radiotherapy | .975 | |||
| Yes‡ | 67 | |||
| No | 35 | 1.01 | 0.58, 1.76 | |
| Time from initial diagnosis to first dose (months)* | — | 1.00 | 0.99, 1.00 | .406 |
| Time since diagnosis (months)/total no. of systemic therapies* | — | 1.02 | 0.99, 1.06 | .233 |
| Time from last auto-SCT to relapse prior to b-v (months)* | — | 1.00 | 0.98, 1.01 | .694 |
| PFS from prior cancer therapy (months)* | — | 0.99 | 0.97, 1.01 | .455 |
| Stage at initial diagnosis,§ n (%) | .094 | |||
| Stage I/II‡ | 51 | |||
| Stage III | 27 | 1.64 | 0.84, 3.19 | |
| Stage IV | 20 | 2.04 | 1.04, 4.01 | |
| Baseline ECOG performance status | .004 | |||
| Grade 0‡ | 42 | |||
| Grade 1 | 60 | 2.37 | 1.32, 4.26 | |
| Baseline electrocardiogram | .423 | |||
| Normal‡ | 60 | |||
| Abnormal | 42 | 1.25 | 0.73, 2.14 | |
| Baseline SPD per investigator (cm2)* | — | 1.04|| | 1.00, 1.08 | .033 |
| Baseline soluble CD30 (ng/mL)* | — | 1.00 | 1.00, 1.00 | .025 |
| Primary refractory status | .931 | |||
| No‡ | 30 | |||
| Yes | 72 | 0.97 | 0.55, 1.73 | |
| Disease status relative to most recent prior therapy | .089 | |||
| Refractory‡ | 43 | |||
| Relapse | 59 | 0.63 | 0.37, 1.07 | |
| Baseline B symptoms | .662 | |||
| No‡ | 67 | |||
| Yes | 35 | 1.13 | 0.65, 1.97 |
Significant factors in the univariate analysis are indicated in italic font.
b-v, brentuximab vedotin.
Continuous variable.
Hazard ratio applies to 10-year increments.
Reference level for the hazard ratio.
Stage was unknown for 4 patients.
Hazard ratio applies to 10-cm2 increments.
Table 6.
Patient characteristics and OS (multivariate analysis)
| Characteristics | Hazard ratio | 95% CI | P |
|---|---|---|---|
| Age* | 1.33 | 1.05, 1.69 | .016 |
| Baseline ECOG performance status | .019 | ||
| Grade 0† | |||
| Grade 1 | 2.05 | 1.13, 3.73 | |
| Baseline SPD per investigator (cm2)‡ | 1.06 | 1.01, 1.10 | .009 |
Continuous variable; hazard ratio applies to 10-year increments.
Reference level for the hazard ratio.
Continuous variable; hazard ratio applies to 10-cm2 increments.
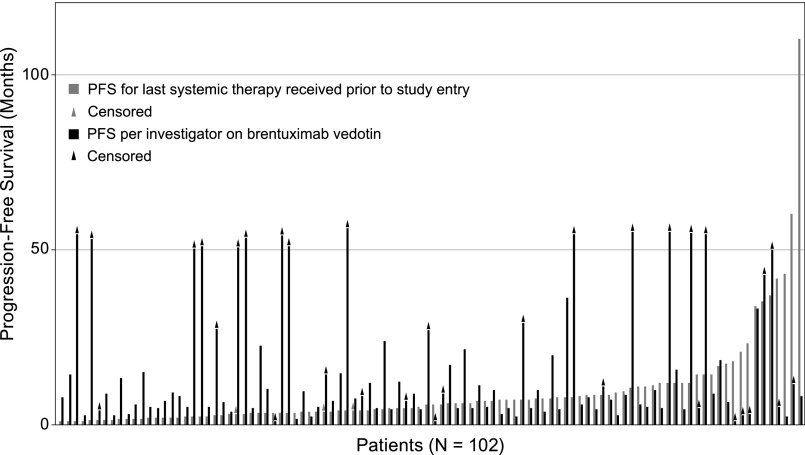
The updated estimated median PFS per the investigator for all patients was 9.3 months (95% CI: 7.1, 12.2; Figure 3), which was approximately 3 months longer than the median PFS of 6.1 months (95% CI: 4.4, 7.2; Table 1) that was observed on the patients’ last systemic therapy prior to brentuximab vedotin. For approximately 64% (65/102) of enrolled patients, the PFS on brentuximab vedotin was longer than that reached on their last prior therapy (Figure 4). Most progression events occurred early and in patients who did not achieve CR: 78% (54/69) of all events occurred within the first year of observation and 87% (47/54) of events during the first year occurred in patients with PR or SD. A total of 16 patients have been followed longer than the last event that occurred at 36.4 months after the first dose of brentuximab vedotin; 15 of these patients (13 with a best response of CR and 2 with a best response of PR) have been followed for at least 50 months without progression. The median PFS for patients who achieved CR has not been reached (95% CI: 21.7 months, —), which is in contrast to a median PFS of 5.8 months (95% CI: 3.8, 8.1) to the last prior therapy for the same group of patients. Thirty-one of the 34 patients (91%) with a best response of CR to brentuximab vedotin had a longer PFS on brentuximab vedotin than their last prior therapy. Three-year PFS was estimated at 58% (95% CI: 41%, 76%) for the 34 patients with a CR to brentuximab vedotin (Figure 3). The estimated 3-year PFS was 80% (95% CI: 45%, 100%) for the subgroup of 6 patients with a CR who received a consolidative allo-SCT and 53% (95% CI: 34%, 73%) for the subgroup of 28 patients with a CR who did not receive a consolidative allo-SCT (Kaplan-Meier curves available in supplemental Figure 1).
