Abstract
Objective:
To determine whether falls can be prevented with minimally supervised exercise targeting potentially remediable fall risk factors, i.e., poor balance, reduced leg muscle strength, and freezing of gait, in people with Parkinson disease.
Methods:
Two hundred thirty-one people with Parkinson disease were randomized into exercise or usual-care control groups. Exercises were practiced for 40 to 60 minutes, 3 times weekly for 6 months. Primary outcomes were fall rates and proportion of fallers during the intervention period. Secondary outcomes were physical (balance, mobility, freezing of gait, habitual physical activity), psychological (fear of falling, affect), and quality-of-life measures.
Results:
There was no significant difference between groups in the rate of falls (incidence rate ratio [IRR] = 0.73, 95% confidence interval [CI] 0.45–1.17, p = 0.18) or proportion of fallers (p = 0.45). Preplanned subgroup analysis revealed a significant interaction for disease severity (p < 0.001). In the lower disease severity subgroup, there were fewer falls in the exercise group compared with controls (IRR = 0.31, 95% CI 0.15–0.62, p < 0.001), while in the higher disease severity subgroup, there was a trend toward more falls in the exercise group (IRR = 1.61, 95% CI 0.86–3.03, p = 0.13). Postintervention, the exercise group scored significantly (p < 0.05) better than controls on the Short Physical Performance Battery, sit-to-stand, fear of falling, affect, and quality of life, after adjusting for baseline performance.
Conclusions:
An exercise program targeting balance, leg strength, and freezing of gait did not reduce falls but improved physical and psychological health. Falls were reduced in people with milder disease but not in those with more severe Parkinson disease.
Classification of evidence:
This study provides Class III evidence that for patients with Parkinson disease, a minimally supervised exercise program does not reduce fall risk. This study lacked the precision to exclude a moderate reduction or modest increase in fall risk from exercise. Trial registration: Australian New Zealand Clinical Trials Registry (ACTRN12608000303347).
People with Parkinson disease (PD) fall frequently, with 60% falling annually and two-thirds of these falling recurrently.1–3 These fall rates are double those in the general older population,3 and the resulting injuries,4 pain,5 activity limitations,3 and fear of falling3 compromise health and well-being.
While evidence from systematic reviews shows that exercise programs are effective in preventing falls in the general older population,6,7 only 5 randomized controlled trials have evaluated exercise programs designed to reduce falls in people with PD. Three underpowered trials8–10 reported no effect on falls, while 2 trials11,12 reported significant reductions in falls when exercises that challenged balance were compared with exercises that did not challenge balance. These positive trials did not, however, target 2 additional independent physical risk factors for falls, i.e., reduced leg strength and freezing of gait,1 which are also potentially remediable.13–16
The outcomes in the 2 positive fall prevention trials in people with PD were achieved using fully supervised exercise. A fully supervised mode of exercise delivery is difficult to sustain in clinical practice, while less-supervised models of exercise delivery may offer a more clinically relevant and sustainable intervention strategy. This approach has been found to reduce falls and fall-related injuries by 35% in the general older population.17 Therefore, we aimed to determine whether a pragmatic, minimally supervised exercise program targeting balance, leg muscle strength, and freezing of gait could reduce falls in community-dwelling people with PD.
METHODS
Design.
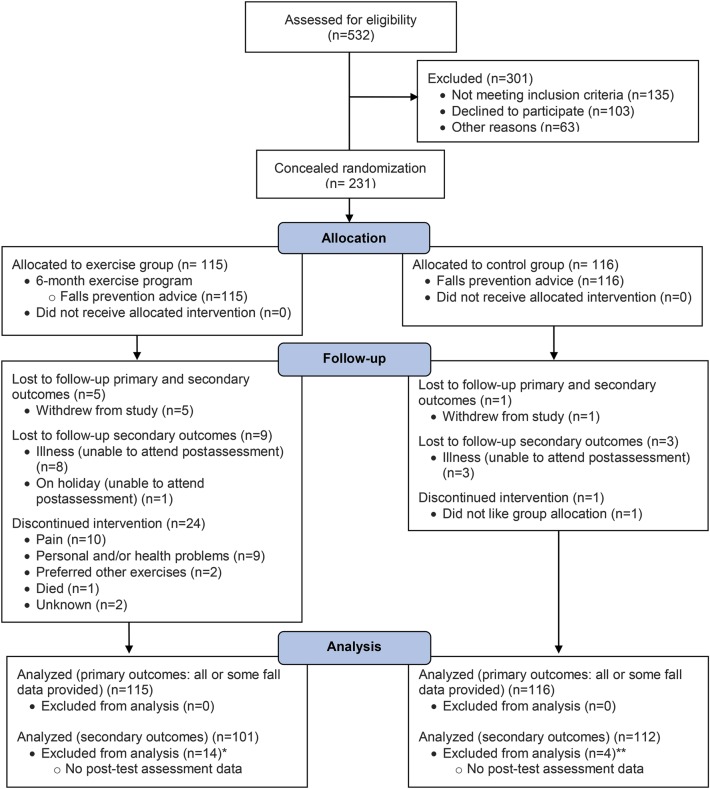
A prospective, assessor-blinded, randomized controlled trial was undertaken from 2008 to 2012. The methods have been described in detail elsewhere.18 We randomly allocated 231 community-dwelling participants with PD to an exercise group (intervention) or usual-care group (control) (figure 1).
