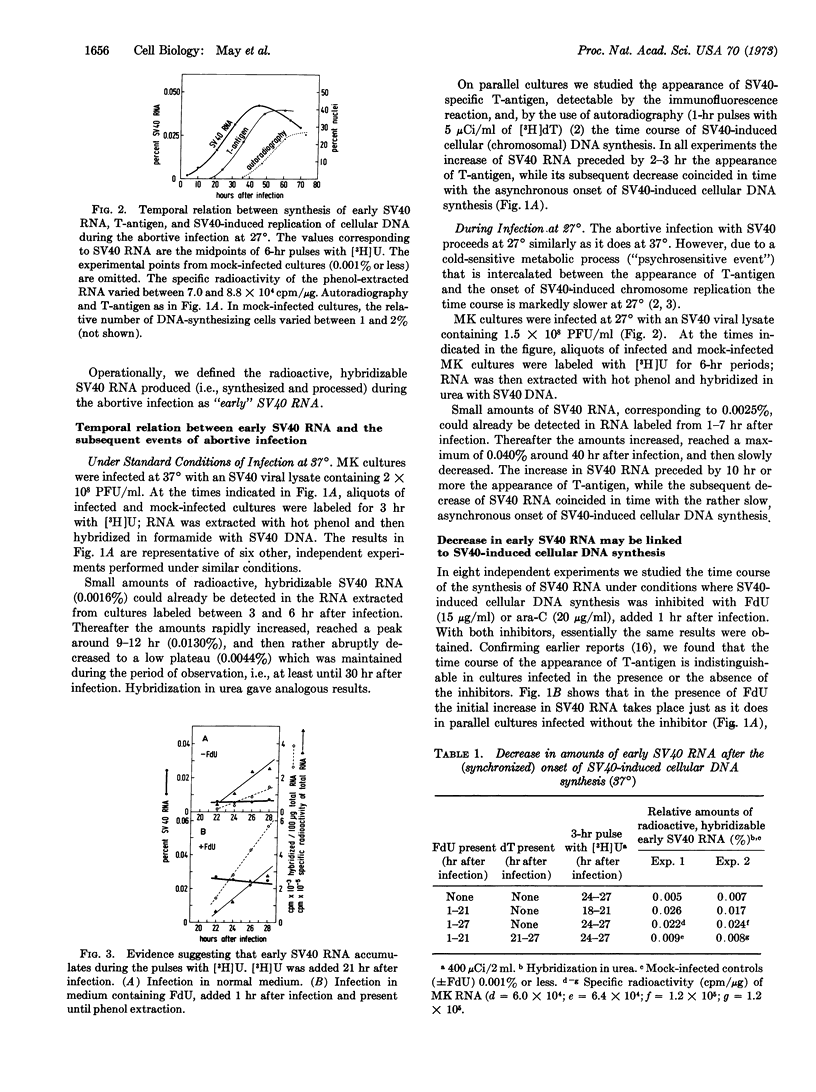
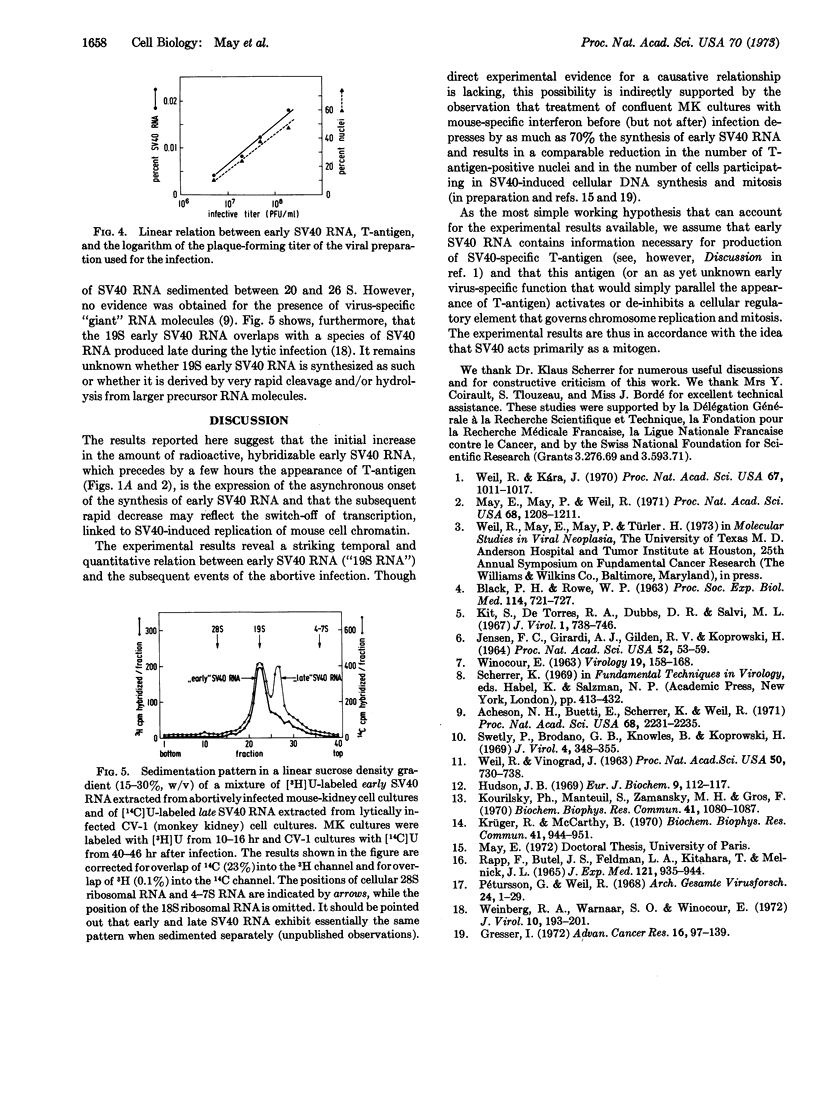
Abstract
Simian Virus 40 (SV40) induces in “contact-inhibited” tissue culture cells of mouse kidney an abortive infection that leads to the appearance of intra-nuclear SV40-specific tumor (T-) antigen, followed by replication of the mouse-cell chromatin and mitosis, while no viral progeny DNA or capsid protein is produced. Synthesis of “early” SV40-specific RNA (“19S RNA”) begins a few hours before the appearance of T-antigen and appears to be switched off after the onset of chromatin replication. As the most simple working hypothesis that can account for the experimental results available, we assume that early SV40 RNA contains information necessary for production of T-antigen and that this antigen (or an unknown early virus-specific function that would simply parallel the appearance of T-antigen) activates or de-inhibits a cellular regulatory element that governs chromosome replication and mitosis. The experimental results agree with the idea that SV40 acts primarily as a mitogen.