Figure 3.
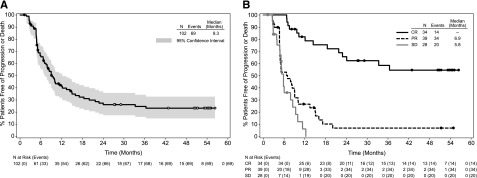
PFS following treatment with brentuximab vedotin. PFS was analyzed using Kaplan-Meier methodology and is shown overall (A) and by best response (B). All censored patients are indicated by dots on the Kaplan-Meier curve. Patients still on study and in remission without the start of new therapy are indicated by open dots on the Kaplan-Meier curve in panel A. One patient was not evaluable for response and is excluded from panel B.
Figure 4.
PFS relative to most recent prior therapy. Includes all enrolled patients (N = 102). PFS was analyzed using Kaplan-Meier methodology and was calculated for each patient’s last systemic therapy received prior to study (gray bar) and on brentuximab vedotin (black). Triangles at the end of bars indicate censored patients. Patients are sorted left to right on the x-axis according to the duration of PFS on their last systemic therapy. Sixty-five patients (64%) had longer PFS on brentuximab vedotin than their last prior therapy.
Discussion
Durable remissions and favorable long-term survival are observed in patients with relapsed/refractory HL who received treatment with brentuximab vedotin. Eighteen patients, 25% of patients with an objective response to brentuximab vedotin, remain on study and in remission without the start of new therapy, other than consolidative allo-SCT, after having been followed for a median of 53.3 months from their first dose. Ongoing remissions of more than 4 years are particularly noteworthy for a single agent in patients who had relapsed or progressed after both combination therapy and auto-SCT. Fifteen of the 18 patients have been followed for more than 4 years, or more than a year after the last progression event, which suggests that brentuximab vedotin may be curative in a fraction of patients. Of course, a decade or more of follow-up may be required before the curative potential of brentuximab vedotin in the multiply relapsed/refractory setting can be confirmed. Although relapses are usually clinically symptomatic and patient-reported signs and symptoms appear reliable in the detection of lymphoma progression,9-15 it is possible that progression based on CT assessment alone and in lieu of clinical symptoms may have been declared for some patients if the requirement for routine CT scanning had been maintained.
The median OS was estimated at 40.5 months after a median follow-up of approximately 3 years for all enrolled patients. A median OS of approximately 3.5 years compares favorably to the historical survival duration range of 10.5 to 27.6 months that has been reported for patients with HL who relapse/progress after auto-SCT.3,4 The median OS is particularly noteworthy given that patients in the present study, who have relapsed/progressed a median of only 6.7 months from their auto-SCT, represent a particularly vulnerable population. Shorter survival durations have been reported for patients who relapse within a year of transplant relative to patients who relapse later.3,16 Of course, given the potential for bias in comparisons to historical data, the ability of brentuximab vedotin to prolong survival in patients with relapsed/refractory HL requires confirmation in a randomized clinical trial.