Figure 1. Flow of participants through the trial.
*The 24 exercise participants who discontinued intervention include 4 of the participants lost to both primary and secondary outcomes and 7 of the participants lost to secondary outcomes; **3 exercise and 3 control participants provided posttest questionnaire data but no posttest physical assessment data.
The primary research question was: does a minimally supervised exercise program targeting balance, leg muscle strength, and freezing of gait reduce falls in community-dwelling people with PD as compared with usual care? The secondary research question was: does the exercise program improve fall risk factors, fear of falling, affect, and quality of life?
This study provides Class III evidence that for patients with PD, a minimally supervised exercise program does not reduce falls risk.
Standard protocol approvals, registrations, and patient consents.
The study protocol was approved by The University of Sydney Human Research Ethics Committee, and written informed consent was obtained from all participants. The trial was registered prospectively with the Australian New Zealand Clinical Trials Registry (ACTRN12608000303347).
Participants.
Participants were recruited from metropolitan Sydney and regional and rural New South Wales (NSW), Australia, via Parkinson's NSW consumer support groups, newspaper advertisements, and referrals from neurologists and physical therapists. Eligibility criteria included a diagnosis of idiopathic PD (confirmed by a medical practitioner), age 40 years or older, ability to walk independently with or without a walking aid, stable antiparkinsonian medication for at least 2 weeks, and one or more falls in the past year or at risk of falls based on physical assessment. Participants were deemed to be at risk of falling if they scored 25 cm or less on the Functional Reach Test19 or if they failed to reach criterion on one of the balance tests in the QuickScreen Clinical Falls Risk Assessments,20 i.e., unable to perform near tandem stand with eyes closed for 10 seconds, unable to complete 8 steps in the alternate step test (18-cm step) in less than 10 seconds, or unable to perform 5 repetitions of sit-to-stand in less than 12 seconds.
Participants were excluded if they had a Mini-Mental State Examination score of <24, unstable cardiovascular disease, or other uncontrolled chronic conditions that would interfere with the safety and conduct of the training and testing protocol. All eligible volunteers received clearance from their medical practitioner.
Randomization and masking.
Participants were randomized to intervention or control groups after baseline assessment. Randomization was stratified by fall history (0–9/≥10 falls in the previous 12 months) using a computer-generated random-number schedule with variable block sizes of 2 and 4. Randomization was performed centrally by an investigator not involved in recruitment or assessments (C.S.). Outcome assessors were masked to group allocation.
Intervention group.
The intervention group undertook the PD-WEBB program (www.webb.org.au). This program18 included 40 to 60 minutes of progressive balance and lower limb strengthening exercises 3 times a week for 6 months, and cueing strategies to reduce freezing of gait15 for participants reporting freezing. Balance exercises included standing with a decreased base of support, graded reaching activities, and forward/sideways/backward stepping. Strengthening exercises included sit-to-stand, forward and lateral step-ups, semisquats, and heel raises, with load progressively increased via weighted vests.21 Participants attended a monthly exercise class led by a physical therapist and performed the remaining exercise sessions at home. The exercises were prescribed and progressed in the class, and 2 to 4 home visits were conducted by the physical therapist over the 6 months. When group sessions were not feasible, participants performed all exercise sessions at home. Eight to 10 of these home sessions were supervised by a physical therapist. For participants with freezing of gait, cueing strategy training was undertaken during an additional 1 or 2 home visits. The intervention group also received a booklet containing standardized fall prevention advice.22
Control group.
The control group received usual care from their medical practitioner and community services. A booklet containing standardized fall prevention advice was provided.22
Outcome measures.
