Abstract
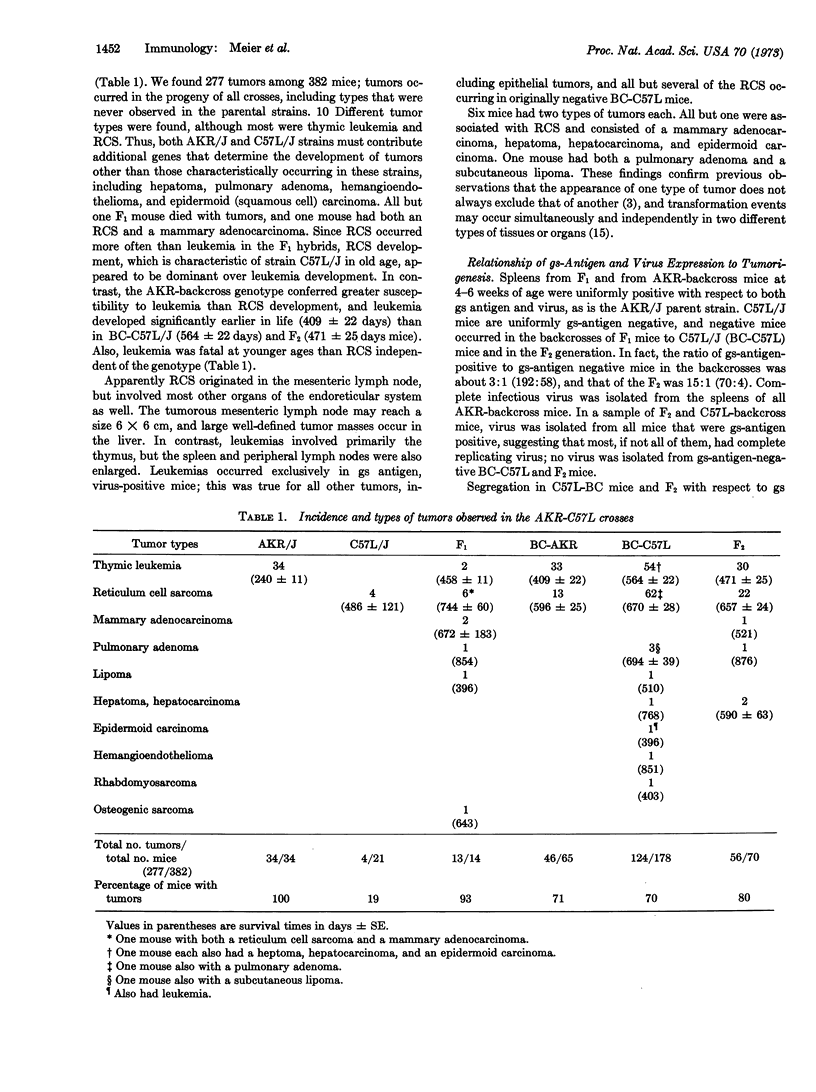
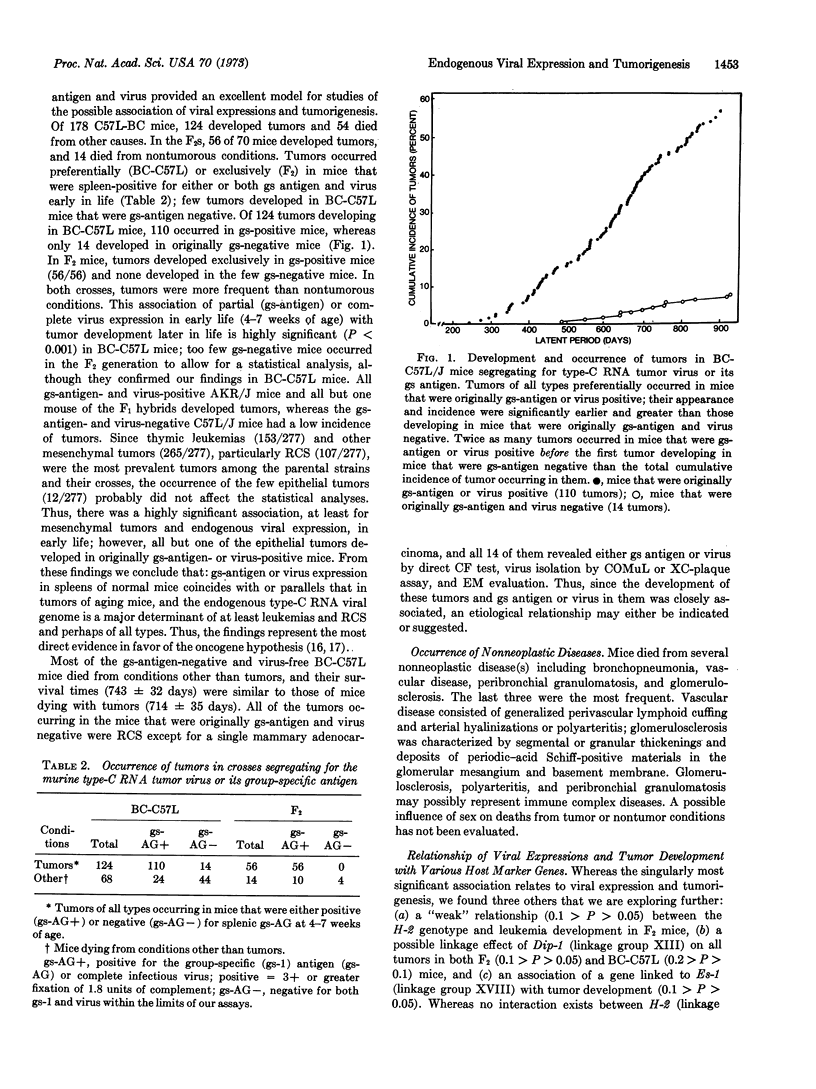
We analyzed the relationship of genetic factors determining the expression of endogenous type-C RNA tumor viruses and other host-gene markers to tumorigenesis. A hybridization experiment was performed with mice of strains AKR/J and C57L, the first filial (F1) generation hybrids, the second filial (F2) generation hybrids, and the backcrosses to the two parental strains. The results demonstrated a highly significant and predictable association between the expression of complete infectious virus or the viral group-specific (gs) antigen in spleens of young mice and tumorigenesis later in life. Most of the tumors were thymic leukemia and reticulum sarcoma, but other mesenchymal, as well as epithelial, tumors were also observed. Tumors occurred preferentially in gs-antigen- or virus-positive mice of all crosses; in the C57L-backcross and F2 mice segregating for gs-antigen and virus expression, a few gs-antigen-negative mice developed reticulum cell sarcomas. At the time of their occurrence, the mice were all gs-antigen-positive, and most had virus as well.
A minor effect of the major histocompatibility locus, H-2, on leukemogenesis was found in the F2 mice. Several tumor types were also found that we have never observed in the two parental strains. Our data provide the most direct biological evidence in favor of the viral oncogene theory. Thus, from the presence or absence of expression in early life of splenic gs antigen or virus, we can predict whether or not a tumor is likely to develop later in life. These findings suggest that the genome of endogenous type-C RNA viruses is the major determinant for tumorigenesis although they provide no clues about the factors responsible for the various histological types.
Keywords: reciprocal backcross progenies, complement-fixing antigens, dominant genes, genetic markers
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen H. W., Meier H., Heiniger H. J., Huebner R. J. Tumorigenesis in strain DW-J mice and induction by prolactin of the group-specific antigen of endogenous C-type RNA tumor virus. J Natl Cancer Inst. 1972 Oct;49(4):1145–1154. [PubMed] [Google Scholar]
- HUTTON J. J., SCHWEET R. S., WOLFE H. G., RUSSELL E. S. HEMOGLOBIN SOLUBILITY AND ALPHA-CHAIN STRUCTURE IN CROSSES BETWEEN TWO INBREAD MOUSE STRAINS. Science. 1964 Jan 17;143(3603):252–253. doi: 10.1126/science.143.3603.252. [DOI] [PubMed] [Google Scholar]
