Abstract
Objective:
Telomere length and telomerase activity are important indicators of cellular senescence and replicative ability. Loss of telomerase is associated with ageing and the development of osteoarthritis. Implantation of telomerase-positive cells, chondrocytes, or stem cells expressing a normal chondrocyte phenotype is desired for cartilage repair procedures. The objective of this study was to identify at what age chondrocytes and at what passage bone marrow–derived mesenchymal stem cells (MSCs) become senescent based on telomerase activity. The effect of osteogenic protein–1 (OP-1) or interleukin-1α (IL-1α) treatment on telomerase activity in chondrocytes was also measured to determine the response to anabolic or catabolic stimuli.
Methods:
Articular cartilage was collected from horses (n = 12) aged 1 month to 18 years. Chondrocytes from prepubescent horses (<15 months) were treated with OP-1 or IL-1α. Bone marrow aspirate from adult horses was collected and cultured for up to 10 days to isolate MSCs. Telomerase activity was measured using the TeloTAGGG Telomerase PCR ELISA kit.
Results:
Chondrocytes from prepubescent horses were positive for telomerase activity. Treatment with IL-1α resulted in a decrease in chondrocyte telomerase activity; however, treatment with OP-1 did not change telomerase activity. One MSC culture sample was positive for telomerase activity on day 2; all samples were negative for telomerase activity on day 10.
Conclusions:
These results suggest that chondrocytes from prepubescent donors are potentially more suitable for cartilage repair procedures and that telomerase activity is diminished by anabolic and catabolic cytokine stimulation. If MSCs are utilized in cartilage repair, minimal passaging should be performed prior to implantation.
Keywords: chondrocyte, telomerase, mesenchymal stem cells
Introduction
Increased age is the greatest risk factor for the development of osteoarthritis (OA).1,2 Cellular senescence occurs with age and is typified by decreased telomere length.3,4 Telomeres are nucleic acid repeats (TTAGGG) found on the ends of all chromosomes in eukaryotic cells and are part of the necessary cellular signals needed to maintain genomic stability.5-7 Without telomeric caps on every chromosome, end-to-end fusion can occur, leading to chromosomal instability.8,9 To avoid chromosomal instability, cells become senescent and cellular division arrests when telomeres become critically shortened.10,11 In chondrocytes, telomere length shortens about 40 base pairs per year.3
To maintain genetic stability, telomeres must be synthesized by the enzyme telomerase. Telomerase is composed of 2 subunits: an RNA template and a telomerase reverse transcriptase (TERT). The RNA template is “read” by TERT, which then extends the telomere sequence on the end of chromosomes, allowing for increased cell replication without degradation of DNA.8,12,13 Enzymatic activity decreases in cells with time, and the lack of telomerase activity signals a reduction in cell proliferation and life span, eventually leading to replicative senescence.8,14 Some cells such as fetal cells or embryonic stem cells have high levels of telomerase.15 Although stem cells collected from peripheral blood11 or bone marrow16 can express the telomerase enzyme, multiple studies have shown that culture-expanded bone marrow–derived mesenchymal stem cells (MSCs) and adipose-derived stem cells have low or no telomerase expression.15,17-20 Telomerase activity has not been found in adult articular cartilage samples, suggesting that adult chondrocytes are senescent,3,17 which is reflected in a 10-fold loss of mitotic activity of chondrocytes over 80 years.3
Cellular senescence is commonly viewed as a challenge to overcome during tissue-engineering approaches in articular cartilage repair. The associations between senescence, loss of the phenotype, ageing, and arthritis may prevent large-scale expansion of cells being used for therapeutic applications.21 The cellular environment can also alter telomerase activity. For example, oxidative stress and physiological stress can decrease telomerase activity,22,23 and in chondrocytes, oxidative stress causes premature chondrocyte senescence.24 The secretory phenotype of senescent chondrocytes includes catabolic cytokines such as interleukin-1α (IL-1α), IL-6, and matrix metalloproteinases.2 This suggests that senescence may contribute to the degeneration of articular cartilage with age,3 resulting in the development and progression of OA.2 In addition, anabolic mediators such as insulin-like growth factor–1 (IGF-1) are synthesized in lower concentrations by aged chondrocytes, and aged chondrocytes become hyporesponsive to the signaling factors present in their surrounding environment, resulting in a further loss of matrix homeostasis.3,25-27 Osteogenic protein–1 (OP-1) is an anabolic protein known to support cartilage health through stimulation of extracellular matrix production and multiple anticatabolic effects.27-29 The ability of these anabolic or catabolic proteins to modulate telomerase activity in chondrocytes is unknown.