Patients’ median PFS on brentuximab vedotin was longer than the median PFS on their most recent prior therapy by more than 3 months (brentuximab vedotin: 9.3 months; most recent prior therapy: 6.1 months). Although shorter PFS durations could be expected to accompany subsequent therapies for patients with multiply relapsed disease, the majority of patients (64%) demonstrated a longer PFS on brentuximab vedotin compared with their most recent prior systemic therapy. Initiation of posttreatment anticancer therapy that included allo-SCT, additional cycles of brentuximab vedotin, as well as additional single-and combination chemotherapy regimens likely contributed to the prolonged survival that was observed relative to the PFS.
Attainment of a CR appeared critical for prolonged disease control. Sixteen of the 18 patients still in remission achieved CR on brentuximab vedotin, representing a long-term remission rate of 47% for patients with CR. The estimated medians for response duration, PFS, and OS have not been reached for patients who achieved CR. The vast majority of progressions occurred within the first year of observation and in patients with PR or SD. Acknowledging that patients must have had relapsed/refractory disease after their last prior therapy in order to enroll in this study, it is notable that patients who attained a CR on brentuximab vedotin did not enjoy longer disease control on their last prior therapy relative to other patients. Patients with a CR on brentuximab vedotin had a median PFS of 5.8 months on their last prior therapy, which was comparable to the median PFS on prior therapy for all patients (6.1 months).
In addition to the majority having had attained a CR on brentuximab vedotin, the 18 patients who are still in remission after extended follow-up tended to be young females with a performance status score of “0” and smaller disease burden who had relapsed disease to their most recent prior therapy and less elapsed time since their HL diagnosis. These characteristics were also representative of the general ability to achieve a CR on brentuximab vedotin, with younger age and shorter elapsed times since diagnosis being even more pronounced in the subgroup of patients with CR who remain in remission. When baseline characteristics were explored across the total enrolled patient population for their association with OS, not surprisingly, younger age, a baseline ECOG performance status score of “0”, and smaller baseline index lesion size emerged as significant favorable independent prognostic factors. The associations between lower age, improved baseline functioning, and lower disease burden with improved survival could suggest that lymphoma may best be treated with brentuximab vedotin in the minimal residual disease state, rather than waiting until frank relapse. Alternatively, these associations could simply reflect a cohort of younger, less impaired patients starting treatment with brentuximab vedotin earlier in their disease course relative to other patients. Results from a phase 3 randomized, double-blind, placebo-controlled study evaluating the potential of brentuximab vedotin to prevent relapse post–auto-SCT in patients at high risk of lymphoma progression (AETHERA trial, ClinicalTrials.gov #NCT01100502) will help determine the benefit of treatment with brentuximab vedotin earlier in the disease course of HL.
Allografting select patients with HL has been used to improve clinical outcomes,5,17-27 and recent case series have started to explore brentuximab vedotin as a potential “bridge” to allo-SCT.28-32 Eight patients (6 in CR and 2 in PR) in the present study received an allo-SCT as their first treatment after discontinuing brentuximab vedotin. Four of 6 patients who received an allo-SCT in CR remain progression-free, with the 3-year PFS rate estimated at 80% for this small population. This rate compares favorably to 3-year PFS rates of 22%24 to 25%20 that have been reported for patients with relapsed/refractory HL who undergo allo-SCT and is consistent with the higher PFS rates of 50% to 62% (4 years) reported for subsets of patients who enter allo-SCT in CR or with minimal residual disease.5,18 The proportion of patients remaining in remission was slightly higher in the subgroup of patients who achieved a CR on brentuximab vedotin and received a consolidative allo-SCT (4 of 6, or 67%) relative to patients who achieved a CR on treatment and who did not undergo transplant (43%). However, the number of patients who received an allo-SCT was small, and additional studies are required to determine whether allo-SCT is the best management approach for patients who achieve remission on brentuximab vedotin. Whether consolidative allo-SCT has a role in improving outcomes for patients who achieve a best response of PR to brentuximab vedotin is worthy of further exploration in light of the observation that both patients in the current study who received an allo-SCT in PR converted to CR and remain in remission.
Brentuximab vedotin can induce durable remissions in a subset of heavily pretreated patients with HL who had relapsed post–auto-SCT, and the OS compares favorably to historical data. The single-arm design is a limitation of the study. Results from the AETHERA trial will provide PFS estimates for brentuximab vedotin relative to best supportive care in patients at risk of relapse after auto-SCT. A randomized phase 3 study is also being conducted to evaluate brentuximab vedotin in combination with AVD (doxorubicin, vinblastine, and dacarbazine) vs ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) for front-line treatment of HL (Echelon-1 trial, ClinicalTrials.gov #NCT01712490). Together, these phase 3 studies will help evaluate the role of brentuximab vedotin alone and in combination with standard chemotherapy in earlier phases of HL.