- Hanna M. G., Jr, Tennant R. W., Yuhas J. M., Clapp N. K., Batzing B. L., Snodgrass M. J. Autogenous immunity to endogenous RNA tumor virus antigens in mice with a low natural incidence of lymphoma. Cancer Res. 1972 Oct;32(10):2226–2234. [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Capps W. I., Huebner R. J. Complement fixation and tissue culture assays for mouse leukemia viruses. Proc Natl Acad Sci U S A. 1965 May;53(5):931–938. doi: 10.1073/pnas.53.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Capps W. I., Huebner R. J. Isolation of naturally occurring viruses of the murine leukemia virus group in tissue culture. J Virol. 1969 Feb;3(2):126–132. doi: 10.1128/jvi.3.2.126-132.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner R. J., Todaro G. J. Oncogenes of RNA tumor viruses as determinants of cancer. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1087–1094. doi: 10.1073/pnas.64.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igel H. J., Huebner R. J., Turner H. C., Kotin P., Falk H. L. Mouse leukemia virus activation by chemical carcinogens. Science. 1969 Dec 26;166(3913):1624–1626. doi: 10.1126/science.166.3913.1624. [DOI] [PubMed] [Google Scholar]
- Klement V., Rowe W. P., Hartley J. W., Pugh W. E. Mixed culture cytopathogenicity: a new test for growth of murine leukemia viruses in tissue culture. Proc Natl Acad Sci U S A. 1969 Jul;63(3):753–758. doi: 10.1073/pnas.63.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIEBERMAN M., HARAN-GHERA N., KAPLAN H. S. POTENTIATION OF VIRUS LEUKAEMOGENESIS IN C57BL MICE BY X-IRRADIATION OR URETHANE. Nature. 1964 Jul 25;203:420–422. doi: 10.1038/203420b0. [DOI] [PubMed] [Google Scholar]
- Lilly F. Fv-2: identification and location of a second gene governing the spleen focus response to Friend leukemia virus in mice. J Natl Cancer Inst. 1970 Jul;45(1):163–169. [PubMed] [Google Scholar]
- Lilly F. The inheritance of susceptibility to the Gross leukemia virus in mice. Genetics. 1966 Mar;53(3):529–539. doi: 10.1093/genetics/53.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly F. The role of genetics in Gross virus leukemogenesis. Bibl Haematol. 1970;(36):213–220. doi: 10.1159/000391710. [DOI] [PubMed] [Google Scholar]
- Markham R. V., Sutherland J. C., Cimino E. F., Drake W. P., Mardiney M. R. Immune complexes localized in the renal glomeruli of AKR mice: the presence of MuLV gs-I and C-type RNA tumor virus gs-3 determinants. Rev Eur Etud Clin Biol. 1972 Aug-Sep;17(7):690–694. [PubMed] [Google Scholar]
- McDevitt H. O., Tyan M. L. Genetic control of the antibody response in inbred mice. Transfer of response by spleen cells and linkage to the major histocompatibility (H-2) locus. J Exp Med. 1968 Jul 1;128(1):1–11. [PMC free article] [PubMed] [Google Scholar]