Telomerase activity is increased in some cells during injury, indicating a role for up-regulation of telomerase in tissue repair and remodeling. Clonal chondroprogenitor cells maintain telomerase activity during culture expansion, suggesting that they might facilitate the meager response of cartilage to injury.30 While telomerase activity might seem like a desirable trait for chondrocytes or stem cells in tissue regeneration, the ability to control telomerase expression to avoid oncogenic transformation is important. Telomerase activity can be gained through increased telomerase enzyme processivity, leading to the increased life span of many types of cancer cells.31 Therefore, finding a method to provide a limited or short-term increase of telomerase activity may be desirable for cartilage repair, such as implantation or cytokine stimulation of chondrocytes or MSCs. The purpose of this study was to determine how age and anabolic (OP-1) or catabolic (IL-1α) stimulation affects telomerase activity in bone marrow–derived MSCs and articular chondrocytes.
Materials and Methods
Chondrocyte Isolation and Treatment
To determine the effect of age on telomerase activity, chondrocytes were isolated32 from horses (n = 12), aged 1 month to 18 years, and divided into prepubescent (0-15 months; n = 6) and postpubescent (≥16 months; n = 6) groups based on serum IGF-1 and IGF-1 binding protein–3 concentrations.33 Chondrocytes were seeded at 5 × 104 cells/cm2 with F-12 media containing 25 mM HEPES, 2 mM L-glutamine, 50 µg/mL ascorbic acid, 30 µg/mL α-ketoglutaric acid, and 10% FBS. Either OP-1 (100 ng/mL) or IL-1α (10 ng/mL) was added for 72 hours, with media being exchanged at 24 hours. The concentration of OP-1 used for the treatment was based on previous published studies in which the effects of OP-1 on cultured chondrocytes were measured.26,34,35 Chondrocytes were removed from the cell culture plate using trypsin and subsequently rinsed, counted, and pelleted. Chondrocyte pellets were frozen per the manufacturer’s protocol (TeloTAGGG Telomerase PCR ELISA kit, Roche, Penzberg, Germany) to determine the presence of telomerase activity.
Fetal Fibroblast Isolation
Cells for positive controls in the telomerase assay were obtained from a 34-day equine conceptus that was collected by uterine lavage. A portion of the body wall was gently homogenized in high-glucose DMEM containing 10% FBS, 100 IU/mL penicillin, and 100 µg/mL streptomycin. The resultant mixture was transferred to a 100-mm tissue culture plate and incubated at 5% CO2, 90% humidity, and 37 °C for 2 days. Media were exchanged after 24 hours. Cells were removed from the cell culture plate using trypsin and subsequently rinsed, counted, and pelleted. Cell pellets were frozen for subsequent telomerase activity analysis.
Bone Marrow Aspirate
Bone marrow–derived MSCs were isolated from bone marrow aspirate obtained from the sternum of 4 mature horses aged 3 to 5 years. Aspirates were diluted 2:1 with phosphate buffered saline and layered on Ficoll-Paque PREMIUM (GE, Uppsala, Sweden) at a 1:2 ratio. Samples were centrifuged for 20 minutes at 500g with no brake during deceleration. The cellular layer was carefully removed and further centrifuged at 500g for 5 minutes. Cell pellets were resuspended in low-glucose DMEM supplemented with 200 mM L-glutamine, 0.0125 µg/mL basic fibroblast growth factor, 10% FBS, 100 IU/mL penicillin, and 100 µg/mL streptomycin. Cells were plated at 200,000 cells/cm2 and incubated at 37 °C with media exchanged on days 1, 3, and 5. Cells were collected and frozen as described for chondrocytes on days 2 and 10.
