Abstract
Hepatic insulin resistance is a key contributor to the pathogenesis of obesity and type 2 diabetes (T2D). Paradoxically the development of insulin resistance in the liver is not universal, but pathway-selective, such that insulin fails to suppress gluconeogenesis but promotes lipogenesis, contributing to the hyperglycemia, steatosis and hypertriglyceridemia that underpin the deteriorating glucose control and microvascular complications in T2D. The molecular basis for the pathway-specific insulin resistance remains unknown. Here we report that oxidative stress accompanying obesity inactivates protein-tyrosine phosphatases (PTPs) in the liver, which activates select signaling pathways that exacerbate disease progression. In obese mice, hepatic PTPN2 (TCPTP) inactivation promoted lipogenesis and steatosis and insulin-STAT-5 signaling. The enhanced STAT-5 signaling increased hepatic IGF-1 production, which suppressed central growth hormone release and exacerbated the development of obesity and T2D. Our studies define a mechanism for the development of selective insulin resistance with wide-ranging implications for diseases characterised by oxidative stress.
INTRODUCTION
The liver is an essential organ in glucose homeostasis serving to produce glucose during periods of fasting to prevent hypoglycemia and maintain brain function and survival. After a meal, insulin acts in the liver via the insulin receptor (IR) protein-tyrosine kinase (PTK) to phosphorylate IR substrate-1/2 and activate the phosphatidylinositol 3-kinase (PI3K)/AKT pathway (Saltiel and Kahn, 2001). AKT2, in turn, phosphorylates Forkhead box protein O1 (Foxo1) and prevents its nuclear translocation, thereby repressing Foxo1-mediated transcription. The resultant inhibition of Foxo1-mediated expression of the gluconeogenic enzymes phosphoenolpyruvate carboxykinase (PEPCK) and glucose 6-phosphatase (G6P) helps to suppress hepatic glucose production and prevent postprandial hyperglycemia and glucose toxicity. Insulin also acts in the liver via AKT2 to promote sterol-regulatory element-binding protein 1c (SREBP-1c) expression and processing (Foretz et al., 1999; Leavens et al., 2009; Li et al., 2010; Lu et al., 2012; Yecies et al., 2011). Processed SREBP-1c in turn promotes the transcription of genes involved in triglyceride (TAG) synthesis, including acetyl-coenzymeA carboxylase (ACC), stearoyl-coenzyme A desaturase (SCD-1) and fatty acid synthase (FAS) (Horton et al., 2002).
Obesity is a key contributor to the development of insulin resistance and T2D (Johnson and Olefsky, 2013). Insulin resistance is associated with defects or aberrations in signaling downstream of the IR PTK. In particular, under conditions of insulin resistance, PI3K/AKT signaling is attenuated, diminishing insulin’s metabolic effects (Defronzo, 2009; Saltiel and Kahn, 2001). Defective insulin-induced P3K/AKT signaling has been attributed to various interrelated factors including intracellular lipid accumulation, inflammation, ER stress and oxidative stress (Johnson and Olefsky, 2013; Samuel and Shulman, 2012). Hepatic insulin resistance is thought to be an early event in the development of T2D (Michael et al., 2000; Turner et al., 2013). The diminished insulin-induced suppression of hepatic gluconeogenesis contributes to the fasting hyperglycaemia evident in patients with T2D. Paradoxically, insulin-induced lipid synthesis in the liver is not diminished, but rather elevated in the insulin resistant state, exacerbating hepatic steatosis (Biddinger et al., 2008; Brown and Goldstein, 2008; Haas et al., 2012; Leavens et al., 2009). Although the basis for the apparent selective insulin resistance and the contrary effects on hepatic gluconeogenesis versus lipogenesis in obesity and T2D remain to be resolved, the bifurcation of insulin signaling appears to be downstream of AKT2 (Leavens et al., 2009). De novo lipogenesis also occurs via insulin-independent pathways, particularly by carbohydrates after feeding. Dietary fatty acids and the flux of peripheral fats from white adipose tissue to the liver can also drive steatosis. Increased lipogenesis and TAG accumulation can, in turn, result in increased very low-density lipoprotein secretion and uptake in muscle, which exacerbates the development of insulin resistance and contributes to the cardiovascular complications of the disease (Postic and Girard, 2008; Samuel and Shulman, 2012).
Chronic reactive oxygen species (ROS) generation and oxidative stress occur in a wide variety of human diseases, including obesity and T2D (Newsholme et al., 2007). Superoxide (O2•−) is a natural byproduct of mitochondrial oxidative phosphorylation, but under normal conditions this is rapidly converted to hydrogen peroxide (H2O2) by superoxide dismutase. H2O2 is thereon eliminated by anti-oxidant enzymes such catalase, peroxiredoxins and glutathione peroxidase 1 (Gpx1) (Fisher-Wellman and Neufer, 2011). In obesity the chronic uptake and oxidation of energy substrates, in particular lipids, is thought to result in enhanced mitochondrial O2•− and H2O2 generation (Fisher-Wellman and Neufer, 2011). ER stress and the unfolded protein response in obesity and the increased expression and activation of NAD(P)H oxidases (NOXs), contribute further to ROS generation (Furukawa et al., 2004; Jiang et al., 2011). Compelling evidence links oxidative stress to the promotion of insulin resistance in humans and rodents (Anderson et al., 2009; Furukawa et al., 2004; Houstis et al., 2006) and oxidative stress in the liver exacerbates inflammation and hepatic fibrosis (Paik et al., 2011; Rolo et al., 2012).
PTPs are important targets for ROS (Tiganis, 2011). Classical tyrosine-specific PTPs contain a conserved signature motif ([I/V]HCSXGXGR[S/T]G) in their catalytic domains wherein the invariant Cys is essential for catalytic activity. The low pKa of the active site Cys facilitates nucleophilic attack of the substrate phosphotyrosine, but also renders PTPs highly susceptible to oxidation by ROS such as H2O2 (Tiganis, 2011). Oxidation of the active site Cys abrogates its nucleophilic properties and induces conformational changes that inhibit PTP activity and prevent substrate-binding. PTP oxidation is thought to occur under both physiological and pathological conditions to promote tyrosine phosphorylation-dependent signaling (Tiganis, 2011).
Here we report that oxidative stress in high fat diet-induced obesity results in pronounced hepatic PTP oxidation. In particular, we demonstrate that under conditions of obesity and insulin resistance, when the PI3K/AKT pathway is otherwise attenuated, PTPN2 (also known as T cell PTP: TCPTP) is oxidised and inactivated to promote lipogenesis and insulin-induced STAT-5 signaling. We report that the selective activation of the insulin-STAT-5 pathway may be instrumental in the promotion of obesity through the STAT-5 transcriptional target insulin-like growth factor-1 (IGF-1) and the repression of growth hormone (GH) release from the pituitary. Our studies have defined a liver-centric mechanism contributing to the development of obesity and T2D.
RESULTS
Hepatic PTP oxidation in high fat-fed mice
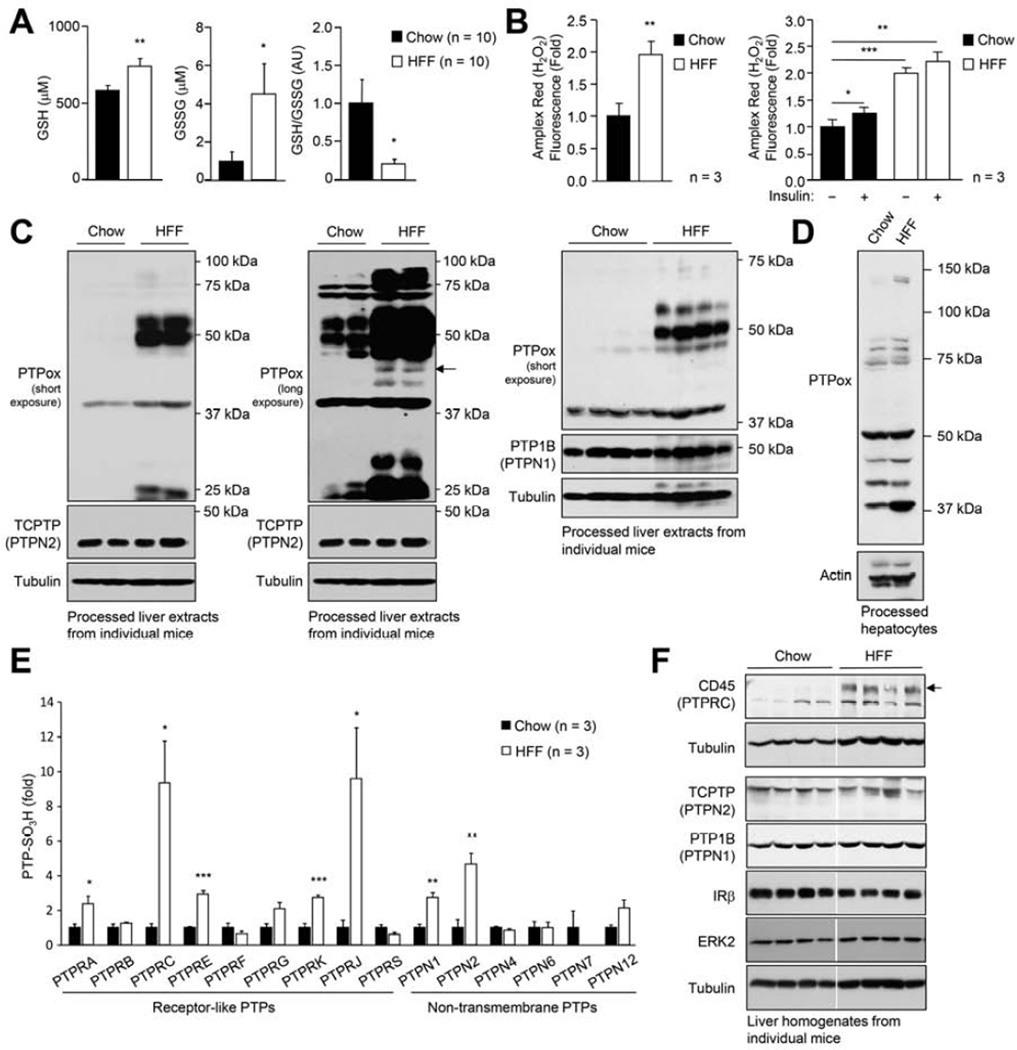
Obesity is associated with systemic oxidative stress in both humans and rodents (Furukawa et al., 2004). To determine whether diet-induced obesity promotes the oxidation of hepatic PTPs, aged-matched C57BL/6 mice were fed either a high fat diet (23% fat) or standard chow diet for 24 weeks, and oxidative stress and PTP oxidation assessed. Consistent with the development of oxidative stress, blood oxidised glutathione (GSSG) levels were increased and the reduced glutathione (GSH):GSSG ratio was decreased, in mice rendered obese by high fat feeding when compared with chow-fed lean controls (Fig. 1a). The systemic oxidative stress was, in turn, associated with increased H2O2 production by hepatocytes (measured using the H2O2-selective probe Amplex red) isolated from high fat-fed (HFF) versus chow-fed mice (Fig. 1b); H2O2 production by hepatocytes from HFF mice was significantly higher than that occurring in insulin-stimulated hepatocytes (Fig. 1b). To assess whether PTPs may be oxidised in the livers of HFF mice, we took advantage of a monoclonal antibody (PTPox) raised against the signature motif peptide of PTP1B with its catalytic cysteinyl residue oxidised to the irreversible sulfonic acid (Persson et al., 2005). Previous studies have established that this antibody can detect virtually all classical tyrosine-specific PTPs when they are oxidised to the sulfonic (−SO3H) state (Karisch et al., 2011). H2O2 promotes the reversible oxidation of PTPs to the sulfenic (−SOH) state, which is accompanied in many classical PTPs by the formation of a sulfenylamide intermediate that limits further oxidation to the irreversible sulfinic (−SO2H) and −SO3H states. At first, we monitored the oxidation status of PTPs by immunoblot analysis (Fig. 1c). Livers from HFF obese and chow-fed lean mice were homogenised in an anaerobic chamber in the presence of N-ethylmaleimide (NEM) to prevent post-lysis oxidation and to alkylate all reduced and active PTPs. Reversibly oxidised PTPs were reduced and hyper-oxidised to the −SO3H state for detection with PTPox by immunoblot analysis. We noted an increase in the oxidation status of several PTPs in the livers from HFF mice (Fig. 1c); PTP oxidation was largely reversible, as several PTPox species were either not detected, or decreased when NEM-treated tissue extracts were immunoblotted without further processing (Supp. Fig. 1a). Oxidised hepatic PTPs in HFF mice included those co-migrating with the prototypic PTP1B (PTPN1) and the closely related TCPTP (PTPN2), both of which have been implicated in the regulation of hepatic insulin sensitivity and gluconeogenesis (Tiganis, 2013). PTP oxidation was not increased further when HFF mice were administered insulin and hepatic PTP oxidation was not altered in chow-fed mice subjected to hyperinsulinemic euglycemic clamps (insulin infused for 4 h; Supp. Fig. 1b–c). Thus, we propose that the heightened hepatic PTP oxidation in obese mice occurs as a consequence of the systemic oxidative stress and the elevated ROS production in the liver parenchyma.