Acknowledgments
The authors thank Lisa Thomson, an employee of Seattle Genetics, Inc., for assistance in manuscript preparation.
Direct funding for this research was issued by Seattle Genetics, Inc. through the joint financial support of Seattle Genetics, Inc. and Takeda Pharmaceuticals International Co. A.K.G. is a Scholar in Clinical Research of the Leukemia and Lymphoma Society and is supported by National Institutes of Health, National Cancer Institute grant P01CA44991, Fred Hutchinson Cancer Research Center/University of Washington Cancer Consortium Cancer Center support grant P30 CA015704, and gifts from Frank and Betty Vandermeer. R.C. is a Tim Nesvig Lymphoma Fellow and is supported by the National Institutes of Health, National Cancer Institute award number K12CA001727. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Presented in abstract form at the 55th annual meeting of the American Society of Hematology, New Orleans, LA, December 7-10, 2013, and the 12th International Conference on Malignant Lymphoma, Lugano, Switzerland, June 19-20, 2013.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.K.G., R.C., E.K.L., X.C., and E.L.S. contributed to the analysis and interpretation of data and wrote the manuscript; A.K.G., R.C., S.E.S., S.M.A., J.D.R., K.J.S., J.M.C., A.E., and A.Y. contributed to the acquisition of the data; S.E.S., S.M.A., J.D.R., K.J.S., J.M.C., A.E., and A.Y. critically reviewed the manuscript; and all authors contributed to the concept and design of the study and approved the final version of the manuscript.
Conflict-of-interest disclosure: Seattle Genetics, Inc. provided research funding to the institutions of A.K.G., R.C., S.E.S., S.M.A., J.D.R., K.J.S., J.M.C., A.E., and A.Y., A.K.G., R.C., and K.S. have acted as consultants for Seattle Genetics, Inc. A.K.G., K.S., A.E., and A.Y. have received honoraria from Seattle Genetics, Inc. A.K.G. has received research funding/grants from BMS, Gilead, Spectrum, Pfizer, Cephalon/Teva, Emergent/Abbott, Gilead, Janssen, Merck, Takeda Pharmaceuticals International Co., Piramal, Biogen Idec, and BioMarin. A.K.G. has acted as a consultant for Pfizer and Sanofi-Aventis and has received honoraria from Takeda Pharmaceuticals International Co. and Sanofi-Aventis. R.C. has participated in a Seattle Genetics, Inc. speakers’ bureau and has received travel expenses from Seattle Genetics, Inc. S.E.S. has participated in a Celgene speakers’ bureau. J.M.C. has received research funding/grants from Roche. A.E. has received research funding/grants and honoraria from Takeda Pharmaceuticals International Co. E.L.S. and E.K.L. are employed by and have equity ownership (including stock options) in Seattle Genetics, Inc. X.C. is employed by and has equity ownership (including stock options) in Takeda Pharmaceuticals International Co. A.Y. has received research funding/grants from Curis, Janssen, and Novartis and has received honoraria from Sanofi-Aventis, Takeda Pharmaceuticals International Co., Celgene, and Bayer.
Correspondence: Ajay Gopal, University of Washington/Seattle Cancer Care Alliance, 825 Eastlake Ave E, G3-200, Seattle, WA 98109; e-mail: agopal@u.washington.edu.