Telomerase Activity
Cells were processed according to the manufacturer’s protocol for the TeloTAGGG Telomerase PCR ELISA kit. Briefly, cell pellets were thawed in lysis reagent, incubated on ice for 30 minutes, and centrifuged at 16,000g for 20 minutes at 4 °C. Telomerase activity was immediately measured in the resultant supernatant using the telomeric repeat amplification protocol in which telomerase, if present in the cell lysate, adds telomeric repeats to the 3′ end of a biotin-labeled synthetic P1-TS primer. Samples were amplified by polymerase chain reaction (PCR), with P1-TS and P2 primers creating an elongated telomere. The PCR product was denatured and hybridized to a digoxigenin-labeled probe that detects telomeric repeats in a subsequent enzyme-linked immunosorbent assay (ELISA). Samples were considered positive for telomerase activity if the ELISA resulted in a background-corrected absorbance of ≥0.2 units, resulting in binary positive/negative data. Telomerase assays were performed in duplicate, and the duplicate mean was used for statistical analysis.
Statistical Analysis
A χ2 test was used to compare categorical responses (telomerase activity as positive/negative) between the 2 independent chondrocyte age groups (prepubescent or postpubescent). The response of telomerase activity in prepubescent chondrocytes to cytokine treatment (OP-1 or IL-1α) was also analyzed using a χ2 test. A paired t test was used to compare the positive/negative presence of telomerase activity in day-2 and day-10 MSC cultures because the MSCs were from the same original population measured along a time continuum. A P value of <0.05 was considered statistically significant. Both statistical tests were chosen for their ideal use with low sample sizes.
Results
Fetal fibroblasts were strongly positive for telomerase activity with a background-corrected absorbance of 3.47, indicating that the TeloTAGGG Telomerase PCR ELISA kit cross-reacted with equine telomerase.
Telomerase Activity in Chondrocytes during Ageing
Telomerase activity was significantly greater in chondrocytes from prepubescent horses (0-15 months of age), with 5 of 6 samples being positive for telomerase activity, than in samples from postpubescent horses (≥16 months of age), of which none of the 6 samples was positive for telomerase activity (P = 0.003) (Fig. 1). Although not tested for in the statistical model, it was observed that telomerase activity declined in horses after 10 months of age (Fig. 2).
Figure 1.
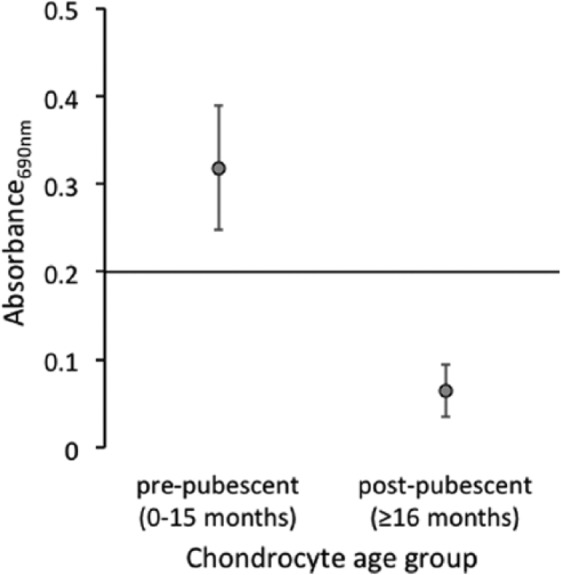
Telomerase activity in prepubescent and postpubescent chondrocytes. Telomerase activity was significantly greater in chondrocytes isolated from prepubescent horses compared to postpubescent chondrocytes (P = 0.003). Data points each represent median telomerase activity (n = 6), and standard error bars are provided. Any point above the x-axis, with an absorbance value greater than 0.2, indicates positive telomerase activity.
Figure 2.
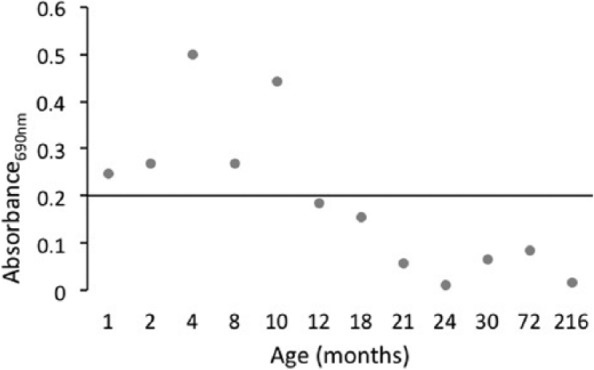
Mean telomerase activity of chondrocytes from individual horses (n = 1) in all age groups, measured in duplicate. Telomerase activity in chondrocytes is no longer positive in horses greater than 10 months of age.