The primary outcome measures were the number of falls and the proportion of fallers recorded during the 6-month intervention period. Falls (defined as unintentionally coming to rest on the ground or other lower surface without overwhelming external force or a major internal event)23 were recorded by the use of a “falls diary.” Participants received calendars on entry to the study, with instructions to record the following: falls; nursing, medical, and allied health appointments; and hospitalizations. Participants returned completed calendars monthly in prepaid envelopes. Participants were also telephoned monthly to record any changes in medications, use of health resources, and to verify fall details.
Secondary outcomes included the following: the PD Fall Risk score1; mean knee extensor muscle strength of both legs24; coordinated stability test of balance25; the Short Physical Performance Battery, which includes walking, standing balance, and sit-to-stand tests26; fast walking velocity over 4 m; the 5-repetition sit-to-stand test; the Freezing of Gait Questionnaire27; the Falls Efficacy Scale–International28; a habitual physical activity questionnaire recording the amount of regular exercise and activities of daily living; quality of life using the mental and physical subscores of the SF-12v2 (Short Form 12 version 2), the SF-6D (Short Form 6 dimensions) utility score, a PD-specific quality-of-life questionnaire (PDQ-39)29; and the positive affect subscale of the Positive and Negative Affect Schedule.30
Home exercise logs were kept by participants, and class records were kept by the physical therapists delivering the intervention. Adverse events (defined as a significant injury or medical event causing the participant to seek attention from a health professional or limit their activities for ≥2 days) occurring during exercise were monitored and recorded throughout the study.
Measurements and procedures.
Secondary outcomes were collected on entry to the study (baseline) and after the 6-month intervention period (posttest) by 1 of 7 trained assessors in participants' homes. The order of outcome measurements was standardized and conducted when participants were optimally medicated, usually 1 hour after ingestion of PD medications.
Statistical analysis.
A statistical analysis plan was developed and certified before unblinding and analysis. An intention-to-treat approach was used for all analyses. A blind review of the falls data revealed that the negative binomial model was not flexible enough to capture both the nonfallers and the large number of multiple fallers. In contrast, the Poisson inverse gaussian (PIG) distribution gave a good fit. Therefore, the intervention effect on the number of falls was assessed using PIG regression, with the logarithm of the days of follow-up included as an exposure term in the model. An analysis adjusted for previous multiple faller status was also performed. The proportion of fallers was compared between groups using a χ2 test.
Prespecified subgroup analyses for the primary outcomes were undertaken to identify any differential impact of the exercise intervention according to fall history (0–9 vs ≥10 falls in the previous year), physical function (based on 4-m comfortable walk speed at baseline, dichotomized at the median), disease severity (based on Unified Parkinson's Disease Rating Scale [UPDRS] motor score at baseline, dichotomized at the median), and cognition (based on Frontal Assessment Battery score at baseline, dichotomized at the median). The main analysis for each subgroup was based on interaction tests in PIG models using continuous interaction terms where possible, and dichotomous interaction terms using prespecified cutoffs were used to assist in the interpretation of subgroup analyses.
Between-group comparisons of final test performance for the continuously scored secondary outcome measures were made using general linear models (analysis of covariance) controlling for baseline performance. The R version 2.15.2 package “gamlss”31 was used to fit the PIG model to the number of falls; SPSS version 20 statistical software (IBM Corp., Armonk, NY) was used for all other analyses.
Sample size.
Because it was our intention to compare fall rates between groups using incidence rate ratios (IRRs) from negative binomial regression models,32 we conducted a sample-size calculation using the nbpower command in the Stata software package.18 Assuming a control group rate of falls of 1 fall/person month1 over 5 months of follow-up, 115 participants per group were required to provide 80% power to detect as significant, at the 5% level, a 30% lower rate of falls for exercise participants than control participants (i.e., IRR = 0.70). Five months was used to account for loss to follow-up.
RESULTS
Flow of participants through the trial.