References
- 1.Sureda A, Arranz R, Iriondo A, et al. Grupo Español de Linformas/Transplante Autólogo de Médula Osea Spanish Cooperative Group. Autologous stem-cell transplantation for Hodgkin’s disease: results and prognostic factors in 494 patients from the Grupo Español de Linfomas/Transplante Autólogo de Médula Osea Spanish Cooperative Group. J Clin Oncol. 2001;19(5):1395–1404. doi: 10.1200/JCO.2001.19.5.1395. [DOI] [PubMed] [Google Scholar]
- 2.Sureda A, Constans M, Iriondo A, et al. Grupo Español de Linfomas/Trasplante Autólogo de Médula Osea Cooperative Group. Prognostic factors affecting long-term outcome after stem cell transplantation in Hodgkin’s lymphoma autografted after a first relapse. Ann Oncol. 2005;16(4):625–633. doi: 10.1093/annonc/mdi119. [DOI] [PubMed] [Google Scholar]
- 3.Arai S, Fanale M, DeVos S, et al. Defining a Hodgkin lymphoma population for novel therapeutics after relapse from autologous hematopoietic cell transplant. Leuk Lymphoma. 2013;54(11):2531–2533. doi: 10.3109/10428194.2013.798868. [DOI] [PubMed] [Google Scholar]
- 4.Crump M. Management of Hodgkin lymphoma in relapse after autologous stem cell transplant. Hematology (Am Soc Hematol Educ Program) 2008:326–333. doi: 10.1182/asheducation-2008.1.326. [DOI] [PubMed] [Google Scholar]
- 5.Sureda A, Canals C, Arranz R, et al. Allogeneic stem cell transplantation after reduced intensity conditioning in patients with relapsed or refractory Hodgkin’s lymphoma. Results of the HDR-ALLO study: a prospective clinical trial by the Grupo Español de Linfomas/Trasplante de Médula Osea (GEL/TAMO) and the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2012;97(2):310–317. doi: 10.3324/haematol.2011.045757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30(18):2183–2189. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheson BD, Pfistner B, Juweid ME, et al. International Harmonization Project on Lymphoma. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 8.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34(2):187–220. [Google Scholar]
- 9.Bestawros A, Foltz L, Srour N, Savage KJ, Connors JM. Patients’ and physicians’ roles in detecting recurrent Hodgkin lymphoma following complete remission. Ann Oncol. 2013;24(5):1359–1363. doi: 10.1093/annonc/mds606. [DOI] [PubMed] [Google Scholar]
- 10.Dryver ET, Jernström H, Tompkins K, Buckstein R, Imrie KR. Follow-up of patients with Hodgkin’s disease following curative treatment: the routine CT scan is of little value. Br J Cancer. 2003;89(3):482–486. doi: 10.1038/sj.bjc.6601052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Galaly TC, Mylam KJ, Brown P, et al. Positron emission tomography/computed tomography surveillance in patients with Hodgkin lymphoma in first remission has a low positive predictive value and high costs. Haematologica. 2012;97(6):931–936. doi: 10.3324/haematol.2011.056010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerlinger M, Rohatiner AZ, Matthews J, Davies A, Lister TA, Montoto S. Surveillance investigations after high-dose therapy with stem cell rescue for recurrent follicular lymphoma have no impact on management. Haematologica. 2010;95(7):1130–1135. doi: 10.3324/haematol.2009.020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guadagnolo BA, Punglia RS, Kuntz KM, Mauch PM, Ng AK. Cost-effectiveness analysis of computerized tomography in the routine follow-up of patients after primary treatment for Hodgkin’s disease. J Clin Oncol. 2006;24(25):4116–4122. doi: 10.1200/JCO.2006.07.0409. [DOI] [PubMed] [Google Scholar]
- 14.Radford JA, Eardley A, Woodman C, Crowther D. Follow up policy after treatment for Hodgkin’s disease: too many clinic visits and routine tests? A review of hospital records. BMJ. 1997;314(7077):343–346. doi: 10.1136/bmj.314.7077.343a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torrey MJ, Poen JC, Hoppe RT. Detection of relapse in early-stage Hodgkin’s disease: role of routine follow-up studies. J Clin Oncol. 1997;15(3):1123–1130. doi: 10.1200/JCO.1997.15.3.1123. [DOI] [PubMed] [Google Scholar]
- 16.Horning S, Fanale M, deVos S, et al. Defining a population of Hodgkin lymphoma patients for novel therapeutics: an international effort. Ann Oncol. 2008;19(suppl 4):118. [Google Scholar]
- 17.Sureda A, Robinson S, Canals C, et al. Reduced-intensity conditioning compared with conventional allogeneic stem-cell transplantation in relapsed or refractory Hodgkin’s lymphoma: an analysis from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2008;26(3):455–462. doi: 10.1200/JCO.2007.13.2415. [DOI] [PubMed] [Google Scholar]