Telomerase Activity in Cytokine-Treated Chondrocytes
Telomerase-positive chondrocytes from prepubescent horses were used for cytokine stimulation experiments so that either an increase or decrease in activity could be detected. Treatment with IL-1α resulted in significantly decreased telomerase activity (P = 0.014) (Fig. 3). Initially, 3 of 3 samples tested positive for telomerase activity; after treatment, none of the samples were positive. Again, 3 of 3 control chondrocyte samples were positive for telomerase activity, but after treatment with OP-1, only 1 sample remained positive. However, the differences with OP-1 treatment were not significant (P = 0.083).
Figure 3.
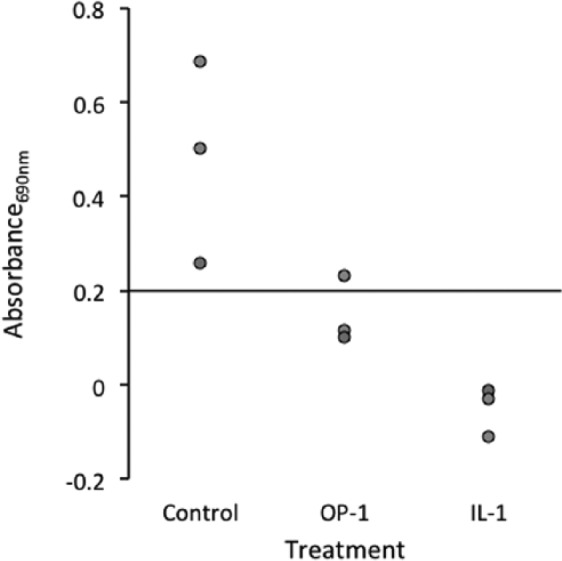
Decreased telomerase activity in cytokine-treated prepubescent chondrocytes (n = 3). There was a decrease in telomerase activity after osteogenic protein–1 (OP-1) treatment, with 2 of the 3 samples becoming negative for telomerase activity; however, the results were not significant (P = 0.083). Interleukin-1α (IL-1α) resulted in a significant decrease in telomerase activity (P = 0.014), with all samples becoming negative for telomerase activity. Any point above the x-axis, with an absorbance value greater than 0.2, indicates that a sample is positive for telomerase activity.
Telomerase Activity in MSCs
Telomerase activity in MSCs was heterogeneous. Only 1 sample from day 2 in culture was positive for telomerase activity, and activity was absent in all 3 samples by day 10 (P = 0.84) (Fig. 4).
Figure 4.
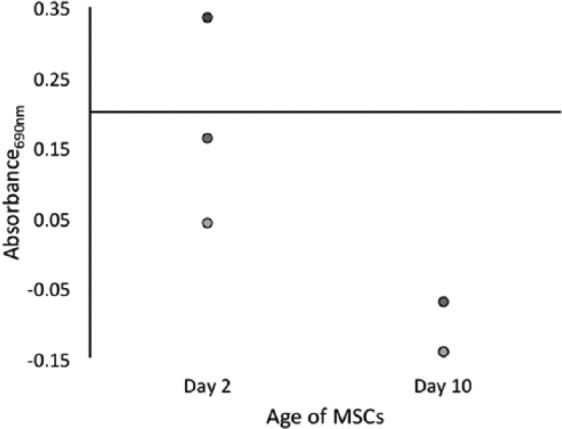
Telomerase activity in bone marrow–derived mesenchymal stem cells (MSCs). Only 1 sample was positive for telomerase at day 2; no samples had telomerase activity at day 10 (n = 3), with 2 samples overlapping due to having the same value of 0.14. There was no statistical difference between day 2 and day 10 (P = 0.82).