Fig. 1. Oxidative stress and hepatic PTP oxidation.
(a) 8 week-old male C57BL/6 mice were chow- or HFF for 24 weeks. Blood GSH and GSSG levels and GSH:GSSG ratios were determined. (b) Hepatocytes from 12-week chow- or HFF mice were either left untreated or serum-starved and stimulated with 100 nM insulin for 30 min and H2O2 production measured. (c, e-f) Livers from 24 week chow- or HFF mice were processed for an assessment of total PTP oxidation by (c) immunoblot analysis with the PTPox antibody, or (e) mass spectrometry determining relative PTP oxidation (PTP-SO3H), or (f) processed for immunoblot analysis for the indicated PTPs. (d) Hepatocytes were processed immediately after isolation for an assessment of total PTP oxidation. In (c-d) arrows correspond to TCPTP. Results are means ± SEM for the indicated number of mice (a, e-f) or experimental repeats (b).
Hepatocytes constitute roughly 80% of the liver volume with the remainder comprising of non-parenchymal cells including Kupffer cells, endothelial cells and stellate cells. We determined whether the increased PTP oxidation in the livers of HFF mice could be ascribed to changes in the oxidation status of PTPs in hepatocytes. PTP oxidation was assessed in hepatocytes that were freshly isolated from chow-versus HFF mice (Fig. 1d); several PTPox species were increased in hepatocytes from HFF mice (Fig. 1d). Thus, the increased PTP oxidation in livers of HFF mice may at least in part be due to changes in PTP oxidation in hepatocytes.
To determine the extent to which individual hepatic PTPs are oxidised in HFF obese versus chow-fed, lean mice, we utilised a recently developed, quantitative proteomic method for the global assessment of classical PTP oxidation (Karisch et al., 2011). In this approach, hyperoxidised PTPs are digested, enriched by PTPox immunoprecipitation and quantified by mass spectrometry (Karisch et al., 2011). Using this approach, 6 of 17 known non-receptor and 9 of 21 known receptor PTPs, were identified (Fig. 1e; data not shown). Of the hepatic PTPs detected in chow-fed and HFF livers, only 8 exhibited an increase in their oxidation status in HFF mice (Fig. 1e). These included the non-receptor phosphatases TCPTP (PTPN2) and PTP1B (PTPN1) and several transmembrane receptor-type PTPs, in particular CD45 (PTPRC), which marks hematopoietic cells, and DEP-1 (PTPRJ), which is expressed in immune and non-immune cells alike (Hermiston et al., 2009). CD45 protein levels were increased in the livers of HFF mice, consistent with the infiltration of immune cells that accompanies hepatosteatosis. These results demonstrate that PTPs can be extensively oxidised in vivo in the context of systemic oxidative stress and raise the possibility that PTP oxidation may contribute to hepatic pathophysiology in obesity/T2D.
Hepatic STAT-1/-3/-5 are hyper-activated in HFF mice
Since PTP1B and TCPTP are implicated in liver physiology (Delibegovic et al., 2009; Fukushima et al., 2010), we focussed our attention on the signaling pathways regulated by these two phosphatases. TCPTP and PTP1B attenuate both IR signaling and Janus-activated kinase (JAK)/STAT signaling (Tiganis, 2013). Both PTPs dephosphorylate and inactivate the IR and JAK family PTKs: PTP1B dephosphorylates JAK-2 and Tyk2 whereas TCPTP dephosphorylates JAK-1 and JAK-3. TCPTP also dephosphorylates STAT family members (including STAT-1, −3 and −5) (Tiganis, 2013). To assess the potential impact of hepatic PTP oxidation on cellular signaling and biological responses in HFF mice, we assessed hepatic IR and JAK/STAT signaling in liver homogenates from chow-fed versus HFF mice. JAK/STAT signaling was assessed by monitoring STAT-1 Y701 (p-STAT-1), STAT-3 Y705 (p-STAT-3) and STAT-5 Y694 (p-STAT-5) phosphorylation. We found that p-STAT-1 and p-STAT-3 were elevated in liver homogenates from HFF (24 week) fasted mice (Fig. 2a; Supp. Fig. 2a); basal p-STAT-5 was also elevated with more prolonged (40 week) high fat feeding (Supp. Fig. 2a). By contrast, we noted no overt differences in general tyrosine phosphorylation in liver homogenates from fasted (4 h) chow-fed versus HFF mice as assessed with phospho-tyrosine (p-Tyr)-specific antibodies (Supp. Fig. 2b). These results demonstrate that enhanced PTP oxidation per se does not result in constitutive pTyr signaling in fasted livers. In keeping with this, there were no overt differences in mitogen-activated protein kinase signaling, as assessed with antibodies to phosphorylated ERK1/2, nor any increase in basal IR β-subunit Y1162/Y1163 (p-IRβ) phosphorylation (Fig. 2a; Supp. Fig. 2a). On the other hand basal p-AKT was reduced (Fig. 2a) in line with the development of pathway-specific insulin resistance. These results are consistent with high fat diet-induced obesity resulting in the promotion of STAT-1/-3/-5 signaling.
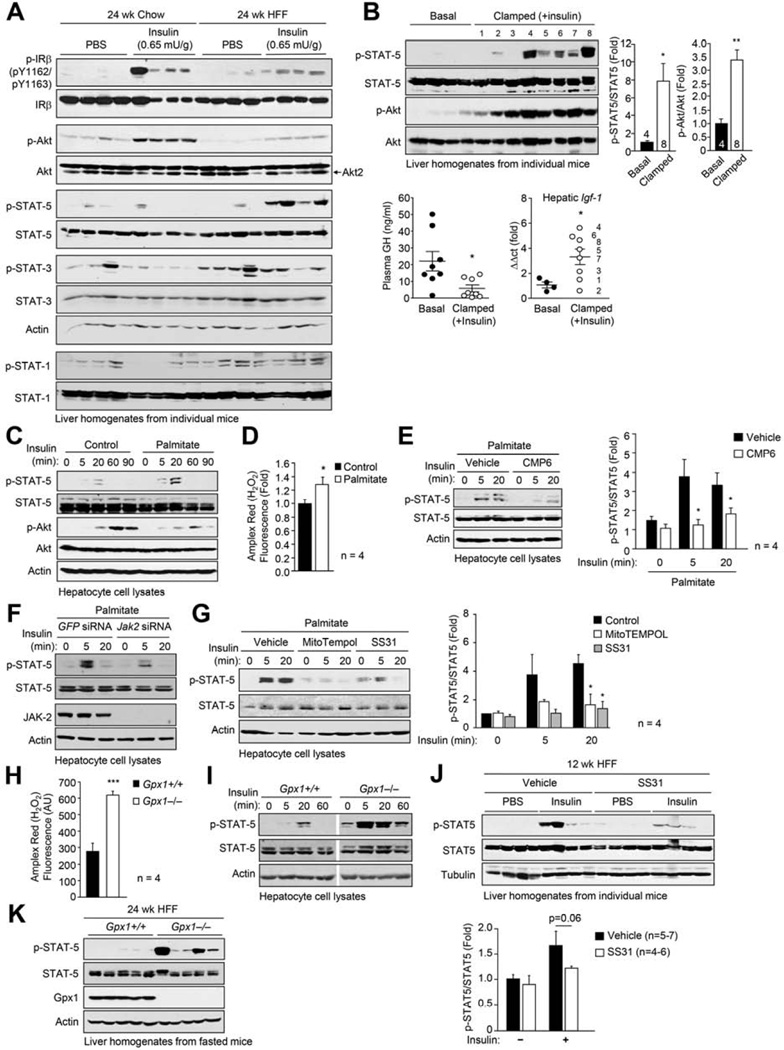
Fig. 2. Oxidative stress promotes insulin-induced STAT-5.
(a) 8 week-old male C57BL/6 mice were chow- or HFF for 24 weeks, fasted, injected with PBS or insulin and livers processed for immunoblotting. (b) 20 week-old male C57BL/6 mice were subjected to hyperinsulinemic euglycemic clamps. Plasma GH levels were measured and livers processed for immunoblotting or quantitative real-time PCR to measure Igf-1. (c-g) Hepatocytes from chow-fed mice were treated with vehicle or 0.5 mM palmitate. (c, e-g) Hepatocytes were serum-starved, stimulated with 100 nM insulin and processed for immunoblotting. (d) H2O2 production was measured. Where indicated hepatocytes were pre-treated with vehicle, the JAK inhibitor CMP6 (10 µM), mitoTempol (10 µM) or SS31 (50 µM) for 2 h. In (f) hepatocytes were transfected with GFP control or Jak-2-specific siRNAs prior to palmitate treatment. (h) H2O2 production and (i) insulin (100 nM)-induced p-STAT-5 signaling in Gpx1+/+ and −/− hepatocytes. (j) 8 week-old male C57BL/6 mice were HFF for 12 weeks and treated with vehicle or SS31 for 10 days. Mice were fasted and injected with PBS or insulin (0.5 mU/g, 10 min) and livers extracted for immunoblotting. (k-l) Gpx1+/+ and −/− male mice were HFF for 24 weeks, fasted and livers processed for immunoblotting. Representative and quantified (means ± SEM) results are shown.
