Abstract
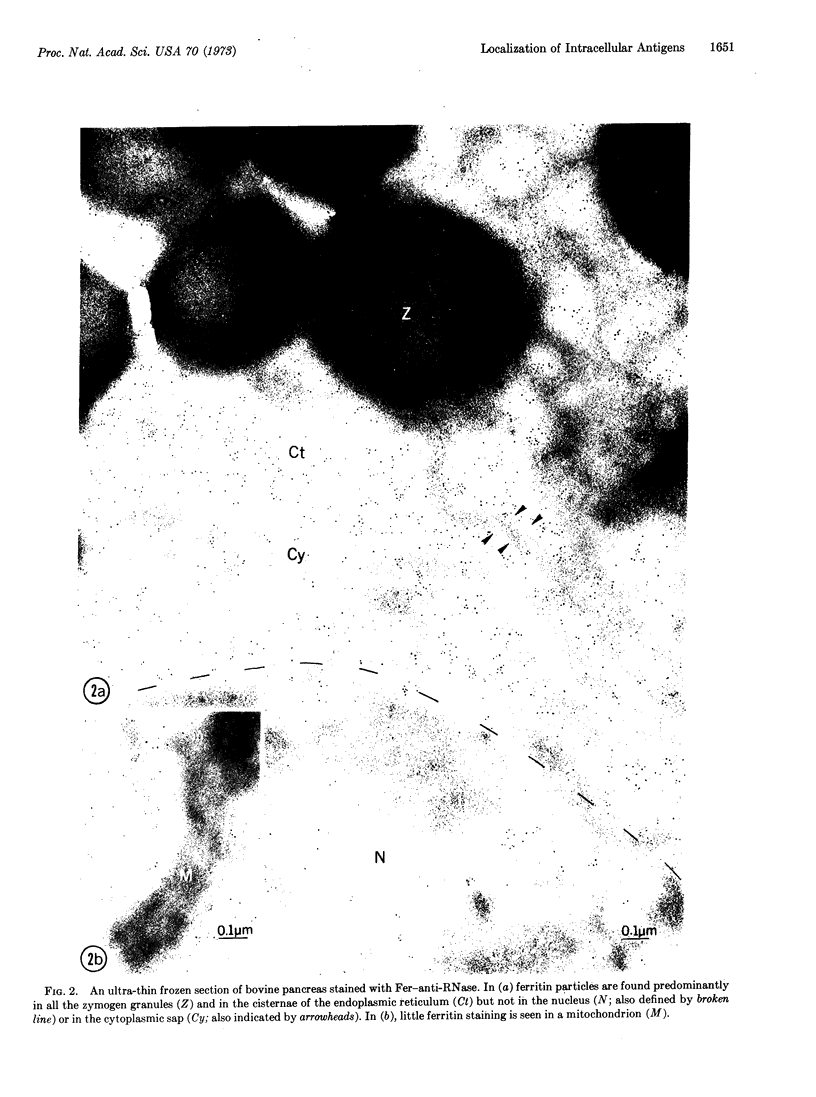
A general method for the ultrastructural localization of intracellular proteins and antigens by immunoferritin techniques has been developed. The method involves direct staining of ultrathin sections of mildly glutaraldehyde-fixed and frozen tissues cut by means of a cryo-ultramicrotome. Bovine pancreatic sections were cut, mounted on grids, and stained with ferritin-rabbit antibovine RNase conjugates. After negative staining with 0.2% phosphotungstic acid, electron micrographs revealed specific labeling of all of the zymogen granules and the cisternae of the rough endoplasmic reticulum. No significant labeling was seen in the nucleus, mitochondria, or cell sap regions. The observation that no significant labeling was found in any region of rat pancreatic sections was consistent with the fact that rat RNase is immunologically non-crossreactive with bovine RNase. In addition, the labeling seen in bovine pancreas was completely absent if the sections were first incubated with free antibody. The method used here avoids prolonged fixation, dehydration, and other harsh chemical or physical treatments, and should extend the usefulness of immunoferritin techniques to the intracellular localization of many protein antigens beyond previously available methods.