Discussion
Telomerase activity was identified for the first time in post-neonatal articular chondrocytes. It was present in chondrocytes from prepubescent but not postpubescent horses. The age at which telomerase activity decreases corresponds with decreasing serum IGF-1 concentrations in horses.33 Time of the peak serum concentration of IGF-1 can be used as a surrogate measure of peak growth hormone concentration and therefore onset of puberty. Serum IGF-1 decreases and remains low after 18 months of age and signifies postpubescence and the beginning of ageing. It is therefore consistent that telomerase activity would be present in chondrocytes from prepubescent, but not postpubescent, ageing horses. Previous studies have shown that telomere length in chondrocytes from adult human articular cartilage decreases with age,25 and no telomerase activity has been found in adult-derived chondrocytes.3,17,36 Combined, these findings suggest that telomere erosion in chondrocytes, senescence, ageing, and increased susceptibility to OA begin as puberty ends. This would imply that chondrocytes and the surrounding cartilage from an early time point, about 11 years in human beings37 and 18 months in horses,33 begin exhibiting age-related changes with a gradual loss of function.25
Bone marrow–derived MSCs from adult horses were essentially devoid of telomerase activity. Only 1 sample in early culture (day 2) was positive for enzyme activity, and no samples were positive by day 10. Telomerase activity is present in hematopoietic stem cells collected from peripheral blood11 and bone marrow.16 This suggests that bone marrow–derived MSCs have less replicative capacity than hematopoietic stem cells, and culture expansion results in a loss of telomerase activity in MSCs, even if it was present in early culture. Additionally, telomeres in adult human MSCs are shorter than in chondrocytes from age-matched donors,17 indicating that MSCs may have less replicative capacity than chondrocytes from mature joints.
In the context of cartilage repair procedures, the findings of this study suggest that chondrocytes from young donors would be superior to MSCs or adult chondrocytes for cartilage repair procedures because of their capacity to replicate and assist in the repair of a cartilage defect. Multiple studies have documented that ectopic expression of telomerase extends a cell’s proliferative life span and function.38,39 However, telomerase gene transfection has limitations and inherent risks to clinical implications.38,40 Use of chondrocytes from prepubescent donors would provide telomerase-positive chondrocytes in which telomerase activity would naturally diminish, thereby avoiding the concern for an oncogenesis-associated sustained or increased cellular replicative life span.
Both OP-1 and IL-1α decreased telomerase activity, but the effect was only significant after IL-1α treatment. OP-1 is a member of the transforming growth factor–β (TGF-β) superfamily, and TGF-β1 has been shown to down-regulate telomerase in injured lungs through the inhibition of rat TERT gene expression.41 The decreased telomerase activity found in articular chondrocytes may be a dose-dependent response towards OP-1. Further studies examining serially diluted OP-1 treatment on articular chondrocytes may yield additional information about the effects on telomerase activity. The association of decreased telomerase with IL-1α treatment supports the concept that cartilage injury, which can be found with increased IL-1α, corresponds with accelerated telomere shortening and contributes to age-related changes in articular cartilage and OA.25 Loss of telomerase activity also implies that methods to decrease IL-1α–associated inflammation should be aggressively pursued, even in young patients, to preserve telomerase activity and chondrocyte function.