Selective activation of insulin-induced STAT-5 signaling
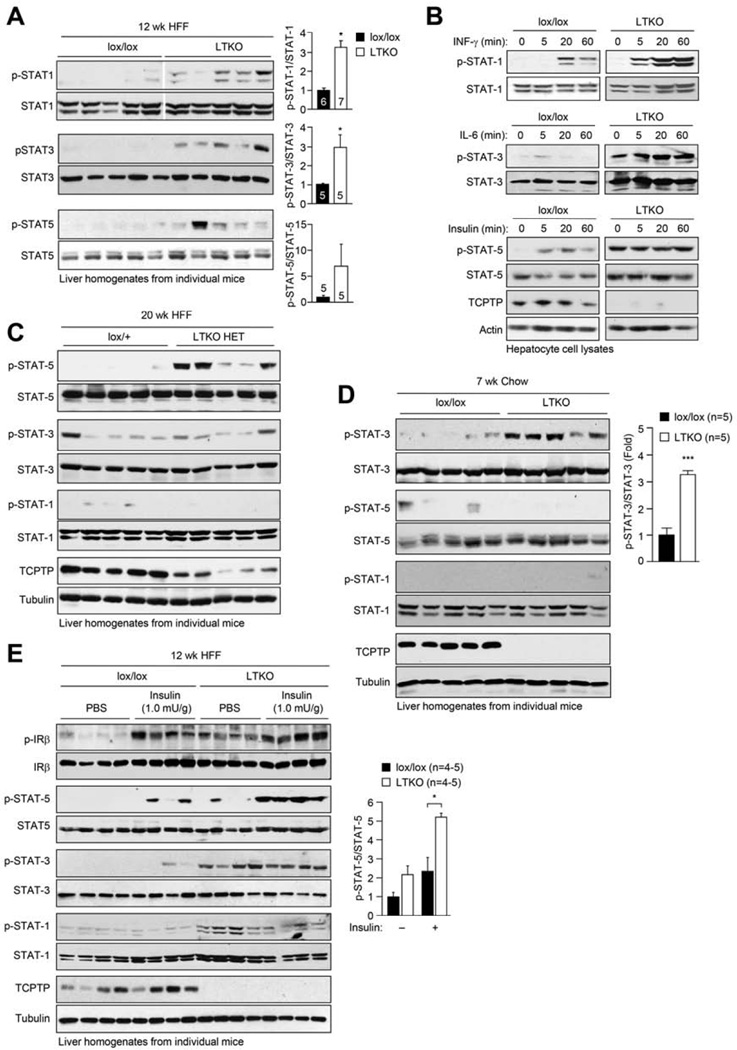
We next assessed the potential impact of hepatic PTP oxidation on insulin signaling in the liver by comparing responses to bolus insulin administration (0.65 mU/g, intraperitoneal, 10 min) in 24 week chow- versus HFF C57BL/6 mice (Fig. 2a). We monitored for p-IRβ, activation of PI3K/AKT signaling with antibodies to Ser-473-phosphorylated AKT and the activation status of STAT-1, −3 and −5 using appropriate phospho-specific antibodies. IR phosphorylation was not overtly altered, whereas AKT phosphorylation was reduced consistent with the development of insulin resistance (Fig. 2a). Surprisingly, we found that insulin-induced p-STAT-5 was robust in the livers from HFF mice and not detectable in chow-fed control mice (Fig. 2a). By contrast, although basal p-STAT-1 and p-STAT-3 were elevated in livers from HFF mice, they were not induced further by insulin (Fig. 2a). To determine whether the insulin-induced activation of the p-STAT-5 pathway was unique to HFF mice, or whether high fat feeding and obesity exacerbated the normal response to insulin, we also challenged 8–10 week-old chow-fed mice with increasing concentrations of insulin and monitored for the status of p-STAT-5. We found that with higher concentrations of insulin (1.3 mU/g), p-STAT-5 was detected in liver homogenates from chow-fed mice (Supp. Fig. 2c).
GH acts in the liver via JAK-2 and STAT-5 to increase IGF-1 production (Lichanska and Waters, 2008). As insulin lowers blood glucose and hypoglycemia can in turn promote the release of GH from the pituitary (Jaffe et al., 1999), the enhanced insulin-induced hepatic p-STAT-5 in chow-fed mice (administered 1.3 mU/g insulin) could have been a consequence of enhanced GH release and signaling. To address this possibility, we subjected chow-fed C57BL/6 mice to hyperinsulinemic euglycemic clamps, wherein a high dose of insulin was infused constantly (60 mU/ml insulin, 10–40 µl/min for 4 h), while euglycemia was maintained by the co-infusion of glucose, and assessed GH levels, p-STAT-5 signaling and hepatic mRNA Igf-1 levels (Fig. 2b; Supp. Fig. 1c). It is well established that insulin can mimic IGF-1 in the suppression of GH release from the anterior pituitary, a negative feedback loop that normally prevents the excessive release of GH (Yamashita and Melmed, 1986). Accordingly, we noted significantly reduced serum GH levels in clamped mice (Fig. 2b). Despite these lower GH levels, p-STAT-5 was increased significantly in livers from clamped mice when compared to saline-infused controls (Fig. 2b). Igf-1 levels also were increased in clamped mice, compared with controls, and the increase in Igf-1 expression correlated largely with the degree of p-STAT-5 (Fig. 2b; numbers in lanes correspond to points in panel below). Complementing these in vivo studies, we found that insulin stimulation also resulted in a modest, but reproducible increase in STAT-5 activation in isolated primary hepatocytes from C57BL/6 mice (Fig. 2c). Taken together, these results demonstrate that the insulin-induced p-STAT-5 pathway occurs in hepatocytes to promote the expression of STAT-5 target genes such as Igf-1 and that the STAT-5 pathway is exacerbated in HFF obese mice, when insulin-induced PI3K/AKT signaling is otherwise attenuated.
ROS promote insulin-induced STAT-5 signaling
Having established that insulin signals via STAT-5 in the liver and that this is exacerbated in obesity, we next sought to define the basis for the exacerbation of the IR-STAT-5 pathway in HFF mice. In particular, we asked whether the hepatic oxidative stress associated with obesity might inactivate PTPs for the selective promotion of insulin-induced STAT-5 signaling. To this end we isolated hepatocytes from chow-fed lean mice and assessed the impact of incubating them with the fatty acid palmitate, which promotes insulin resistance and generates high rates of mitochondrial H2O2 emission (Fisher-Wellman and Neufer, 2011). We found that treatment with palmitate-BSA promoted H2O2 production and resulted in increased insulin-induced p-STAT-5 (Fig. 2c–d). The increase in p-STAT-5 was accompanied by a decrease in PI3K/AKT signaling (Fig. 2c), consistent with palmitate promoting insulin resistance. The insulin-induced p-STAT-5 in untreated and palmitate-treated hepatocytes was reliant on JAK-2, since a highly selective JAK PTK inhibitor (Fig. 2e), or JAK-2 knockdown using specific siRNAs (Fig. 2f), attenuated insulin-induced p-STAT-5 signaling. To determine if the palmitate-mediated increase in insulin-induced p-STAT-5 was mediated by the increase in mitochondrial ROS emission we took advantage of the mitochondrial-targeted anti-oxidant mitoTEMPOL and the mitochondrial-targeted peptide SS31 (Bendavia™; Stealth Peptides, MA) that accumulates in mitochondria and reduces mitochondrial O2•−/H2O2 generation/release (Anderson et al., 2009). We found that treating hepatocytes with mitoTEMPOL or SS31 significantly attenuated the palmitate-induced increase in p-STAT-5 signaling in response to insulin (Fig. 2g). Finally, we assessed whether the oxidation status of PTPs may be increased in response to palmitate in hepatocytes. For these experiments we took advantage of non-transformed AML12 hepatocytes. Treatment of AML12 cells with palmitate increased both H2O2 production and PTP oxidation (Supp Fig. 1e).
To further explore the role of H2O2 in the promotion of insulin-induced p-STAT-5 signaling we took advantage of mice lacking the antioxidant enzyme Gpx1, which converts H2O2 to water. Hepatocytes were isolated from 12 week-old male Gpx1−/− and Gpx1+/+ mice and H2O2 levels and insulin-induced p-STAT-5 signaling assessed (Fig. 2h–i). Gpx1-deficiency resulted in a 2-fold increase in H2O2 production in hepatocytes (Fig. 2h) and this was accompanied by a striking increase in basal and insulin-induced p-STAT-5 signaling (Fig. 2i), which was increased further when cells were treated with palmitate (data not shown).
Having established that H2O2 can promote insulin-induced p-STAT-5 signaling in hepatocytes ex vivo we next assessed whether this might occur in vivo. First we determined if the mitochondrial-targeted SS31 could attenuate insulin-induced p-STAT-5 signaling in HFF mice. Mice that had been HFF for 12 weeks were administered vehicle control or SS31 (2.0 mg/kg/day intraperitioneal) for 12 days, and insulin-induced (0.5 mU/g) STAT-5 signaling assessed (Fig. 2j). Insulin-induced p-STAT-5 signaling trended lower in SS31-treated mice (Fig. 2j), consistent with mitochondrial-derived oxidative stress contributing to the promotion of STAT-5 signaling. We next determined if exacerbating oxidative stress could have the opposite effect and enhance insulin-induced STAT-5 signaling. For this we took advantage of Gpx1−/− mice and assessed if Gpx1-deficiency and elevated H2O2 could exacerbate high fat diet-induced oxidative stress, PTP oxidation and p-STAT-5 signaling (Supp. Fig. 1f–g). Blood GSH:GSSG ratios in Gpx1−/− versus Gpx1+/+ HFF (24 week) mice were reduced (Supp. Fig. 1f), consistent with the promotion of systemic oxidative stress; alterations in GSH:GSSG ratios were not evident in chow-fed mice. The increased oxidative stress was in turn associated with elevated hepatic PTP oxidation (Supp. Fig. 1g), as assessed by immunoblot analysis with the PTPox antibody; the increase in PTP oxidation occurred for several species including one co-migrating with TCPTP (Supp. Fig. 1g). Furthermore, the increased hepatic PTP oxidation coincided with the detection of hepatic p-STAT-5 in the livers of fasted Gpx1−/− mice (Fig. 2k). Taken together these results provide evidence for hepatic oxidative stress promoting insulin-induced hepatic p-STAT-5 signaling in vivo. Moreover, these results are consistent with the possibility that the ROS-mediated promotion of insulin-p-STAT-5 signaling might be reliant on the oxidation of PTPs such as TCPTP.