- 18.Sobol U, Rodriguez T, Smith S, et al. Seven-year follow-up of allogeneic transplant using BCNU, etoposide, cytarabine and melphalan chemotherapy in patients with Hodgkin lymphoma after autograft failure: importance of minimal residual disease. Leuk Lymphoma. 2014;55(6):1281–1287. doi: 10.3109/10428194.2013.838233. [DOI] [PubMed] [Google Scholar]
- 19.Sarina B, Castagna L, Farina L, et al. Gruppo Italiano Trapianto di Midollo Osseo. Allogeneic transplantation improves the overall and progression-free survival of Hodgkin lymphoma patients relapsing after autologous transplantation: a retrospective study based on the time of HLA typing and donor availability. Blood. 2010;115(18):3671–3677. doi: 10.1182/blood-2009-12-253856. [DOI] [PubMed] [Google Scholar]
- 20.Robinson SP, Sureda A, Canals C, et al. Lymphoma Working Party of the EBMT. Reduced intensity conditioning allogeneic stem cell transplantation for Hodgkin’s lymphoma: identification of prognostic factors predicting outcome. Haematologica. 2009;94(2):230–238. doi: 10.3324/haematol.13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devetten MP, Hari PN, Carreras J, et al. Unrelated donor reduced-intensity allogeneic hematopoietic stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Biol Blood Marrow Transplant. 2009;15(1):109–117. doi: 10.1016/j.bbmt.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen R, Palmer JM, Popplewell L, et al. Reduced intensity allogeneic hematopoietic cell transplantation can induce durable remission in heavily pretreated relapsed Hodgkin lymphoma. Ann Hematol. 2011;90(7):803–808. doi: 10.1007/s00277-010-1146-3. [DOI] [PubMed] [Google Scholar]
- 23.Burroughs LM, O’Donnell PV, Sandmaier BM, et al. Comparison of outcomes of HLA-matched related, unrelated, or HLA-haploidentical related hematopoietic cell transplantation following nonmyeloablative conditioning for relapsed or refractory Hodgkin lymphoma. Biol Blood Marrow Transplant. 2008;14(11):1279–1287. doi: 10.1016/j.bbmt.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armand P, Kim HT, Ho VT, et al. Allogeneic transplantation with reduced-intensity conditioning for Hodgkin and non-Hodgkin lymphoma: importance of histology for outcome. Biol Blood Marrow Transplant. 2008;14(4):418–425. doi: 10.1016/j.bbmt.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderlini P, Saliba R, Acholonu S, et al. Fludarabine-melphalan as a preparative regimen for reduced-intensity conditioning allogeneic stem cell transplantation in relapsed and refractory Hodgkin’s lymphoma: the updated M.D. Anderson Cancer Center experience. Haematologica. 2008;93(2):257–264. doi: 10.3324/haematol.11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvarez I, Sureda A, Caballero MD, et al. Nonmyeloablative stem cell transplantation is an effective therapy for refractory or relapsed hodgkin lymphoma: results of a spanish prospective cooperative protocol. Biol Blood Marrow Transplant. 2006;12(2):172–183. doi: 10.1016/j.bbmt.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Peggs KS, Hunter A, Chopra R, et al. Clinical evidence of a graft-versus-Hodgkin’s-lymphoma effect after reduced-intensity allogeneic transplantation. Lancet. 2005;365(9475):1934–1941. doi: 10.1016/S0140-6736(05)66659-7. [DOI] [PubMed] [Google Scholar]
- 28.Chen R, Forman S, Palmer JM, et al. Two-year follow-up of patients with relapsed/refractory hodgkin treated with brentuximab vedotin prior to reduced intensity allogeneic hematopoietic cell transplantation [abstract]. Hematol Oncol. 2013;31(S1):a140. [Google Scholar]
- 29.Chen R, Palmer JM, Thomas SH, et al. Brentuximab vedotin enables successful reduced-intensity allogeneic hematopoietic cell transplantation in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2012;119(26):6379–6381. doi: 10.1182/blood-2012-03-418673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garciaz S, Coso D, Peyrade F, et al. Brentuximab vedotin followed by allogeneic transplantation as salvage regimen in patients with relapsed and/or refractory Hodgkin’s lymphoma. Hematol Oncol. 2014;32(4):187–191. doi: 10.1002/hon.2119. [DOI] [PubMed] [Google Scholar]
- 31.Gibb A, Jones C, Bloor A, et al. Brentuximab vedotin in refractory CD30+ lymphomas: a bridge to allogeneic transplantation in approximately one quarter of patients treated on a Named Patient Programme at a single UK center. Haematologica. 2013;98(4):611–614. doi: 10.3324/haematol.2012.069393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Illidge T, Bouabdallah R, Chen R, et al. Allogeneic transplant following brentuximab vedotin in patients with relapsed or refractory hodgkin lymphoma and systemic anaplastic large cell lymphoma. Leuk Lymphoma. 2014:1–34. doi: 10.3109/10428194.2014.930852. [DOI] [PubMed] [Google Scholar]