Keywords: early SV40-specific RNA, tumor antigen, mitogen
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H., Buetti E., Scherrer K., Weil R. Transcription of the polyoma virus genome: synthesis and cleavage of giant late polyoma-specific RNA. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2231–2235. doi: 10.1073/pnas.68.9.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACK P. H., ROWE W. P. SV-40 INDUCED PROLIFERATION OF TISSUE CULTURE CELLS OF RABBIT, MOUSE, AND PORCINE ORIGIN. Proc Soc Exp Biol Med. 1963 Dec;114:721–727. doi: 10.3181/00379727-114-28780. [DOI] [PubMed] [Google Scholar]
- Gresser I. Antitumor effects of interferon. Adv Cancer Res. 1972;16:97–140. doi: 10.1016/s0065-230x(08)60339-5. [DOI] [PubMed] [Google Scholar]
- Hudson J. B. An analysis of the polyoma virus DNA-RNA hybridization reaction in vitro. Eur J Biochem. 1969 May 1;9(1):112–117. doi: 10.1111/j.1432-1033.1969.tb00583.x. [DOI] [PubMed] [Google Scholar]
- JENSEN F. C., GIRARDI A. J., GILDEN R. V., KOPROWSKI H. INFECTION OF HUMAN AND SIMIAN TISSUE CULTURES WITH ROUS SARCOMA VIRUS. Proc Natl Acad Sci U S A. 1964 Jul;52:53–59. doi: 10.1073/pnas.52.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., De Torres R. A., Dubbs D. R., Salvi M. L. Induction of cellular deoxyribonuleic acid synthesis by simian virus 40. J Virol. 1967 Aug;1(4):738–746. doi: 10.1128/jvi.1.4.738-746.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourilsky P., Manteuil S., Zamansky M. H., Gros F. DNA-RNA hybridization at low temperature in the presence of urea. Biochem Biophys Res Commun. 1970 Nov 25;41(4):1080–1087. doi: 10.1016/0006-291x(70)90196-8. [DOI] [PubMed] [Google Scholar]
- Krueger R. G., McCarthy B. J. Hybridization studies with nucleic acids from murine plasma cell tumors. Biochem Biophys Res Commun. 1970 Nov 25;41(4):944–951. doi: 10.1016/0006-291x(70)90175-0. [DOI] [PubMed] [Google Scholar]
- May E., May P., Weil R. Analysis of the events leading to SV40-induced chromosome replication and mitosis in primary mouse kidney cell cultures. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1208–1211. doi: 10.1073/pnas.68.6.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pétursson G., Weil R. A study on the mechanism of polyoma-induced activation of the cellular DNA-synthesizing apparatus. Synchronization by FUdR of virus-induced DNA synthesis. Arch Gesamte Virusforsch. 1968;24(1):1–29. doi: 10.1007/BF01242898. [DOI] [PubMed] [Google Scholar]
- RAPP F., BUTEL J. S., FELDMAN L. A., KITAHARA T., MELNICK J. L. DIFFERENTIAL EFFECTS OF INHIBITORS ON THE STEPS LEADING TO THE FORMATION OF SV40 TUMOR AND VIRUS ANTIGENS. J Exp Med. 1965 Jun 1;121:935–944. doi: 10.1084/jem.121.6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swetly P., Brodano G. B., Knowles B., Koprowski H. Response of simian virus 40-transformed cell lines and cell hybrids to superinfection with simian virus 40 and its deoxyribonucleic acid. J Virol. 1969 Oct;4(4):348–355. doi: 10.1128/jvi.4.4.348-355.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIL R., VINOGRAD J. THE CYCLIC HELIX AND CYCLIC COIL FORMS OF POLYOMA VIRAL DNA. Proc Natl Acad Sci U S A. 1963 Oct;50:730–738. doi: 10.1073/pnas.50.4.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINOCOUR E. Purification of polyoma virus. Virology. 1963 Feb;19:158–168. doi: 10.1016/0042-6822(63)90005-9. [DOI] [PubMed] [Google Scholar]
- Weil R., Kára J. Polyoma "tumor antigen": an activator of chromosome replication? Proc Natl Acad Sci U S A. 1970 Oct;67(2):1011–1017. doi: 10.1073/pnas.67.2.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A., Warnaar S. O., Winocour E. Isolation and characterization of simian virus 40 ribonucleic acid. J Virol. 1972 Aug;10(2):193–201. doi: 10.1128/jvi.10.2.193-201.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