Footnotes
Acknowledgments and Funding: The research was funded by the Harry M. Zweig Memorial Fund for Equine Research.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1. Antonelli MC, Starz TW. Assessing for risk and progression of osteoarthritis: the nurse’s role. Am J Nurs. 2012;112(3 Suppl 1):S26-31. [DOI] [PubMed] [Google Scholar]
- 2. Shane Anderson A, Loeser RF. Why is osteoarthritis an age-related disease? Best Pract Res Clin Rheumatol. 2010;24(1):15-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martin JA, Buckwalter JA. Telomere erosion and senescence in human articular cartilage chondrocytes. J Gerontol A Biol Sci Med Sci. 2001;56(4):B172-9. [DOI] [PubMed] [Google Scholar]
- 4. Zhao Z, Pan X, Liu L, Liu N. Telomere length maintenance, shortening, and lengthening. J Cell Physiol. Epub 2013. Dec 20. [DOI] [PubMed] [Google Scholar]
- 5. Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345(6274):458-60. [DOI] [PubMed] [Google Scholar]
- 6. Allsopp RC, Chang E, Kashefi-Aazam M, Rogaev EI, Piatyszek MA, Shay JW, Harley CB. Telomere shortening is associated with cell division in vitro and in vivo. Exp Cell Res. 1995;220(1):194-200. [DOI] [PubMed] [Google Scholar]
- 7. Moyzis RK, Buckingham JM, Cram LS, Dani M, Deaven LL, Jones MD, et al. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A. 1988;85(18):6622-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blackburn EH. Structure and function of telomeres. Nature. 1991;350(6319):569-73. [DOI] [PubMed] [Google Scholar]
- 9. Bailey SM, Goodwin EH. DNA and telomeres: beginnings and endings. Cytogenet Genome Res. 2004;104(1-4):109-15. [DOI] [PubMed] [Google Scholar]
- 10. Yu GL, Bradley JD, Attardi LD, Blackburn EH. In vivo alteration of telomere sequences and senescence caused by mutated tetrahymena telomerase RNAs. Nature. 1990;344(6262):126-32. [DOI] [PubMed] [Google Scholar]
- 11. Notaro R, Cimmino A, Tabarini D, Rotoli B, Luzzatto L. In vivo telomere dynamics of human hematopoietic stem cells. Proc Natl Acad Sci U S A. 1997;94(25):13782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Counter CM, Avilion AA, LeFeuvre CE, Stewart NG, Greider CW, Harley CB, Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992;11(5):1921-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, et al. The RNA component of human telomerase. Science. 1995;269(5228):1236-41. [DOI] [PubMed] [Google Scholar]
- 14. Masutomi K, Yu EY, Khurts S, Ben-Porath I, Currier JL, Metz GB, et al. Telomerase maintains telomere structure in normal human cells. Cell. 2003;114(2):241-53. [DOI] [PubMed] [Google Scholar]
- 15. Hiyama E, Hiyama K. Telomere and telomerase in stem cells. Br J Cancer. 2007;96(7):1020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Broccoli D, Young JW, de Lange T. Telomerase activity in normal and malignant hematopoietic cells. Proc Natl Acad Sci U S A. 1995;92(20):9082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Parsch D, Fellenberg J, Brummendorf TH, Eschlbeck AM, Richter W. Telomere length and telomerase activity during expansion and differentiation of human mesenchymal stem cells and chondrocytes. J Mol Med (Berl). 2004;82(1):49-55. [DOI] [PubMed] [Google Scholar]
- 18. Greenwood MJ, Lansdorp PM. Telomeres, telomerase, and hematopoietic stem cell biology. Arch Med Res. 2003;34(6):489-95. [DOI] [PubMed] [Google Scholar]
- 19. Neri S, Bourin P, Peyrafitte JA, Cattini L, Facchini A, Mariani E. Human adipose stromal cells (ASC) for the regeneration of injured cartilage display genetic stability after in vitro culture expansion. PLoS One. 2013;8(10):e77895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ryu E, Hong S, Kang J, Woo J, Park J, Lee J, Seo JS. Identification of senescence-associated genes in human bone marrow mesenchymal stem cells. Biochem Biophys Res Commun. 2008;371(3):431-6. [DOI] [PubMed] [Google Scholar]
- 21. Li J, Pei M. Cell senescence: a challenge in cartilage engineering and regeneration. Tissue Eng Part B Rev. 2012;18(4):270-87. [DOI] [PubMed] [Google Scholar]
- 22. von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27(7):339-44. [DOI] [PubMed] [Google Scholar]