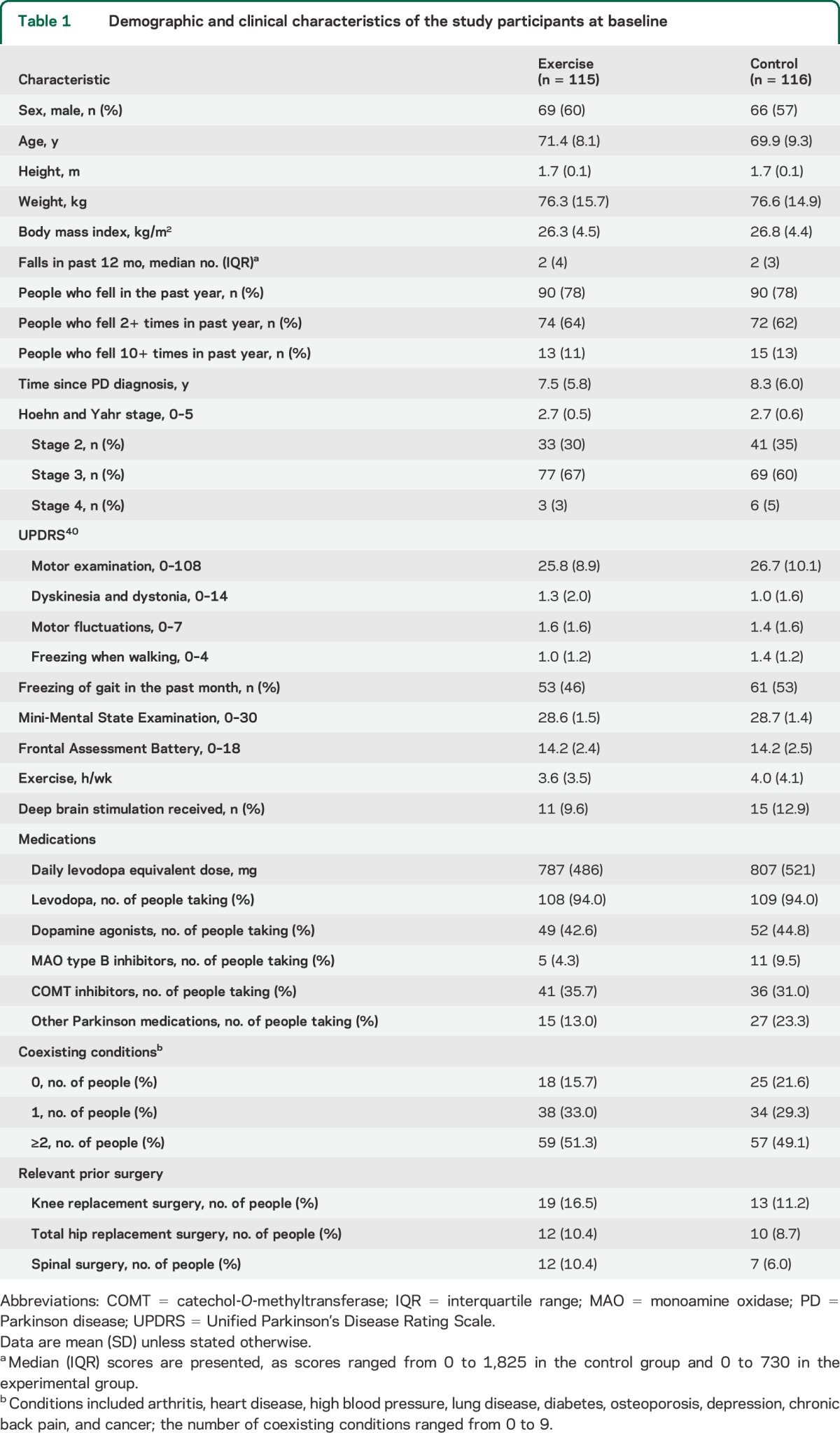
The trial flow of participants is summarized in figure 1. A total of 231 participants (135 male) with an average age of 71 (SD 9) years were recruited. The 115 exercise participants and 116 control participants were similar in demographic characteristics, levodopa equivalent dosages, and comorbidities at baseline (table 1).
Table 1.
Demographic and clinical characteristics of the study participants at baseline
Intervention.
Group exercise was offered at 22 locations. Seventy-eight participants attended an exercise class once per month, with an average of 3.5 participants per group (range 2–6). The remaining 37 exercise participants performed all exercise sessions at home. On average, 13% of the exercise sessions were supervised by a physical therapist.
Adherence to exercise protocol.
Exercise adherence records were available for 108 (94%) of the 115 exercise participants. The exercise group completed a mean of 72% (SD 38%) of prescribed exercise sessions. Twenty-five participants (22%) performed a modified program to account for pain and coexisting conditions, while 24 participants (21%) discontinued the exercise program.
Adverse events.