Hepatic TCPTP deficiency exacerbates obesity, steatosis and insulin resistance
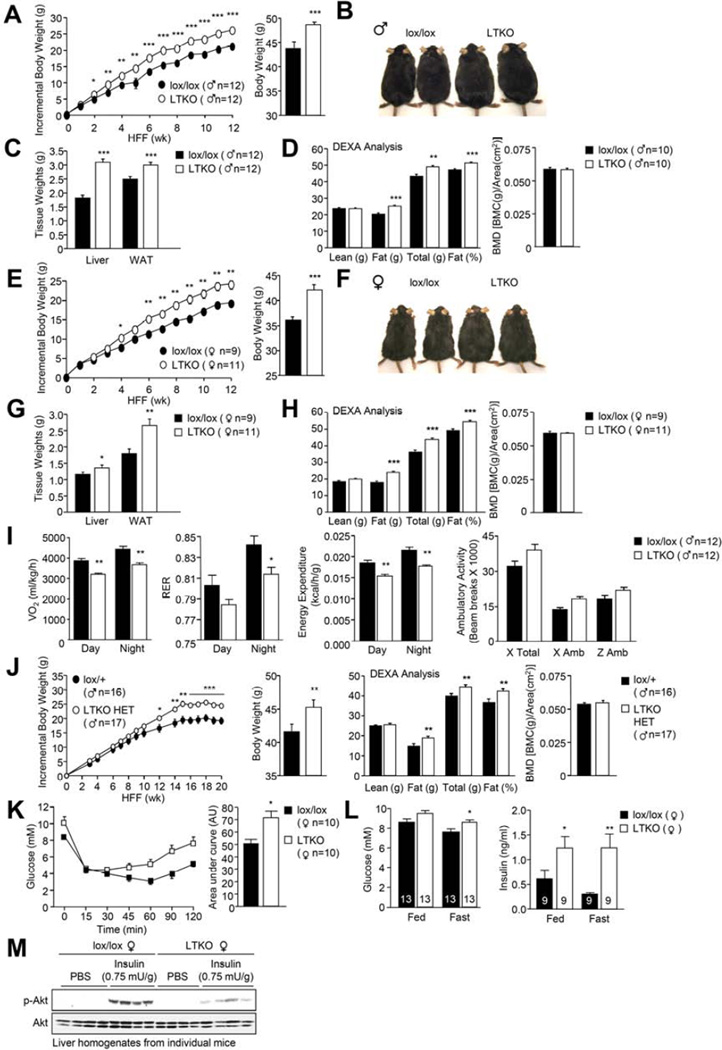
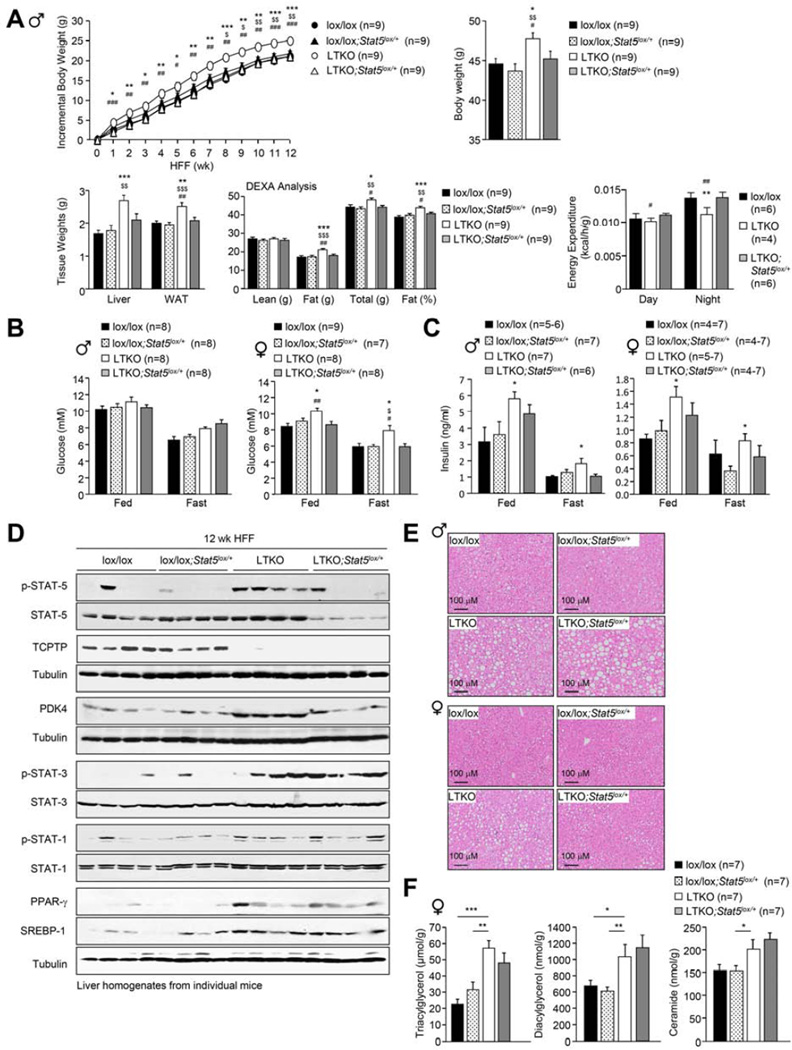
To ascertain if the inactivation of hepatic PTPs might contribute to the promotion of STAT-1, −3 and −5 signaling and the development of obesity and T2D, we sought to delete those hepatic PTPs that were oxidised in HFF mice. We focussed our attention on TCPTP for several reasons. First, the relative increase in TCPTP oxidation in HFF obese mice was greater than that of other non-transmembrane PTPs (Fig. 1e–f). Second, TCPTP has the capacity to attenuate STAT-5 signaling (Loh et al., 2011) and third, TCPTP has been implicated in the attenuation of hepatic STAT-3 signaling (Fukushima et al., 2010). Ptpn2lox/lox (lox/lox) mice were bred with Alb-Cre mice to generate liver-specific TCPTP knockout (Alb-Cre;Ptpn2lox/lox: LTKO) mice (Supp. Fig. 3a). Seven-week-old LTKO mice appeared normal and did not exhibit differences in body weight/adiposity or overt alterations in glucose tolerance (data not shown). To assess the impact of hepatic TCPTP-deficiency on the development of obesity and T2D, 6 week-old male and female lox/lox and LTKO mice were fed standard chow versus high fat diets for 12 weeks and their body weights and metabolic status assessed (Fig. 3; Supp. Fig. 4). Strikingly both male and female LTKO mice fed a high fat diet gained more weight than their lox/lox counterparts and this was associated increased adiposity, as assessed by measuring fat pad weights and by monitoring body composition by dual-energy x-ray absorptiometry (DEXA) (Fig. 3a–h). Although liver weights were higher in HFF LTKO mice, gross differences in body length were not evident and no significant differences were noted in lean mass or bone density (Fig. 3a–h; data not shown). The increased adiposity was accompanied by decreased energy expenditure in both sexes and decreased a respiratory exchange ratio (RER) in male mice (Fig. 3i; Supp. Fig. 4a). No differences were seen in ambulatory activity (Fig. 3i; Supp. Fig. 4a) or food intake (data not shown), arguing against overt differences in the central control of body weight. Increased weight gain and adiposity and decreased energy expenditure were also observed in female LTKO mice fed a standard chow diet, but not in chow-fed male mice (Supp. Fig. 4b–c; data not shown). These results indicate that TCPTP deficiency in the liver promotes diet-induced weight gain. Moreover, male mice with heterozygous hepatic TCPTP deficiency (Alb-Cre;Ptpn2lox/+; LTKO HET) also gained more weight when fed a high fat diet and this was associated with increased adiposity (Fig. 3j). Thus, even partial hepatic TCPTP deficiency, or TCPTP inactivation, as might occur as a consequence of oxidation, is sufficient to promote obesity. In keeping with their increased adiposity, HFF LTKO and LTKO HET mice exhibited decreased insulin sensitivity, as monitored in insulin tolerance tests, and glucose intolerance, as assessed in glucose tolerance tests (Fig. 3k; Supp. Fig. 4d–e, g). HFF LTKO mice also exhibited elevated fasting blood glucose levels, accompanied by elevated plasma insulin levels in fed (female) and fasted (male and female) mice (Fig. 3l; Supp. Fig. 4f) and decreased insulin-induced PI3K/AKT signaling in liver and muscle (Fig. 3m; Supp. Fig. 4h–i).
Fig. 3. Hepatic TCPTP deletion promotes obesity and insulin resistance.
7 week-old lox/lox and LTKO mice were HFF for 12 weeks and (a, e) body weights, (b, f) gross morphology, (c, g) tissue weights, (d, h) body composition and (i) oxygen consumption, RER, energy expenditure and ambulatory activity assessed. (j) 7 week-old lox/+ and LTKO HET mice were HFF for 20 weeks and body weights and composition determined. (k) HFF mice were subjected to insulin tolerance tests and (l) fed and fasted blood glucose levels and plasma insulin levels determined. (m) Fasted HFF mice were injected with insulin and livers extracted for immunoblot analysis. Representative and quantified (means ± SEM) results are shown.
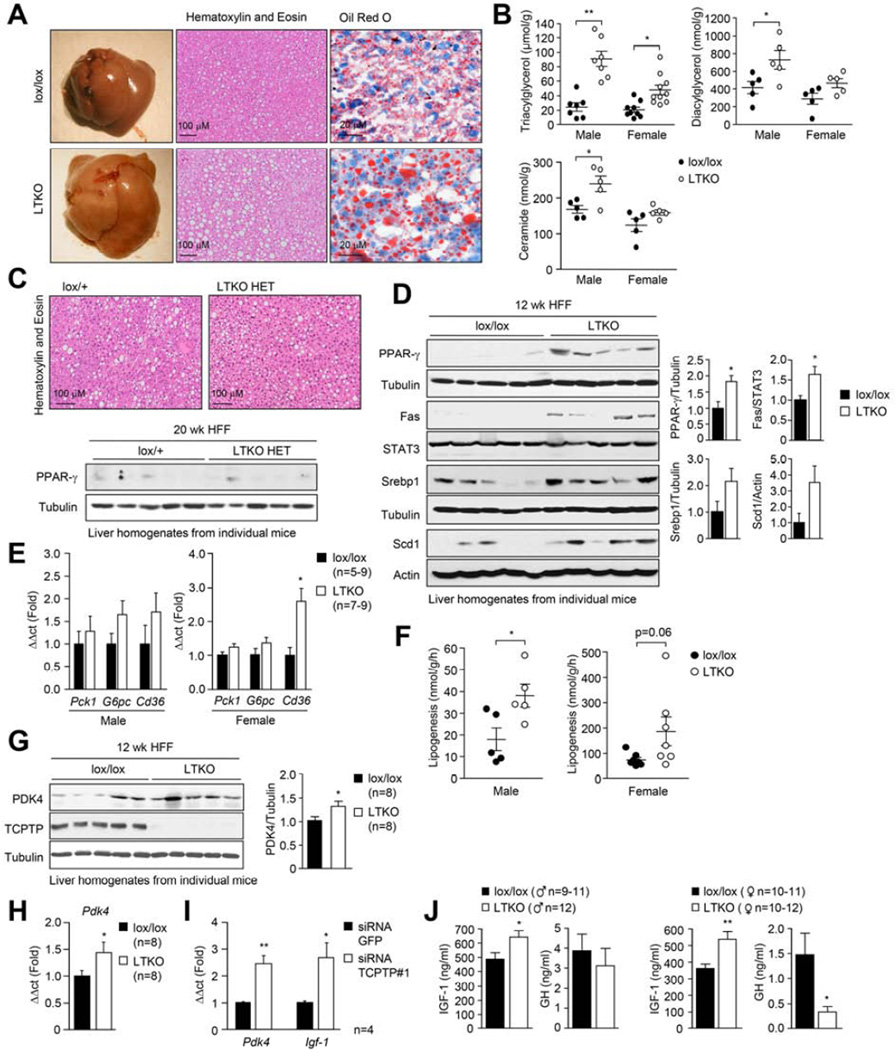
Next we assessed the impact of hepatic TCPTP deletion on liver morphology (Fig. 4a; Supp. Fig. 5a). As noted earlier, livers from male and female 12 week HFF LTKO mice were heavier than their lox/lox counterparts. This was associated with increased steatosis, as assessed by gross morphology, histology (hematoxylin and eosin staining) and Oil Red O staining (stains lipid droplets) and by measuring hepatic lipids (Fig. 4a–b). The steatotic phenotype was not accompanied by alterations in circulating free fatty acids (FFAs) and TAGs (Supp. Fig. 5b). The increased steatosis was not evident in 20 week HFF LTKO HET livers (Fig. 4c), even though these mice exhibited increased diet-induced weight gain and insulin resistance (Fig. 3j; Supp. Fig. 4g). These findings suggest that the promotion of obesity and the development of steatosis in HFF LTKO mice might be regulated by independent processes.
Fig. 4. LTKO mice exhibit steatosis and alterations in IGF-1 and GH.
7 week-old male lox/lox and LTKO or lox/+ and LTKO HET mice were HFF for 12 and 20 weeks respectively. Livers were extracted from fasted mice and either (a, c) processed for histology, (b) lipid analyses, (c, d, g) immunoblotting, or (e, h) real time PCR. (f) Lipogenesis was assessed ex vivo in liver slices. (i) Hepatocytes from chow-fed C57BL/6 were transfected with GFP- or Ptpn2-specific siRNAs and after 48 h processed for real time PCR. (j) Lox/lox and LTKO mice were HFF for 12 weeks, fasted overnight and serum IGF-1 and GH levels determined. Results are means ± SEM.
Steatosis in obesity is accompanied by increased hepatic levels of the transcription factors SREBP-1c and peroxisome proliferator-activated receptor-γ (PPAR-γ) (Horton et al., 2002; Memon et al., 2000). Hepatic overexpression of SREBP-1c or PPAR-γ is sufficient to promote steatosis (Shimano et al., 1997; Yu et al., 2003), whereas PPAR-γ deletion in hepatocytes can attenuate both genetic and high fat diet-induced fatty liver disease (Matsusue et al., 2003; Moran-Salvador et al., 2011). SREBP-1 and PPAR-γ were elevated in the livers of HFF male and female LTKO mice (Fig. 4d; Supp. Fig. 5c), but interestingly not in HFF LTKO HET mice (Fig. 4c; data not shown). SREBP-1c promotes the expression of lipogenic enzymes, such as FAS and SCD-1 (Horton et al., 2002) and PPAR-γ promotes the transcription of fatty acid transporters such as CD36/FAT to promote the uptake of FFAs (Barclay et al., 2011). SCD-1 and FAS protein levels were elevated in the livers of HFF male and female LTKO mice (Fig. 4d), as was the expression of Cd36 mRNA in female mice (Fig. 4e). Moreover, hepatic lipogenesis, as assessed in liver slices ex vivo, also was increased in HFF LTKO mice (Fig. 4f). Taken together, these results demonstrate that the deletion of TCPTP in the liver promotes diet-induced obesity and insulin resistance as well as hepatic lipogenesis and steatosis.