- Mellors R. C., Aoki T., Huebner R. J. Further implication of murine leukemia-like virs in the disorders of NZB mice. J Exp Med. 1969 May 1;129(5):1045–1062. doi: 10.1084/jem.129.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers D. D., Meier H., Huebner R. J. Prevalence of murine C-type RNA virus group specific antigen in inbred strains of mice. Life Sci II. 1970 Sep 22;9(18):1071–1080. doi: 10.1016/0024-3205(70)90016-0. [DOI] [PubMed] [Google Scholar]
- Myers D. D., Meier H., Huebner R. J. Prevalence of murine C-type RNA virus group specific antigen in inbred strains of mice. Life Sci II. 1970 Sep 22;9(18):1071–1080. doi: 10.1016/0024-3205(70)90016-0. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Aoki T., Dixon F. J. The antibody response of mice to murine leukemia virus in spontaneous infection: absence of classical immunologic tolerance (AKR mice-complement-fixing antibodies-lymphocytic choriomeningitis virus-immunofluorescence-glomerular deposits of antigen-antibody complexes). Proc Natl Acad Sci U S A. 1972 Jan;69(1):134–138. doi: 10.1073/pnas.69.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. I. Tissue culture studies of naturally occurring viruses. J Exp Med. 1971 Jun 1;133(6):1219–1233. doi: 10.1084/jem.133.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus T., Rowe W. P., Lilly F. A major genetic locus affecting resistance to infection with murine leukemia viruses. II. Apparent identity to a major locus described for resistance to friend murine leukemia virus. J Exp Med. 1971 Jun 1;133(6):1234–1241. doi: 10.1084/jem.133.6.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Shaw C. R., Prasad R. Starch gel electrophoresis of enzymes--a compilation of recipes. Biochem Genet. 1970 Apr;4(2):297–320. doi: 10.1007/BF00485780. [DOI] [PubMed] [Google Scholar]
- Snell G. D., Démant P., Cherry M. Hemagglutination and cytotoxic studies of H-2. I. H-2.1 and related specificities in the EK crossover regions. Transplantation. 1971 Mar;11(3):210–237. doi: 10.1097/00007890-197103000-00002. [DOI] [PubMed] [Google Scholar]
- Taylor B. A., Meier H., Myers D. D. Host-gene control of C-type RNA tumor virus: inheritance of the group-specific antigen of murine leukemia virus. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3190–3194. doi: 10.1073/pnas.68.12.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Huebner R. J. N.A.S. symposium: new evidence as the basis for increased efforts in cancer research. Proc Natl Acad Sci U S A. 1972 Apr;69(4):1009–1015. doi: 10.1073/pnas.69.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]