Two participants fell while exercising at home. One fell while putting on the weighted vest and one fell while turning on completion of a stepping exercise. Neither fall resulted in injury requiring medical attention or restriction of activities.
Primary outcomes.
Six months of falls data were available for 225 participants (97%) and one or more months of falls data were available for the remaining 6 participants. During the intervention period, 467 falls (4.1 falls/person) were reported in the exercise group and 810 (7.0 falls/person) in the control group (figure 2, table 2). This 27% difference in fall rate in the exercise group compared with the control group was not statistically significant (IRR = 0.73, 95% confidence interval [CI] 0.45–1.17, p = 0.18). There was no significant difference in the proportion of fallers with 75 (65%) of the exercise participants and 81 (70%) of the control participants reporting at least one fall (relative risk [RR] = 0.93, 95% CI 0.78–1.12, p = 0.45). The results of the primary analyses were essentially unchanged after adjustment for baseline faller status. Medical attention was sought for 21 falls in the exercise group and 19 falls in the control group (table e-1 on the Neurology® Web site at Neurology.org).
Figure 2. Falls reported by all participants, participants with lower disease severity, and participants with higher disease severity.
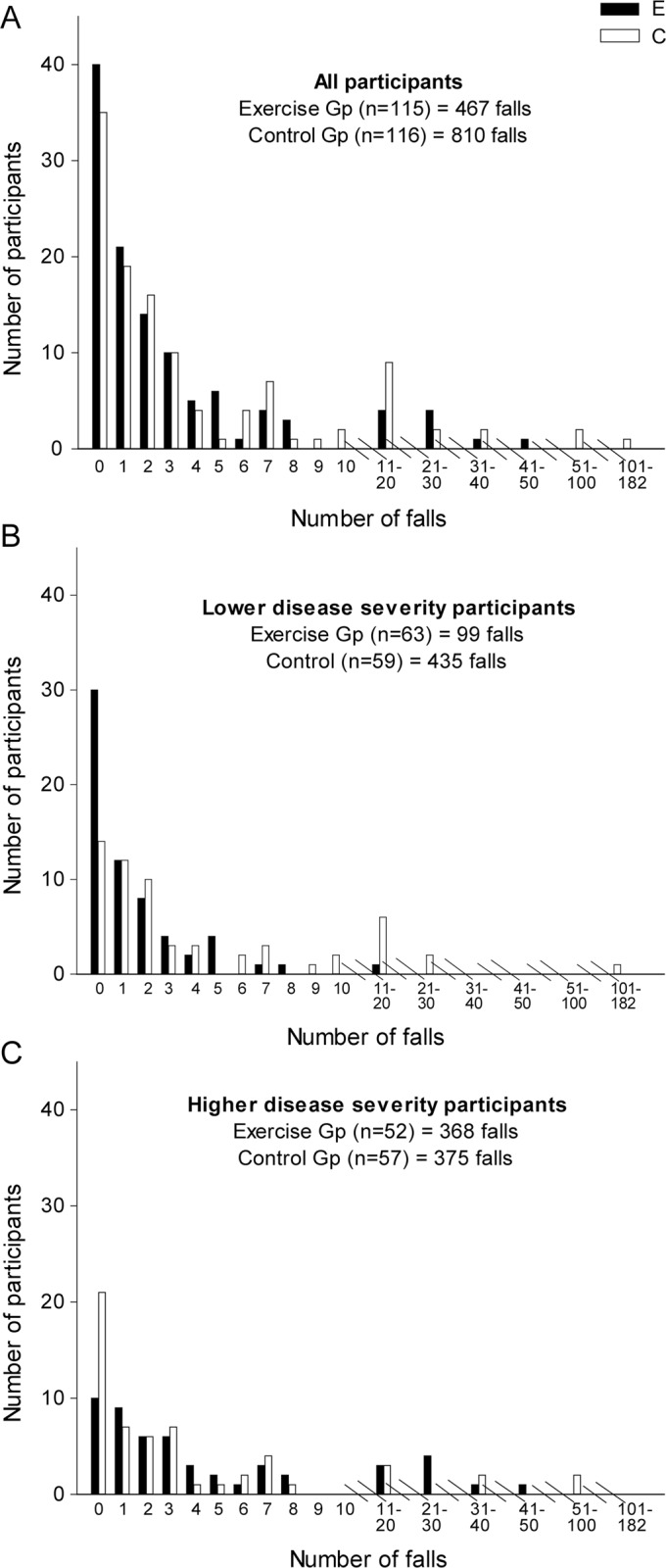
Table 2.