Hepatic TCPTP deficiency promotes STAT-1/-3/-5 signaling
To understand how hepatic TCPTP deletion/inactivation might be promoting obesity/T2D and steatosis, we sought to delineate the tyrosine phosphorylation-dependent pathways that might be altered in 12 week HFF LTKO mice and 20 week LTKO HET mice. We did not observe any significant change in global tyrosine phosphorylation, basal ERK1/2 or p38 activation, or in the activation of the ER stress response, as monitored with antibodies to Thr-980 phosphorylated PERK or Ser-51 phosphorylated eIFα in the livers of fasted mice (Supp. Fig. 6a; data not shown). Similarly, TCPTP-deficiency did not result in a global increase in the phosphorylation of TCPTP substrates, including that of Src family kinases (SFKs), as monitored with antibodies to the Y416 site on c-Src (Supp. Fig. 6a), and JAK-1, as monitored with antibodies to the Y1022/Y1023 PTK activation loop site (Supp. Fig. 5f). Thus, hepatic signaling in fasted mice was not elevated in general as a consequence of TCPTP deficiency. Previous studies have established that STAT-1, STAT-3 and STAT-5 may serve as substrates of TCPTP (Tiganis, 2013; Tiganis and Bennett, 2007). We found that p-STAT-1, p-STAT-3 and p-STAT-5 were elevated in the livers of fasted male and female LTKO mice that were HFF for 12 weeks, when compared to lox/lox controls (Fig. 5a, e; Supp. Fig. 5d–e). The elevated STAT-1/3/5 signaling in HFF LTKO liver homogenates was not associated with overt alterations in the expression of PTP1B, SHP-1 and SHP-2 (Supp. Fig. 6b) that have also been implicated in the control of JAK/STAT signaling. Importantly, interferon (IFN) γ-induced p-STAT-1 was increased in hepatocytes isolated from LTKO mice, p-STAT-3 and −5 were elevated constitutively in LTKO hepatocytes (Fig. 5b) and p-STAT-1 and −5 were elevated in C57BL/6 hepatocytes after TCPTP knockdown (Supp. Fig. 6c), consistent with the enhanced signaling in vivo being hepatocyte intrinsic. Interestingly only p-STAT-5 was elevated in the livers of 20 week HFF LTKO HET mice, highlighting the sensitivity of the STAT-5 pathway to Ptpn2 gene dosage in the context of obesity (Fig. 5c). Moreover, as p-STAT-1 and p-STAT-5 were not detected in the livers of seven week-old chow-fed LTKO male mice (Fig. 5d), these results point towards TCPTP-deficiency promoting p-STAT-1 and p-STAT-5 only in the context of high fat feeding/obesity.
Fig. 5. Hepatic TCPTP deletion exacerbates insulin-induced STAT-5 signaling.
(a) 7 week-old male lox/lox and LTKO mice were HFF for 12 weeks. Livers from 4 h fasted mice were processed for immunoblotting. (b) Hepatocytes were serum-starved and stimulated with either 50 U/ml INF-γ, 100 U/ml IL-6 or 100 nM insulin and processed for immunoblotting. (c) 7 week-old male lox/+ and LTKO HET mice were HFF for 20 weeks. Livers from fasted mice were processed for immunoblotting. (d) Livers from 7 week-old fasted male lox/lox and LTKO mice were processed for immunoblotting. (e) Mice were HFF for 12 weeks, fasted and injected with PBS or insulin and livers processed for immunoblotting. Representative and quantified (means ± SEM) results are shown.
Our studies indicate that high fat feeding C57BL/6 mice promotes hepatic insulin-induced p-STAT-5 signaling. Accordingly, we next examined whether the inactivation of hepatic TCPTP by ROS in diet-induced obesity might be responsible for the promotion of insulin-induced p-STAT-5 signaling. To this end we assessed the impact of TCPTP-deficiency on hepatic insulin signaling in mice that had been HFF for 12 weeks (Fig. 5e; Supp. Fig. 5e). Despite insulin-induced PI3K/AKT signaling being attenuated in HFF LTKO livers (Fig. 3m; Supp Fig. 4h), we found that the induction of p-STAT-5 was significantly enhanced in male and female LTKO mice (Fig. 5e; Supp. Fig. 5e). Although IR β-subunit Y1162/Y1163 phosphorylation was elevated in the livers of fasted HFF LTKO male mice (not female mice), insulin-induced IR phosphorylation (Fig. 5e; Supp. Fig. 5e) was not altered by TCPTP-deficiency. These results are consistent with previous studies demonstrating that TCPTP regulates basal, but not insulin-induced IR phosphorylation in the liver (Fukushima et al., 2010). Moreover while insulin-induced STAT-5 signaling was enhanced in LTKO livers, JAK-1 Y1022/Y1023 (p-JAK-1) and JAK-2 Y1007/Y1008 (p-JAK-2) phosphorylation was not altered (Supp. Fig. 5f–g). Therefore these results are consistent with the induction of STAT-5 signaling in response to insulin occurring downstream of the IR and JAK PTKs. Taken together, our results indicate that the inactivation of TCPTP might contribute to the promotion of basal STAT-1 and STAT-3 signaling and insulin-induced STAT-5 signaling in the context of obesity and insulin resistance.
Hepatic TCPTP deficiency alters the IGF-1/GH axis
We next explored the potential molecular mechanisms by which hepatic TCPTP-deficiency might promote obesity and T2D. We noted that p-STAT-5, but not p-STAT-1 or −3, was elevated in the livers of HFF LTKO HET mice (Fig. 5d). Because HFF LTKO HET male mice gained more weight and exhibited greater insulin resistance than controls, we reasoned that the enhanced p-STAT-5 might be sufficient to drive the expression of genes that promote obesity and T2D. In particular, we focussed on two STAT-5 transcriptional targets, Igf-1 (Cui et al., 2007) and the gene encoding pyruvate dehydrogenase kinase 4 (Pdk4) (White et al., 2007). PDK4 phosphorylates pyruvate dehydrogenase complex to conserve glucose and divert three carbon compounds for gluconeogenesis (Kim et al., 2012). In keeping with the elevated hepatic p-STAT-5 in HFF LTKO mice we found that PDK4 protein and Pdk4 mRNA were increased in both male and female mice (Fig. 4g–h; Supp. Fig. 5f–g). Furthermore, Ptpn2 knockdown in hepatocytes using siRNAs increased Pdk4 message (Fig. 4i) consistent with the increased PDK4 in vivo being directly attributable to TCPTP deficiency. Despite the increase in PDK4, we did not detect any differences in the expression of the gluconeogenic genes for PEPCK (Pck1) or G6P (G6pc) in HFF LTKO livers (Fig. 4e), nor differences in glucose excursions in response to the gluconeogenic substrate pyruvate (data not shown), suggesting that hepatic glucose production was not altered. Thus, changes in PDK4 expression may not be sufficient to influence the development obesity and insulin resistance in LTKO mice.
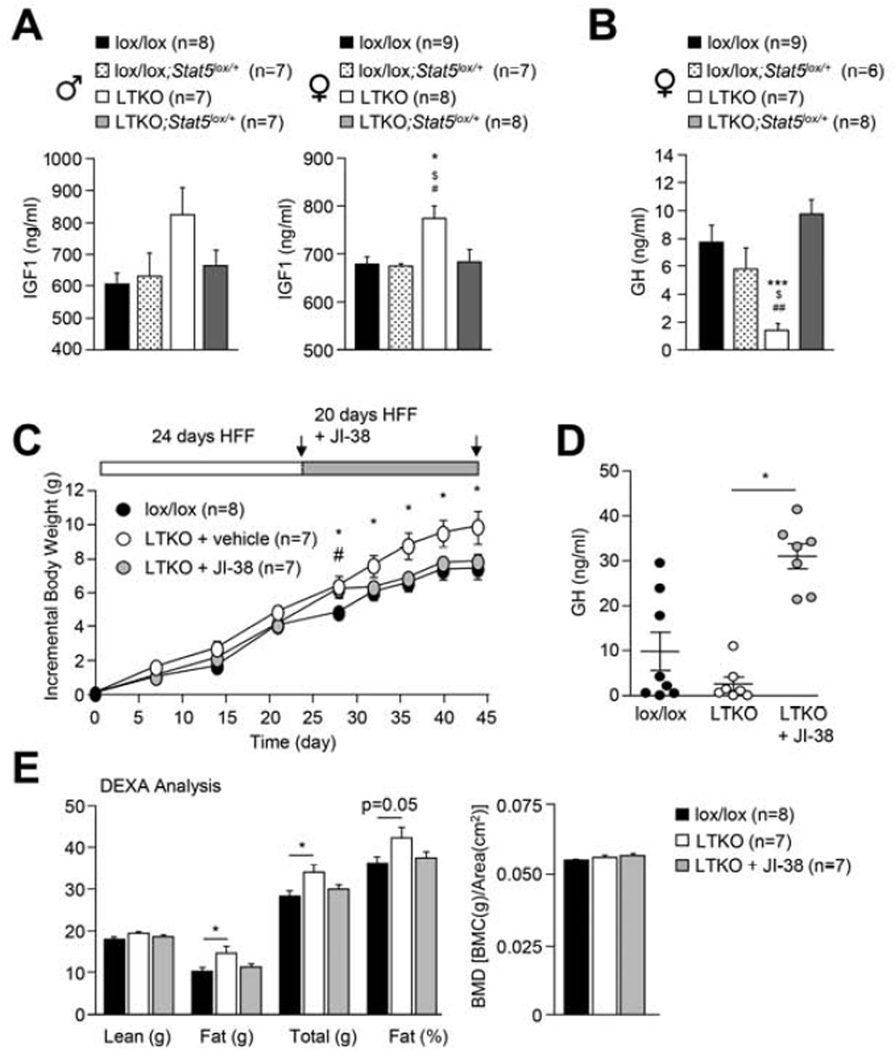
STAT-5 is both necessary and sufficient for the GH-induced transcription of Igf-1 in the liver (Cui et al., 2007). IGF-1 can in turn feed back onto hypothalamic neurons and somatotrophs to inhibit GH release from the pituitary (Romero et al., 2010). We found that serum IGF-1 levels were increased in male and female mice fasted overnight (Fig. 4j). Moreover, in keeping with TCPTP’s potential to regulate STAT-5 signaling and Igf-1 expression, we found that Ptpn2 knockdown using siRNAs increased Igf-1 expression in C57BL/6 hepatocytes (Fig. 4i). Finally, consistent with circulating IGF-1 levels being increased, we found that serum GH levels were decreased in female mice and trended lower in male mice (Fig. 4j); the difference in GH levels is in keeping with the continuous versus pulsatile nature of GH secretion in female versus male mice (Lichanska and Waters, 2008). GH levels are reduced in obese rodents and humans and this can promote fat accumulation (Lichanska and Waters, 2008). Thus, elevated hepatic STAT-5 signaling associated with hepatic TCPTP-deficiency/inactivation could promote IGF-1 expression for the suppression of GH release and the promotion of adiposity in non-hepatic tissues.
Hepatic STAT-5 heterozygosity corrects obesity but not steatosis
To assess the importance of hepatic STAT-5 signaling in the development of obesity and T2D we determined the effect of super imposing STAT-5-deficiency on LTKO mice. To this end we crossed male and female LTKO mice onto a hepatic STAT-5lox/+ (Cui et al., 2007) background (generating LTKO;STAT-5lox/+ mice) to specifically reduce STAT-5 in the liver by 50%, and then fed mice a high fat diet for 12 weeks (Fig. 6; Supp. Fig. 7a). Remarkably, hepatic STAT-5 heterozygosity largely corrected the obesity phenotype in LTKO male and female mice (Fig. 6a; Supp. Fig. 7b). The decreased weight gain in LTKO;STAT-5lox/+ mice was attributable to decreased adiposity, as assessed by DEXA and by weighing fad pads and was accompanied by increased energy expenditure (Fig. 6a; Supp. Fig. 7b), but unaltered food intake and activity (data not shown). Moreover, in female mice the decreased adiposity in LTKO;STAT-5lox/+ mice was accompanied by improved glucose homeostasis, as reflected by the reduced fasted blood glucose levels in females (Fig. 6b); fed and fasted plasma insulin levels also trended lower in both male and female mice (Fig. 6c). Notably, when we assessed p-STAT-5 levels in 4 h fasted LTKO;STAT-5lox/+ mice we found that p-STAT-5 approximated that seen in lox/lox and lox/lox;STAT-5lox/+ controls (Fig. 6d). In keeping with the decreased STAT-5 signaling we found that the otherwise increased hepatic PDK4 levels in LTKO mice were corrected by STAT-5 heterozygosity (Fig. 6d), as were circulating IGF-1 (in male and female mice) and GH (in female mice; levels in male mice not determined) levels (Fig. 7a–b). Therefore the heightened STAT-5 signaling in HFF LTKO mice alters IGF-1 and GH levels and promotes obesity.
