Abstract
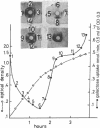
The synthesis of the periplasmic galactose-binding protein of E. coli is regulated by events occurring during its cell cycle, and proceeds in synchronized cells for only a short period after cell division is completed. Transport activity mediated by the β-methylgalactoside transport system follows closely the synthesis pattern of the binding protein.
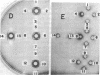
A mutant, E. coli BUG-6, exhibits temperature-sensitive cell division [Reeve et al. (1970) J. Bacteriol. 104, 1052-1064], synthesizing galactose-binding protein at the permissive but not at the nonpermissive temperature. Galactose-binding protein synthesized at the permissive temperature is not degraded after the culture is shifted to the nonpermissive temperature. Polyacrylamide gel electrophoresis of the periplasmic proteins of BUG-6 grown at the permissive and nonpermissive temperatures suggests that several, but not all, periplasmic proteins are subject to the same regulatory control by the cell cycle as the galactose-binding protein.
Keywords: cell division mutant, periplasmic proteins
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W. Transduction of chromosomal genes and episomes in Escherichia coli. Virology. 1960 May;11:273–288. doi: 10.1016/0042-6822(60)90066-0. [DOI] [PubMed] [Google Scholar]
- Anraku Y. Transport of sugars and amino acids in bacteria. I. Purification and specificity of the galactose- and leucine-binding proteins. J Biol Chem. 1968 Jun 10;243(11):3116–3122. [PubMed] [Google Scholar]
- Boos W., Gordon A. S. Transport properties of the galactose-binding protein of Escherichia coli. Occurrence of two conformational states. J Biol Chem. 1971 Feb 10;246(3):621–628. [PubMed] [Google Scholar]
- Boos W., Lengeler J., Hermann K. O., Unsöld H. J. The regulation of the beta-methylgalactoside transport system and of the galactose binding protein of Escherichia coli K12. Eur J Biochem. 1971 Apr 30;19(4):457–470. doi: 10.1111/j.1432-1033.1971.tb01336.x. [DOI] [PubMed] [Google Scholar]
- Boos W., Sarvas M. O. Close linkage between a galactose binding protein and the beta-methylgalactoside permease in Escherichia coli. Eur J Biochem. 1970 Apr;13(3):526–533. doi: 10.1111/j.1432-1033.1970.tb00956.x. [DOI] [PubMed] [Google Scholar]
- Boos W. Structurally defective galactose-binding protein isolated from a mutant negative in the -methylgalactoside transport system of Escherichia coli. J Biol Chem. 1972 Sep 10;247(17):5414–5424. [PubMed] [Google Scholar]
- Boos W. The galactose binding protein and its relationship to the beta-methylgalactoside permease from Escherichia coli. Eur J Biochem. 1969 Aug;10(1):66–73. doi: 10.1111/j.1432-1033.1969.tb00656.x. [DOI] [PubMed] [Google Scholar]
- Brockman R. W., Heppel L. A. On the localization of alkaline phosphatase and cyclic phosphodiesterase in Escherichia coli. Biochemistry. 1968 Jul;7(7):2554–2562. doi: 10.1021/bi00847a016. [DOI] [PubMed] [Google Scholar]
- Cutler R. G., Evans J. E. Synchronization of bacteria by a stationary-phase method. J Bacteriol. 1966 Feb;91(2):469–476. doi: 10.1128/jb.91.2.469-476.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitschek H. E. Constancy of uptake during the cell cycle in Escherichia coli. Biophys J. 1968 Dec;8(12):1401–1412. doi: 10.1016/S0006-3495(68)86562-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitschek H. E., Freedman M. L., Silver S. Potassium uptake in synchronous and synchronized cultures of Escherichia coli. Biophys J. 1971 Oct;11(10):787–797. doi: 10.1016/S0006-3495(71)86254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Novick A., Weiner M. ENZYME INDUCTION AS AN ALL-OR-NONE PHENOMENON. Proc Natl Acad Sci U S A. 1957 Jul 15;43(7):553–566. doi: 10.1073/pnas.43.7.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oki M. Correlation between metabolism of phosphatidylglycerol and membrane synthesis in Escherichia coli. J Mol Biol. 1972 Jul 21;68(2):249–264. doi: 10.1016/0022-2836(72)90212-4. [DOI] [PubMed] [Google Scholar]
- Reeve J. N., Clark D. J. Cell division of Escherichia coli BUG-6: effect of varying the length of growth at the nonpermissive temperature. J Bacteriol. 1972 Apr;110(1):117–121. doi: 10.1128/jb.110.1.117-121.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J. N., Clark D. J. Cell division of Escherichia coli BUG-6: effect of varying the temperature used as the nonpermissive growth condition. J Bacteriol. 1972 Apr;110(1):122–125. doi: 10.1128/jb.110.1.122-125.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J. N., Groves D. J., Clark D. J. Regulation of Cell Division in Escherichia coli: Characterization of Temperature-Sensitive Division Mutants. J Bacteriol. 1970 Dec;104(3):1052–1064. doi: 10.1128/jb.104.3.1052-1064.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciuti C. P. Synchronized division in Escherichia coli: an integral portion of culture growth. J Bacteriol. 1972 Oct;112(1):643–645. doi: 10.1128/jb.112.1.643-645.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. R., Rotman B. Inhibition of methylgalactoside transport in Escherichia coli upon the cessation of unsaturated fatty acid biosynthesis. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2125–2129. doi: 10.1073/pnas.69.8.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TORRIANI A., ROTHMAN F. Mutants of Escherichia coli constitutive for alkaline phosphatase. J Bacteriol. 1961 May;81:835–836. doi: 10.1128/jb.81.5.835-836.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Wilson T. H. The role of energy coupling in the transport of beta-galactosides by Escherichia coli. J Biol Chem. 1966 May 25;241(10):2200–2211. [PubMed] [Google Scholar]
- Wu H. C., Boos W., Kalckar H. M. Role of the galactose transport system in the retention of intracellular galactose in Escherichia coli. J Mol Biol. 1969 Apr 14;41(1):109–120. doi: 10.1016/0022-2836(69)90129-6. [DOI] [PubMed] [Google Scholar]