- 23. Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101(49):17312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martin JA, Klingelhutz AJ, Moussavi-Harami F, Buckwalter JA. Effects of oxidative damage and telomerase activity on human articular cartilage chondrocyte senescence. J Gerontol A Biol Sci Med Sci. 2004;59(4):324-37. [DOI] [PubMed] [Google Scholar]
- 25. Martin JA, Buckwalter JA. The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair. J Bone Joint Surg Am. 2003;85-A Suppl 2:106-10. [DOI] [PubMed] [Google Scholar]
- 26. Chubinskaya S, Kumar B, Merrihew C, Heretis K, Rueger DC, Kuettner KE. Age-related changes in cartilage endogenous osteogenic protein-1 (OP-1). Biochim Biophys Acta. 2002;1588(2):126-34. [DOI] [PubMed] [Google Scholar]
- 27. Chubinskaya S, Otten L, Soeder S, Borgia JA, Aigner T, Rueger DC, Loeser RF. Regulation of chondrocyte gene expression by osteogenic protein-1. Arthritis Res Ther. 2011;13(2):R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chubinskaya S, Kuettner KE. Regulation of osteogenic proteins by chondrocytes. Int J Biochem Cell Biol. 2003;35(9):1323-40. [DOI] [PubMed] [Google Scholar]
- 29. Flechtenmacher J, Huch K, Thonar EJ, Mollenhauer JA, Davies SR, Schmid TM, et al. Recombinant human osteogenic protein 1 is a potent stimulator of the synthesis of cartilage proteoglycans and collagens by human articular chondrocytes. Arthritis Rheum. 1996;39:1896-904. [DOI] [PubMed] [Google Scholar]
- 30. Khan IM, Bishop JC, Gilbert S, Archer CW. Clonal chondroprogenitors maintain telomerase activity and Sox9 expression during extended monolayer culture and retain chondrogenic potential. Osteoarthritis Cartilage. 2009;17(4):518-28. [DOI] [PubMed] [Google Scholar]
- 31. D’Souza Y, Lauzon C, Chu TW, Autexier C. Regulation of telomere length and homeostasis by telomerase enzyme processivity. J Cell Sci. 2013;126(Pt 2):676-87. [DOI] [PubMed] [Google Scholar]
- 32. Nixon AJ, Lust G, Vernier-Singer M. Isolation, propagation and cryopreservation of equine articular chondrocytes. Am J Vet Res. 1992;53:2364-70. [PubMed] [Google Scholar]
- 33. Fortier LA, Kornatowski MA, Mohammed HO, Jordan MT, O’Cain LC, Stevens WB. Age-related changes in serum insulin-like growth factor-I, insulin-like growth factor-I binding protein-3 and articular cartilage structure in thoroughbred horses. Equine Vet J. 2010;37(1):37-42. [DOI] [PubMed] [Google Scholar]
- 34. Loeser RF, Pacione CA, Chubinskaya S. The combination of insulin-like growth factor 1 and osteogenic protein 1 promotes increased survival of and matrix synthesis by normal and osteoarthritic human articular chondrocytes. Arthritis Rheum. 2003;48(8):2188-96. [DOI] [PubMed] [Google Scholar]
- 35. Chubinskaya S, Segalite D, Pikovsky D, Hakimiyan AA, Rueger DC. Effects induced by BMPS in cultures of human articular chondrocytes: comparative studies. Growth Factors. 2008;26(5):275-83. [DOI] [PubMed] [Google Scholar]
- 36. Brandl A, Angele P, Roll C, Prantl L, Kujat R, Kinner B. Influence of the growth factors PDGF-BB, TGF-beta1 and bFGF on the replicative aging of human articular chondrocytes during in vitro expansion. J Orthop Res. 2010;28(3):354-60. [DOI] [PubMed] [Google Scholar]
- 37. Patton GC, Viner R. Pubertal transitions in health. Lancet. 2007;369(9567):1130-9. [DOI] [PubMed] [Google Scholar]
- 38. Rufer N, Migliaccio M, Antonchuk J, Humphries RK, Roosnek E, Lansdorp PM. Transfer of the human telomerase reverse transcriptase (TERT) gene into T lymphocytes results in extension of replicative potential. Blood. 2001;98(3):597-603. [DOI] [PubMed] [Google Scholar]
- 39. Simonsen JL, Rosada C, Serakinci N, Justesen J, Stenderup K, Rattan SI, et al. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat Biotechnol. 2002;20(6):592-6. [DOI] [PubMed] [Google Scholar]
- 40. Robbins PD, Ghivizzani SC. Viral vectors for gene therapy. Pharmacol Ther. 1998;80(1):35-47. [PubMed] [Google Scholar]
- 41. Hu B, Tack DC, Liu T, Wu Z, Ullenbruch MR, Phan SH. Role of Smad3 in the regulation of rat telomerase reverse transcriptase by TGFbeta. Oncogene. 2006;25(7):1030-41. [DOI] [PubMed] [Google Scholar]