Fig. 6. Liver-specific STAT-5 heterozygosity corrects obesity but not steatosis.
7 week-old male lox/lox, Ptpn2lox/lox;STAT-5lox/+ (lox/lox;STAT-5lox/+), LTKO, and Alb-Cre;Ptpn2lox/lox;STAT-5lox/+ (LTKO;STAT-5lox/+) mice were HFF for 12 weeks and (a) body and tissue weights, body composition and energy expenditure, determined, (b-c) fed and fasted blood glucose and plasma insulin measured and (d-f) livers extracted for (d) immunoblotting, (e) histology or (f) lipid analyses. Results shown are means ± SEM; significance (* LTKO and lox/lox, $ LTKO and lox/lox;STAT-5 lox/+, # LTKO and LTKO;STAT-5lox/+) determined using a (a-c) one-way ANOVA or (f) a 2-tailed student’s t-test.
Fig. 7. JI-38 corrects the obesity in LTKO mice.
(a-b) 7 week-old lox/lox, lox/lox;STAT-5 lox/+, LTKO, and LTKO;STAT-5lox/+ mice were HFF for 12 weeks, fasted overnight and serum IGF-1 and GH levels (at 8 am) measured. (c-e) 7 week-old female lox/lox and LTKO mice were HFF for 24 days and administered vehicle or the synthetic GH releasing hormone agonist JI-38 (50 µg/kg in 100 µl) subcutaneously twice daily (8 am and 6 pm) for 20 days and body weights recorded. Serum GH levels (8 am; 30 min after JI-38) and body composition were determined. Results are means ± SEM; significance in a-b (* LTKO and lox/lox, $ LTKO and lox/lox;STAT-5lox/+, # LTKO and LTKO;STAT-5lox/+) and c-d (* LTKO and lox/lox, # LTKO + JI-38 and lox/lox) determined using one-way ANOVA.
In contrast to the effects on obesity, STAT-5 heterozygosity did not correct the steatotic phenotype evident in HFF LTKO male mice (Fig. 6e); in female HFF LTKO;STAT-5lox/+ mice, steatosis appeared to be decreased (Fig. 6e) and hepatic TAG levels trended lower, but this was not significant (Fig. 6f). Moreover p-STAT-1 and p-STAT-3 signaling, as well as PPARγ and SREBP-1 protein levels remained elevated and were indistinguishable from those in LTKO mice (Fig. 6d), in line with these being STAT-5 independent events. Interestingly, we found that heterozygous STAT-5 deletion alone in the liver (Alb-Cre;STAT-5lox/+) promoted steatosis and modestly increased adiposity in HFF mice (Supp. Fig. 7c–e); thus the decreased adiposity in HFF LTKO;STAT-5lox/+ mice was not due to an unrelated effect of STAT-5-deficiency. The increased adiposity and steatosis in HFF Alb-Cre;STAT-5lox/+ mice is consistent with the results of previous studies (Cui et al., 2007). Irrespective, these results indicate that the promotion of hepatic STAT-5 signaling in LTKO mice promotes obesity, whereas alternate pathways promote steatosis.
The altered IGF-1/GH axis in LTKO mice promotes obesity
Having established the importance of STAT-5 signaling in the development of obesity, we next sought to determine the extent to which perturbations in the IGF-1/GH axis might contribute to the obesity phenotype. In particular, we determined if the elevated IGF-1 and lower GH levels in LTKO mice were responsible for the high fat diet-induced obesity by asking whether the promotion of endogenous GH secretion could correct the increased weight gain (Fig. 7c–e). These studies were undertaken in female mice where GH secretion is continuous, rather than in male mice where interventions could be confounded by the pulsatile nature of GH secretion (Lichanska and Waters, 2008). HFF LTKO mice were administered either vehicle control or the synthetic GH releasing hormone agonist JI-38 (Kanashiro-Takeuchi et al., 2010). JI-38 was administered at the beginning of the light and dark cycles for 20 days and body weights were monitored every 4–5 days. We found that JI-38 administration promoted GH release and corrected the obesity phenotype in LTKO mice, preventing weight gain and the increase in adiposity, without affecting lean mass or bone density (Fig. 7c–e). Importantly, the effects of JI-38 administration were reversible and HFF LTKO mice regained weight upon cessation of JI-38 administration (data not shown). Taken together, these results are consistent with the STAT-5/IGF-1/GH axis playing a pivotal role in the development of obesity in LTKO mice.
DISCUSSION
It has long been known that the development of insulin resistance in the liver can be pathway-selective, such that insulin fails to suppress gluconeogenesis but promotes lipogenesis, contributing to the development of fatty liver disease, dyslypidemia and atherosclerosis (Brown and Goldstein, 2008). We establish the capacity of ROS in obesity to inactivate hepatic PTPs for the activation of select signaling pathways in an otherwise insulin-resistant state. In particular, we demonstrate that ROS inactivate TCPTP to promote the insulin-STAT-5 pathway and exacerbate the development of obesity and insulin resistance and thus the hasten progression towards morbid obesity and T2D. In addition, our studies suggest that the oxidation of PTPs such as TCPTP in obesity might also function through independent pathways, possibly in concert with insulin-induced and AKT2-mediated signaling (Biddinger et al., 2008; Haas et al., 2012; Leavens et al., 2009), to promote lipogenesis and the development of fatty liver disease.
The inhibition of PTPs by ROS is thought to represent a key mechanism for the regulation of PTP function and the coordination of tyrosine phosphorylation-dependent cellular signaling (Tiganis, 2011). Cell-based studies have established that the inhibition of PTPs by ROS such as H2O2 is essential for pTyr signaling in response to growth factors, cytokines, hormones and cell adhesion (Tiganis, 2011). Moreover increases in H2O2 in muscle, occurring as a consequence of Gpx1 deficiency, can promote insulin signaling in vivo to attenuate the development of insulin resistance (Loh et al., 2009). However, to date there is no direct evidence for PTPs being oxidised in vivo in response to a physiological stimulus. Similarly, although others have reported that PTPs can be oxidised under pathological conditions in vivo, including ischemia/reperfusion injury and hepatocarcinogenesis, evidence for this has been indirect, relying on the measurement of phosphatase activity (He et al., 2010; Sandin et al., 2011), or undertaken in mice with a deficiency in Gpx1 (Merry et al., 2013). Our studies directly assess the oxidation status of PTPs in the context of an important disease, demonstrating that classical PTPs in the liver undergo substantial oxidation in obese mice characterised by systemic and hepatic oxidative stress. Prominent non-transmembrane PTPs undergoing oxidation included PTP1B and TCPTP. The differential oxidation of PTPs may result from intrinsic differences in PTP sensitivity to oxidation (Lou et al., 2008; Ross et al., 2007) and/or reflect differences in PTP versus antioxidant enzyme subcellular localisations.
Notably the oxidation of such PTPs did not result in global increases in tyrosine phosphorylation in fasted mice. Although we cannot exclude additional pTyr pathways being elevated in response to specific stimuli, our findings are consistent with hepatic PTP oxidation in HFF mice promoting the activation of select signaling pathways. In particular, we found that basal p-STAT-1, and −3 and insulin-induced p-STAT-5 were increased in the livers of HFF mice. This is in keeping with TCPTP being one of the most abundantly oxidised PTPs in the livers of HFF mice, and TCPTP’s capacity to dephosphorylate STAT family members (Tiganis, 2013; Tiganis and Bennett, 2007). Although in this study we focussed our attention on TCPTP, it is probable that the oxidation of multiple PTPs contributes to the promotion of hepatic STAT-1/3/5 signaling in obesity. Previous studies have reported that STAT-5 can interact directly with the IR and that the IR directly or indirectly via JAK2 can phosphorylate and activate STAT-5 in cell lines (Chen et al., 1997; Le et al., 2002). Our results indicate that JAK-2 is essential for the ROS-mediated and insulin-induced activation of STAT-5 in hepatocytes. We speculate that the concomitant oxidation of PTP1B and TCPTP in the livers of HFF mice might promote the coordinated activation of hepatic JAK-2 and STAT-5 in response to insulin. This is in keeping with previous studies that have shown that PTP1B and TCPTP can function in concert to attenuate hypothalamic JAK-2/STAT-3 signaling in vivo (Loh et al., 2011). Although liver-specific PTP1B deficiency does not promote obesity (Delibegovic et al., 2009), PTP1B knockout mice fed a high fat diet for 19.5 weeks are more steatotic that controls (Miraldi et al., 2013). Thus the oxidation of PTP1B and TCPTP may at least cooperate in the promotion of steatosis.
Our studies indicate that the selective activation of the insulin-STAT-5 pathway in the livers of HFF/obese mice was attributable to the accompanying oxidative stress, since i) palmitate-induced mitochondrial H2O2 generation in isolated hepatocytes enhanced insulin-induced p-STAT-5, whilst repressing PI3K/AKT signaling, ii) basal and insulin-induced p-STAT-5 were exacerbated by the increased H2O2 accompanying Gpx1 deficiency, and iii) insulin-induced hepatic p-STAT-5 in HFF mice was at least partially attenuated by SS31. It remains unclear if mitochondria are solely responsible for the ROS generation, PTP oxidation and promotion of p-STAT-5 in HFF mice. Increased NOX activity and expression can contribute to hepatic ROS generation in the fibrotic liver (Aoyama et al., 2012; Paik et al., 2011), but it remains to be seen if NOXs contribute to hepatic oxidative stress in obesity.
Aberrations in the IGF-1/GH axis have been implicated in the development of obesity. GH levels are markedly reduced in obese humans with lower GH levels correlating with increased visceral adiposity (Rasmussen, 2010). Similarly, GH deficiency (Donahue and Beamer, 1993), or defects in GH receptor-STAT-5 signaling (Rowland et al., 2005) in mice are associated with the development of obesity. The precise mechanisms by which decreased GH levels contribute to obesity are not fully understood, but may be attributable to defective lipolytic responses and decreased fatty acid oxidation (Lichanska and Waters, 2008). In humans the increased adiposity may also be due to decreases in energy expenditure (Moller and Jorgensen, 2009; Snel et al., 1995; Wolthers et al., 1996). This is consistent with the decreased energy expenditure seen in HFF LTKO mice; although corrected by Stat5 heterozygosity, it remains to be determined if the effects on energy expenditure were mediated by the IGF-1/GH axis. The underlying cause(s) of the lower GH levels in human obesity is/are contentious and include the suppression of GH secretion by elevated plasma insulin (Steyn et al., 2013) and IGF-1 (Romero et al., 2010). Total plasma IGF-1 is not reduced to the degree predicted by the low GH levels, and circulating IGF-1 is in the normal-high range in obese humans (Frystyk et al., 1999; Nam et al., 1997). The association between IGF-1 and GH is especially evident in adolescent obesity, where physiological insulin resistance and elevated circulating IGF-1 (Moran et al., 2002) are exaggerated in the obese state to result in reduced GH levels (Vanderschueren-Lodeweyckx, 1993). Our studies indicate that the oxidative stress and the inactivation of hepatic PTPs in obesity might promote the insulin-STAT-5-IGF-1 pathway to reduce GH levels and exacerbate the development of obesity and T2D.