Primary intention-to-treat analysis and disease severity subgroup analysisa
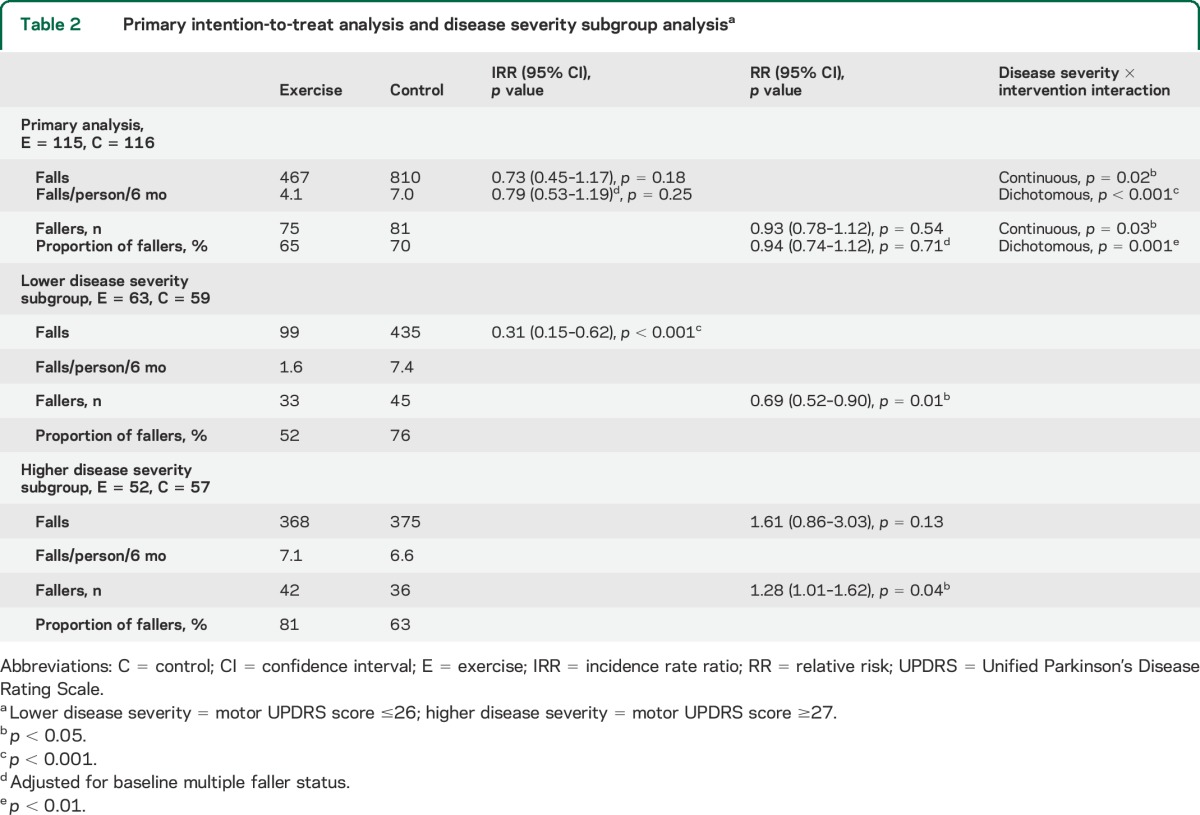
Prespecified subgroup analysis revealed a significant interaction effect for disease severity (rate of falls p < 0.001, proportion of fallers p = 0.001) (figure 2, table 2). Participants with lower disease severity (motor UPDRS score ≤26) demonstrated a 69% reduction in falls in the exercise group (IRR = 0.31, 95% CI 0.15–0.62, p < 0.001) and a lower proportion of fallers (RR = 0.69, 95% CI 0.52–0.90, p = 0.01) compared with the control group. In contrast, participants with higher disease severity (motor UPDRS score ≥27) displayed a trend toward more falls in the exercise group (IRR = 1.61, 95% CI 0.86–3.03, p = 0.13) with a higher proportion of fallers (RR = 1.28, 95% CI 1.01–1.62, p = 0.04) compared with the control group. A marginally significant interaction effect was found for cognition on rate of falls as a continuous variable (p = 0.048), but this interaction was not significant when data were dichotomized (p = 0.45) (table e-2). No significant interaction effect was found for fall history or physical function on rate of falls.
Secondary outcomes.