Keywords: electron microscopy, ribonuclease, pancreas, anti-ribonuclease, affinity chromatography
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDRES G. A., MORGAN C., HSU K. C., RIFKIND R. A., SEEGAL B. C. Electron microscopic studies of experimental nephritis with ferritin-conjugated antibody. The basement membranes and cisternae of visceral epithelial cells in nephritic rat glomeruli. J Exp Med. 1962 May 1;115:929–936. doi: 10.1084/jem.115.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- DICKMAN S. R., MORRILL G. A. Intracellular localization and chromatography of mouse pancreas ribonucleases. Ann N Y Acad Sci. 1959 Sep 4;81:585–598. doi: 10.1111/j.1749-6632.1959.tb49339.x. [DOI] [PubMed] [Google Scholar]
- GREENE L. J., HIRS C. H., PALADE G. E. On the protein composition of bovine pancreatic zymogen granules. J Biol Chem. 1963 Jun;238:2054–2070. [PubMed] [Google Scholar]
- Gordon J. Ribonucleases of the rat. I. Purification of liver and pancreatic ribonuclease. Arch Biochem Biophys. 1965 Dec;112(3):421–428. doi: 10.1016/0003-9861(65)90074-3. [DOI] [PubMed] [Google Scholar]
- HIRS C. H. W., MOORE S., STEIN W. H. A chromatographic investigation of pancreatic ribonuclease. J Biol Chem. 1953 Feb;200(2):493–506. [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. II. Transport to condensing vacuoles and zymogen granules. J Cell Biol. 1967 Aug;34(2):597–615. doi: 10.1083/jcb.34.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALNITSKY G., HUMMEL J. P., DIERKS C. [Some factors which affect the enzymatic digestion of ribonucleic acid]. J Biol Chem. 1959 Jun;234(6):1512–1516. [PubMed] [Google Scholar]
- KELLER P. J., COHEN E. Enzymic composition of some cell fractions of bovine pancrease. J Biol Chem. 1961 May;236:1407–1413. [PubMed] [Google Scholar]
- Kawarai Y., Nakane P. K. Localization of tissue antigens on the ultrathin sections with peroxidase-labeled antibody method. J Histochem Cytochem. 1970 Mar;18(3):161–166. doi: 10.1177/18.3.161. [DOI] [PubMed] [Google Scholar]
- Kraehenbuhl J. P., Jamieson J. D. Solid-phase conjugation of ferritin to Fab-fragments of immunoglobulin G for use in antigen localization on thin sections. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1771–1775. doi: 10.1073/pnas.69.7.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc E. H., Scott G. B., Avrameas S. Ultrastructural localization of intracellular immune globulins in plasma cells and lymphoblasts by enzyme-labeled antibodies. J Histochem Cytochem. 1969 Apr;17(4):211–224. doi: 10.1177/17.4.211. [DOI] [PubMed] [Google Scholar]
- McLean J. D., Singer S. J. A general method for the specific staining of intracellular antigens with ferritin-antibody conjugates. Proc Natl Acad Sci U S A. 1970 Jan;65(1):122–128. doi: 10.1073/pnas.65.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean J. D., Singer S. J. A technique for the specific staining of macromolecules and viruses with ferritin-antibody conjugates. J Mol Biol. 1971 Mar 28;56(3):633–635. doi: 10.1016/0022-2836(71)90407-4. [DOI] [PubMed] [Google Scholar]
- Nakane P. K., Pierce G. B., Jr Enzyme-labeled antibodies for the light and electron microscopic localization of tissue antigens. J Cell Biol. 1967 May;33(2):307–318. doi: 10.1083/jcb.33.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L., Marchesi V. T., Singer S. J. The localization of spectrin on the inner surface of human red blood cell membranes by ferritin-conjugated antibodies. J Cell Biol. 1971 Oct;51(1):265–272. doi: 10.1083/jcb.51.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHICK A. F., SINGER S. J. On the formation of covalent linkages between two protein molecules. J Biol Chem. 1961 Sep;236:2477–2485. [PubMed] [Google Scholar]
- SIEKEVITZ P., PALADE G. E. A cytochemical study on the pancreas of the guinea pig. II. Functional variations in the enzymatic activity of microsomes. J Biophys Biochem Cytol. 1958 May 25;4(3):309–318. doi: 10.1083/jcb.4.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]