Although the enhanced hepatic insulin-induced STAT-5 pathway exacerbated the development of obesity, our studies indicate that other pathways might contribute to the enhanced lipogenesis and steatosis in LTKO mice. We observed elevated STAT-1 signaling, which can drive PPARγ to promote lipogenesis via SREBP-1c, SCD-1 and ACC and FFA uptake via CD36 (Barclay et al., 2011; Matsusue et al., 2003; Moran-Salvador et al., 2011). Thus, the increased steatosis in LTKO mice might result from the high p-STAT-1, occurring as a direct consequence of TCPTP deficiency, promoting lipogenesis and at least in female mice increasing FFA uptake via CD36. The heightened STAT-3 signaling in LTKO mice might also contribute to steatosis, since STAT-3 overexpression increases ACC and FAS and inhibits acyl-CoA oxidase (involved in β-oxidation) expression and promotes steatosis (Kinoshita et al., 2008). Obesity is associated with systemic inflammation and there is evidence for alteration of the inflammatory cytokine millieu in the livers of obese rodents (Li et al., 2005). Determining the extent to which TCPTP oxidation/inactivation might exacerbate inflammatory STAT-1/3 signaling in the liver to promote steatosis will require further study.
The results of this study identify a mechanism for the development of selective hepatic insulin resistance, define the contributions of the insulin-STAT-5 pathway in the liver to the development obesity and T2D and highlight the potential utility of targeted anti-oxidants in combating the associated hepatic complications. Many human diseases, including inflammatory and neurological disorders, cancer, obesity and T2D are characterised by oxidative stress. Thus the results of this study may have wide-ranging implications highlighting the potential for oxidative stress and PTP oxidation to alter cellular signaling in diverse pathological contexts.
EXPERIMENTAL PROCEDURES
Mice
We maintained mice on a 12 h light-dark cycle in a temperature-controlled high barrier facility with free access to food and water. Aged- and sex-matched mice were used for experiments. Ptpn2lox/lox (C57BL/6) described previously (Loh et al., 2012; Wiede et al., 2011) were mated with Alb-Cre (C57BL/6) mice (JAX) for the postnatal deletion of Ptpn2 in hepatocytes. STAT-5lox/lox (C57BL/6) mice described previously (Cui et al., 2007) were mated with Alb-Cre;Ptpn2lox/lox mice. Gpx1−/− (C57BL6) mice have been described previously (Loh et al., 2009). Mice were fed a standard chow or a high fat diet (23% fat) as indicated. Experiments were approved by the Monash University School of Biomedical Sciences Animal Ethics Committee.
Metabolic measures
Insulin and glucose tolerance tests and hyperinsulinemic euglycemic clamps were performed as described previously (Loh et al., 2009). Metabolic measures were undertaken in an environmentally controlled Comprehensive Lab Animal Monitoring System (Columbus Instruments) and body composition measured by DEXA (Lunar PIXImus2; GE Healthcare).
Cell culture and RNA interference
Hepatocytes were isolated by a two-step collagenase A (0.05% w/v; Roche Diagnostics) perfusion method as described previously (Fukushima et al., 2010). Where indicated cells were serum starved in M199 medium for 2–4 h and then stimulated with bovine insulin, GH, IFN-γ or IL-6. Alternatively, cells were treated with 0.5 mM sodium palmitate in the presence of 1% w/v fatty acid free BSA overnight and then serum starved and stimulated as indicated. Ptpn2 or Jak-2 were knocked down transiently in primary murine hepatocytes using Ptpn2- or Jak2-specific siRNAs; enhanced GFP siRNA was used as a control.
ROS determinations
H2O2 production in live hepatocytes was determined using the Amplex® Red hydrogen peroxide assay kit (Invitrogen). Total (GSH) and oxidised (GSSG) glutathione levels were measured using a BIOXYTECH GSH/GSSG-412 assay kit (Oxis International).
PTP oxidation
Total (reversible and irreversible) PTP oxidation was assessed essentially as described previously (Karisch et al., 2011) with some modifications. Briefly, frozen liver tissue or freshly isolated hepatocytes were homogenised under anaerobic conditions in de-gassed, ice-cold PTPox lysis buffer containing 10 mM N-ethylmaleimide to prevent post-lysis oxidation and to alkylate all reduced and active PTPs, and incubated for 1 h at 4°C. Cell lysates and liver homogenates were clarified by centrifugation at 16,000 g and 50,000 g respectively for 20 min and the buffer exchanged (NAP™-5 columns, GE Healthcare) to 20 mM HEPES containing 10 mM DTT to reduce oxidised PTPs. Reduced PTPs were then hyperoxidised to their sulfonic (−SO3H) state by exchanging the buffer to 20 mM HEPES containing 100 µM pervanadate and resolved by SDS-PAGE and immunoblotted with PTPox antibody. Alternatively pervanadate-treated samples were made to 9 M urea and 4.5 mM DTT, incubated at 60°C for 30 min and then treated with 10 mM iodoacetamide before being diluted to a final concentration of 2 M urea for digestion with TPCK-trypsin (Thermo Scientific) and processing for PTPox immunoprecipitation and analysis by mass spectrometry as described previously (Karisch et al., 2011).
Statistical Analysis
Statistical significance was determined by a two-tailed paired Student’s t-test or where indicated ANOVA with Bonferroni correction. P values < 0.05 were considered statistically significant (*p<0.05, **p<0.01, ***p<0.001).
Supplementary Material
HIGHLIGHTS.
Reactive oxygen species oxidise and inactivate hepatic PTPs in obesity.
PTPN2 inactivation in the liver promotes insulin-STAT-5 signaling.
PTPN2 inactivation and STAT5 signaling in the liver promotes obesity.
PTPN2 inactivation in the liver promotes steatosis and insulin resistance.
ACKNOWLEDGMENTS
The work was supported by NHMRC Australia (TT, MJW, MW, and SA), AICR (TT; 12-1172), NIH (BGN; R37 CA49152), the Ontario Ministry of Health and Long Term Care and the Princess Margaret Cancer Foundation (BGN). BGN is a Canada Research Chair, Tier 1. TT, MJW, MW, and SA are NHMRC Fellows.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, Kang L, Rabinovitch PS, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119:573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama T, Paik YH, Watanabe S, Laleu B, Gaggini F, Fioraso-Cartier L, Molango S, Heitz F, Merlot C, et al. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology. 2012;56:2316–2327. doi: 10.1002/hep.25938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay JL, Nelson CN, Ishikawa M, Murray LA, Kerr LM, McPhee TR, Powell EE, Waters MJ. GH-dependent STAT5 signaling plays an important role in hepatic lipid metabolism. Endocrinology. 2011;152:181–192. doi: 10.1210/en.2010-0537. [DOI] [PubMed] [Google Scholar]
- Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Aleman JO, Suzuki R, Scapa EF, Agarwal C, Carey MC, Stephanopoulos G, et al. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008;7:125–134. doi: 10.1016/j.cmet.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Chen J, Sadowski HB, Kohanski RA, Wang LH. Stat5 is a physiological substrate of the insulin receptor. Proc Natl Acad Sci USA. 1997;94:2295–2300. doi: 10.1073/pnas.94.6.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Hosui A, Sun R, Shen K, Gavrilova O, Chen W, Cam MC, Gao B, Robinson GW, Hennighausen L. Loss of signal transducer and activator of transcription 5 leads to hepatosteatosis and impaired liver regeneration. Hepatology. 2007;46:504–513. doi: 10.1002/hep.21713. [DOI] [PubMed] [Google Scholar]
- Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–795. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delibegovic M, Zimmer D, Kauffman C, Rak K, Hong EG, Cho YR, Kim JK, Kahn BB, Neel BG, Bence KK. Liver-specific deletion of protein-tyrosine phosphatase 1B improves metabolic syndrome and attenuates diet-induced ER Stress. Diabetes. 2009;58:590–599. doi: 10.2337/db08-0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue LR, Beamer WG. Growth hormone deficiency in 'little' mice results in aberrant body composition, reduced insulin-like growth factor-I and insulinlike growth factor-binding protein-3 (IGFBP-3), but does not affect IGFBP-2, −1 or −4. J Endocrinol. 1993;136:91–104. doi: 10.1677/joe.0.1360091. [DOI] [PubMed] [Google Scholar]
- Fisher-Wellman KH, Neufer PD. Linking mitochondrial bioenergetics to insulin resistance via redox biology. Trends Endocrinol Metab. 2011;23:142–153. doi: 10.1016/j.tem.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foretz M, Guichard C, Ferre P, Foufelle F. Sterol regulatory element binding protein-1c is a major mediator of insulin action on the hepatic expression of glucokinase and lipogenesis-related genes. Proc Natl Acad Sci USA. 1999;96:12737–12742. doi: 10.1073/pnas.96.22.12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frystyk J, Skjaerbaek C, Vestbo E, Fisker S, Orskov H. Circulating levels of free insulin-like growth factors in obese subjects: the impact of type 2 diabetes. Diabetes Metab Res Rev. 1999;15:314–322. doi: 10.1002/(sici)1520-7560(199909/10)15:5<314::aid-dmrr56>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Fukushima A, Loh K, Galic S, Fam B, Shields B, Wiede F, Tremblay ML, Watt MJ, Andrikopoulos S, Tiganis T. T-cell protein tyrosine phosphatase attenuates STAT3 and insulin signaling in the liver to regulate gluconeogenesis. Diabetes. 2010;59:1906–1914. doi: 10.2337/db09-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas JT, Miao J, Chanda D, Wang Y, Zhao E, Haas ME, Hirschey M, Vaitheesvaran B, Farese RV, Jr., Kurland IJ, et al. Hepatic insulin signaling is required for obesity-dependent expression of SREBP-1c mRNA but not for feeding-dependent expression. Cell Metab. 2012;15:873–884. doi: 10.1016/j.cmet.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He G, Yu GY, Temkin V, Ogata H, Kuntzen C, Sakurai T, Sieghart W, Peck-Radosavljevic M, Leffert HL, Karin M. Hepatocyte IKKβ/NF-κB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation. Cancer Cell. 2010;17:286–297. doi: 10.1016/j.ccr.2009.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermiston ML, Zikherman J, Zhu JW. CD45, CD148, and Lyp/Pep: critical phosphatases regulating Src family kinase signaling networks in immune cells. Immunol Rev. 2009;228:288–311. doi: 10.1111/j.1600-065X.2008.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- Jaffe CA, Huffman BW, Demott-Friberg R. Insulin hypoglycemia and growth hormone secretion in sheep: a paradox revisited. Am J Physiol. 1999;277:E253–E258. doi: 10.1152/ajpendo.1999.277.2.E253. [DOI] [PubMed] [Google Scholar]
- Jiang F, Lim HK, Morris MJ, Prior L, Velkoska E, Wu X, Dusting GJ. Systemic upregulation of NADPH oxidase in diet-induced obesity in rats. Redox Rep. 2011;16:223–229. doi: 10.1179/174329211X13049558293713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AM, Olefsky JM. The origins and drivers of insulin resistance. Cell. 2013;152:673–684. doi: 10.1016/j.cell.2013.01.041. [DOI] [PubMed] [Google Scholar]