At 6 months, the exercise group performed significantly better than the control group on several physical outcomes (Short Physical Performance Battery and sit-to-stand), psychological outcomes (Falls Efficacy Scale International and Positive Affect Scale), and overall quality of life (SF-6D) after adjusting for baseline values (table e-3).
Post hoc analysis.
To assist in understanding the differential impact of the intervention on falls by disease severity, post hoc subgroup analyses were undertaken for the secondary outcomes (table e-4). The only significant interaction effect was found for amount of regular exercise including prescribed exercises (p = 0.04). For participants with lower disease severity, a greater amount of habitual exercise was performed by the exercise group compared with the control group (1.5 h/wk, 95% CI 0.03–3.05, p = 0.046), but there was no significant between-group difference (−0.4 h/wk, 95% CI −1.2 to 0.5, p = 0.38) for participants with higher disease severity. Post hoc analysis of adherence data showed that the exercise participants with lower disease severity completed 76% (SD 39%) of prescribed exercise sessions, while those with higher disease severity completed 67% (SD 36%) of prescribed exercise sessions, and this −9% (95% CI −23 to 6) difference in adherence was not statistically significant (p = 0.24). Participant characteristics according to disease severity subgroup are presented in table e-5.
DISCUSSION
This randomized controlled trial of a 6-month, minimally supervised exercise program targeting physical fall risk factors, i.e., impaired balance, impaired leg strength, and freezing of gait, did not reduce falls, proportion of fallers, or serious fall-related injuries in community-dwelling people with PD. Despite the lack of significant effect on several fall risk factors (PD Fall Risk score, knee strength, coordinated stability test, and Freezing of Gait Questionnaire), this exercise program resulted in improvements in balance and mobility (Short Physical Performance Battery and 5-repetition sit-to-stand), fear of falling, positive affect, and overall quality of life relative to the control group. These findings may be explained by the nature of the balance and strength exercises and are consistent with recent evidence highlighting the task-specific effect of exercise in people with PD.33 These findings add weight to the evidence supporting the efficacy of exercise for people with PD13,34 but, unlike previous trials of supervised exercise,35 were achieved with an exercise program in which more than 87% of the prescribed exercise sessions were undertaken independently.
The nonsignificant effect on overall fall rate found in our study may be explained by the differential effects of the exercise program according to disease severity. Our prespecified subgroup analysis indicated that this exercise program was effective in reducing falls and proportion of fallers in people with milder PD but marginally (p = 0.04) increased the proportion of fallers in people with more severe disease. This group had good adherence to the prescribed program but overall did no more exercise per week than the control group. If the overall dose of exercise is important (i.e., prescribed program + usual physical activity), then the dose of exercise more broadly may have been insufficient in our study. It is also possible that the more severely affected participants were potentially placed at higher risk of falls, through increased exposure to fall risk situations resulting from improved mobility and reduced fear of falling achieved in the program. The lack of a differential effect of the intervention on other secondary outcomes for those of lower and higher disease severity may simply reflect low statistical power of interaction tests. However, given the differential impact according to disease severity on falls themselves, the lack of a differential impact on secondary outcomes may also suggest a role of other fall risk factors that were not measured.
The significant 69% reduction in fall rate for the lower disease severity subgroup achieved in our trial is comparable to the 67% reduction in fall rate in a 6-month, fully supervised trial of tai chi.11 The majority of participants in the tai chi trial had relatively mild PD (i.e., 1–2 on the Hoehn and Yahr scale), while the majority of participants in our trial scored 3–4 on the Hoehn and Yahr scale. In contrast, however, a reduction in falls in people with PD in Hoehn and Yahr stages 3–4 was reported when a 7-week, fully supervised, balance-demanding exercise program was delivered.12 Taken together, this evidence suggests that minimally supervised exercise programs aimed at reducing falls in people with PD should be implemented early in the disease process. With the progression of disease affecting both motor and nonmotor systems (as evidenced by the higher fall risk in participants with higher disease severity at baseline, shown in table e-5), people with more severe disease may derive more benefit from a multifactorial, closely supervised intervention. Further adequately powered trials and meta-analyses are required to confirm these suggestions.