- Kanashiro-Takeuchi RM, Tziomalos K, Takeuchi LM, Treuer AV, Lamirault G, Dulce R, Hurtado M, Song Y, Block NL, Rick F, et al. Cardioprotective effects of growth hormone-releasing hormone agonist after myocardial infarction. Proc Natl Acad Sci USA. 2010;107:2604–2609. doi: 10.1073/pnas.0914138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karisch R, Fernandez M, Taylor P, Virtanen C, St-Germain JR, Jin LL, Harris IS, Mori J, Mak TW, et al. Global proteomic assessment of the classical protein-tyrosine phosphatome and "Redoxome". Cell. 2011;146:826–840. doi: 10.1016/j.cell.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YD, Kim YH, Tadi S, Yu JH, Yim YH, Jeoung NH, Shong M, Hennighausen L, Harris RA, et al. Metformin inhibits growth hormone-mediated hepatic PDK4 gene expression through induction of orphan nuclear receptor small heterodimer partner. Diabetes. 2012;61:2484–2494. doi: 10.2337/db11-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita S, Ogawa W, Okamoto Y, Takashima M, Inoue H, Matsuki Y, Watanabe E, Hiramatsu R, Kasuga M. Role of hepatic STAT3 in the regulation of lipid metabolism. Kobe J Med Sci. 2008;54:E200–E208. [PubMed] [Google Scholar]
- Le MN, Kohanski RA, Wang LH, Sadowski HB. Dual mechanism of signal transducer and activator of transcription 5 activation by the insulin receptor. Mol Endocrinol. 2002;16:2764–2779. doi: 10.1210/me.2002-0017. [DOI] [PubMed] [Google Scholar]
- Leavens KF, Easton RM, Shulman GI, Previs SF, Birnbaum MJ. Akt2 is required for hepatic lipid accumulation in models of insulin resistance. Cell Metab. 2009;10:405–418. doi: 10.1016/j.cmet.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci USA. 2010;107:3441–3446. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Soloski MJ, Diehl AM. Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology. 2005;42:880–885. doi: 10.1002/hep.20826. [DOI] [PubMed] [Google Scholar]
- Lichanska AM, Waters MJ. How growth hormone controls growth, obesity and sexual dimorphism. Trends Genet. 2008;24:41–47. doi: 10.1016/j.tig.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Loh K, Deng H, Fukushima A, Cai X, Boivin B, Galic S, Bruce C, Shields BJ, Skiba B, Ooms LM, et al. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 2009;10:260–272. doi: 10.1016/j.cmet.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh K, Fukushima A, Zhang X, Galic S, Briggs D, Enriori PJ, Simonds S, Wiede F, Reichenbach A, et al. Elevated hypothalamic TCPTP in obesity contributes to cellular leptin resistance. Cell Metab. 2011;14:684–699. doi: 10.1016/j.cmet.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh K, Merry TL, Galic S, Wu BJ, Watt MJ, Zhang S, Zhang ZY, Neel BG, Tiganis T. T cell protein tyrosine phosphatase deficiency in muscle does not alter insulin signaling and glucose homeostasis in mice. Diabetologia. 2012;55:468–478. doi: 10.1007/s00125-011-2386-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou YW, Chen YY, Hsu SF, Chen RK, Lee CL, Khoo KH, Tonks NK, Meng TC. Redox regulation of the protein tyrosine phosphatase PTP1B in cancer cells. FEBS J. 2008;275:69–88. doi: 10.1111/j.1742-4658.2007.06173.x. [DOI] [PubMed] [Google Scholar]
- Lu M, Wan M, Leavens KF, Chu Q, Monks BR, Fernandez S, Ahima RS, Ueki K, Kahn CR, Birnbaum MJ. Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat Med. 2012;18:388–395. doi: 10.1038/nm.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsusue K, Haluzik M, Lambert G, Yim SH, Gavrilova O, Ward JM, Brewer B, Jr., Reitman ML, Gonzalez FJ. Liver-specific disruption of PPARγ in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J Clin Invest. 2003;111:737–747. doi: 10.1172/JCI17223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memon RA, Tecott LH, Nonogaki K, Beigneux A, Moser AH, Grunfeld C, Feingold KR. Up-regulation of peroxisome proliferator-activated receptors (PPARα) and PPARγ mRNA expression in the liver in murine obesity: troglitazone induces expression of PPARγ-responsive adipose tissue-specific genes in the liver of obese diabetic mice. Endocrinology. 2000;141:4021–4031. doi: 10.1210/endo.141.11.7771. [DOI] [PubMed] [Google Scholar]
- Merry TL, Tran M, Stathopoulos M, Wiede F, Fam BC, Dood GT, Clarke I, Watt MJ, Andrikopoulos S, Tiganis T. High fat fed obese Gpx1-deficient mice exhibit defective insulin secretion but protection from hepatic steatosis and liver damage. Antioxid Redox Signal. 2013 doi: 10.1089/ars.2013.5428. [DOI] [PubMed] [Google Scholar]
- Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- Miraldi ER, Sharfi H, Friedline RH, Johnson H, Zhang T, Lau KS, Ko HJ, Curran TG, Haigis KM, et al. Molecular network analysis of phosphotyrosine and lipid metabolism in hepatic PTP1b deletion mice. Integr Biol. 2013;5:940–963. doi: 10.1039/c3ib40013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller N, Jorgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009;30:152–177. doi: 10.1210/er.2008-0027. [DOI] [PubMed] [Google Scholar]
- Moran A, Jacobs DR, Steinberger J, Cohen P, Hong CP, Prineas R, Sinaiko AR. Association between the insulin resistance of puberty and the IGF-I/growth hormone axis. J Clin Endocrinol Metab. 2002;87:4817–4820. doi: 10.1210/jc.2002-020517. [DOI] [PubMed] [Google Scholar]
- Moran-Salvador E, Lopez-Parra M, Garcia-Alonso V, Titos E, Martinez-Clemente M, Gonzalez-Periz A, Lopez-Vicario C, Barak Y, Arroyo V, Claria J. Role for PPARγ in obesity-induced hepatic steatosis as determined by hepatocyte- and macrophage-specific conditional knockouts. Faseb J. 2011;25:2538–2550. doi: 10.1096/fj.10-173716. [DOI] [PubMed] [Google Scholar]
- Nam SY, Lee EJ, Kim KR, Cha BS, Song YD, Lim SK, Lee HC, Huh KB. Effect of obesity on total and free insulin-like growth factor (IGF)-1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone. Int J Obes Relat Metab Disord. 1997;21:355–359. doi: 10.1038/sj.ijo.0800412. [DOI] [PubMed] [Google Scholar]
- Newsholme P, Haber EP, Hirabara SM, Rebelato EL, Procopio J, Morgan D, Oliveira-Emilio HC, Carpinelli AR, Curi R. Diabetes associated cell stress and dysfunction: role of mitochondrial and non-mitochondrial ROS production and activity. J Physiol. 2007;583:9–24. doi: 10.1113/jphysiol.2007.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik YH, Iwaisako K, Seki E, Inokuchi S, Schnabl B, Osterreicher CH, Kisseleva T, Brenner DA. The nicotinamide adenine dinucleotide phosphate oxidase (NOX) homologues NOX1 and NOX2/gp91phox mediate hepatic fibrosis in mice. Hepatology. 2011;53:1730–1741. doi: 10.1002/hep.24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson C, Kappert K, Engstrom U, Ostman A, Sjoblom T. An antibody-based method for monitoring in vivo oxidation of protein tyrosine phosphatases. Methods. 2005;35:37–43. doi: 10.1016/j.ymeth.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118:829–838. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen MH. Obesity, growth hormone and weight loss. Mol Cell Endocrinol. 2010;316:147–153. doi: 10.1016/j.mce.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Rolo AP, Teodoro JS, Palmeira CM. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med. 2012;52:59–69. doi: 10.1016/j.freeradbiomed.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Romero CJ, Ng Y, Luque RM, Kineman RD, Koch L, Bruning JC, Radovick S. Targeted deletion of somatotroph IGF-I signaling in a cell-specific knockout mouse model. Mol Endocrinol. 2010;24:1077–1089. doi: 10.1210/me.2009-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SH, Lindsay Y, Safrany ST, Lorenzo O, Villa F, Toth R, Clague MJ, Downes CP, Leslie NR. Differential redox regulation within the PTP superfamily. Cell Signal. 2007;19:1521–1530. doi: 10.1016/j.cellsig.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Rowland JE, Lichanska AM, Kerr LM, White M, d'Aniello EM, Maher SL, Brown R, Teasdale RD, Noakes PG, Waters MJ. In vivo analysis of growth hormone receptor signaling domains and their associated transcripts. Mol Cell Biol. 2005;25:66–77. doi: 10.1128/MCB.25.1.66-77.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel AR, Kahn CR. Insulin signaling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandin A, Dagnell M, Gonon A, Pernow J, Stangl V, Aspenstrom P, Kappert K, Ostman A. Hypoxia followed by re-oxygenation induces oxidation of tyrosine phosphatases. Cell Signal. 2011;23:820–826. doi: 10.1016/j.cellsig.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Shimano H, Horton JD, Shimomura I, Hammer RE, Brown MS, Goldstein JL. Isoform 1c of sterol regulatory element binding protein is less active than isoform 1a in livers of transgenic mice and in cultured cells. J Clin Invest. 1997;99:846–854. doi: 10.1172/JCI119248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snel YE, Doerga ME, Brummer RJ, Zelissen PM, Zonderland ML, Koppeschaar HP. Resting metabolic rate, body composition and related hormonal parameters in growth hormone-deficient adults before and after growth hormone replacement therapy. Eur J Endocrinol. 1995;133:445–450. doi: 10.1530/eje.0.1330445. [DOI] [PubMed] [Google Scholar]
- Steyn FJ, Xie TY, Huang L, Ngo ST, Veldhuis JD, Waters MJ, Chen C. Increased adiposity and insulin correlates with the progressive suppression of pulsatile GH secretion during weight gain. J Endocrinol. 2013;218:233–244. doi: 10.1530/JOE-13-0084. [DOI] [PubMed] [Google Scholar]
- Tiganis T. Reactive oxygen species and insulin resistance: the good, the bad and the ugly. Trends Pharmacol Sci. 2011;32:82–89. doi: 10.1016/j.tips.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Tiganis T. PTP1B and TCPTP - nonredundant phosphatases in insulin signaling and glucose homeostasis. FEBS J. 2013;280:445–458. doi: 10.1111/j.1742-4658.2012.08563.x. [DOI] [PubMed] [Google Scholar]
- Tiganis T, Bennett AM. Protein tyrosine phosphatase function: the substrate perspective. Biochem J. 2007;402:1–15. doi: 10.1042/BJ20061548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner N, Kowalski GM, Leslie SJ, Risis S, Yang C, Lee-Young RS, Babb JR, Meikle PJ, Lancaster GI, et al. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia. 2013;56:1638–1648. doi: 10.1007/s00125-013-2913-1. [DOI] [PubMed] [Google Scholar]
- Vanderschueren-Lodeweyckx M. The effect of simple obesity on growth and growth hormone. Horm Res. 1993;40:23–30. doi: 10.1159/000183763. [DOI] [PubMed] [Google Scholar]
- White UA, Coulter AA, Miles TK, Stephens JM. The STAT5A-mediated induction of pyruvate dehydrogenase kinase 4 expression by prolactin or growth hormone in adipocytes. Diabetes. 2007;56:1623–1629. doi: 10.2337/db06-1286. [DOI] [PubMed] [Google Scholar]
- Wiede F, Shields BJ, Chew SH, Kyparissoudis K, van Vliet C, Galic S, Tremblay ML, Russell SM, Godfrey DI, Tiganis T. T cell protein tyrosine phosphatase attenuates T cell signaling to maintain tolerance in mice. J Clin Invest. 2011;121:4758–4774. doi: 10.1172/JCI59492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolthers T, Groftne T, Moller N, Christiansen JS, Orskov H, Weeke J, Jorgensen JO. Calorigenic effects of growth hormone: the role of thyroid hormones. J Clin Endocrinol Metab. 1996;81:1416–1419. doi: 10.1210/jcem.81.4.8636344. [DOI] [PubMed] [Google Scholar]
- Yamashita S, Melmed S. Effects of insulin on rat anterior pituitary cells. Inhibition of growth hormone secretion and mRNA levels. Diabetes. 1986;35:440–447. doi: 10.2337/diab.35.4.440. [DOI] [PubMed] [Google Scholar]
- Yecies JL, Zhang HH, Menon S, Liu S, Yecies D, Lipovsky AI, Gorgun C, Kwiatkowski DJ, Hotamisligil GS, et al. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 2011;14:21–32. doi: 10.1016/j.cmet.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Matsusue K, Kashireddy P, Cao WQ, Yeldandi V, Yeldandi AV, Rao MS, Gonzalez FJ, Reddy JK. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome PPARγ1 overexpression. J Biol Chem. 2003;278:498–505. doi: 10.1074/jbc.M210062200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.