Our study has several limitations. The control group did not receive an intervention to control for the Hawthorne effect, and all participants continued their usual medical care including adjustment of medications and deep brain stimulation parameters as required. Nevertheless, these results provide a pragmatic, clinically relevant evaluation of the impact of a minimally supervised exercise program compared with usual care. We chose to focus on physical risk factors and acknowledge that interventions designed to target nonmotor fall risk factors, such as impaired cognition,1,36 in combination with exercise may have a more significant impact on falls.37
Future research is required to gain greater insight into successful fall reduction programs as well as the potential for increasing fall risk in some individuals. Given the heterogeneity of PD, interventions aiming to reduce falls in PD should consider tailoring multifactorial interventions according to motor and nonmotor risk factors, absolute risk of falls,38 disease severity, and motor phenotype. In addition, promising pharmacologic therapies39 in combination with multifactorial interventions warrant investigation.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the people with PD who participated in the study and Parkinson's NSW for assisting with recruitment, in particular, Miriam Dixon and Trish Morgan. The authors thank the following physical therapists who delivered the intervention: Leah Burton, Cathy Chittenden, Diane Davidson, Paul Dean, Kate Godfrey, Larraine Griffin, Jill Hall, Diane Hemsworth, Marty Hewer, Mary Leavesley, Fiona Mackey, Jennifer Mannell, Melissa McConaghy, Rajal Pandya, Neroli Page, Megan Perry, Jessica Pike, Renee Pirozzi, Elisabeth Preston, Wendy Robinson, Elizabeth Shannon, Rebecca Snow, Angela Stark, Judy Sunderland, and Krystle Tate. The authors thank the following physical therapists who assisted with data collection: Wendy Robinson, Lauren Wade, and Geraldine Wallbank. The authors thank the following people who assisted with recruitment: Kay Double, Glenda Halliday, Mariese Hely, Simon Lewis, Joan Perkins, and Connie Vogler.
GLOSSARY
- CI
confidence interval
- IRR
incidence rate ratio
- NSW
New South Wales
- PD
Parkinson disease
- PIG
Poisson inverse gaussian
- RR
relative risk
- UPDRS
Unified Parkinson's Disease Rating Scale
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Colleen Canning contributed to the conception and design of the trial, analyzed and interpreted the data, drafted the manuscript, and revised the manuscript for important intellectual content. Catherine Sherrington contributed to the conception and design of the trial, interpreted the data, and revised the manuscript for important intellectual content. Stephen Lord contributed to the conception and design of the trial, interpreted the data, and revised the manuscript for important intellectual content. Jacqueline Close contributed to the conception and design of the trial, interpreted the data, and revised the manuscript for important intellectual content. Stephane Heritier wrote the statistical analysis plan, analyzed and interpreted the data, and revised the manuscript for important intellectual content. Gillian Heller analyzed and interpreted the data and revised the manuscript for important intellectual content. Kirsten Howard analyzed and interpreted the data and revised the manuscript for important intellectual content. Natalie Allen contributed to the conception and design of the trial, interpreted the data, and revised the manuscript for important intellectual content. Mark Latt contributed to the conception and design of the trial, interpreted the data, and revised the manuscript for important intellectual content. Susan Murray contributed to the design of the study and revised the manuscript for important intellectual content. Sandra O'Rourke contributed to the design of the study and revised the manuscript for important intellectual content. Serene Paul interpreted the data and revised the manuscript for important intellectual content. Jooeun Song analyzed and interpreted the data, drafted the manuscript, and revised the manuscript for important intellectual content. Victor Fung contributed to the conception and design of the trial, interpreted the data, and revised the manuscript for important intellectual content.
STUDY FUNDING
This study was funded by the Australian National Health and Medical Research Council (NHMRC ID: 512326), and the Harry Secomb Foundation.
DISCLOSURE
C. Canning has received travel expenses and honoraria for lectures and educational activities not funded by industry; and research support from the Australian Government National Health and Medical Research Council, the Harry Secomb Foundation, and Parkinson's NSW. C. Sherrington has received travel expenses and honoraria for lectures and educational activities not funded by industry; and research support from the Australian Government National Health and Medical Research Council, the Consortium national de formation en santé (Canada), Arthritis New South Wales, NSW Ministry of Health, University of Sydney, Motor Accidents Authority of New South Wales, and The Trust Company. S. Lord has received travel expenses and honoraria for lectures not funded by industry; a consultancy payment for methodologic advice by Eli Lilly Ltd.; and research support from the Australian Government National Health and Medical Research Council, The Australian Research Council, Multiple Sclerosis Australia, and the NSW Ministry of Health. J. Close has received travel expenses and honoraria for lectures not funded by industry; and research support from the Australian Government National Health and Medical Research Council, Bupa Health Foundation, and the NSW Ministry of Health. S. Heritier has received funding for 2 Australian Government National Health and Medical Research Council grants unrelated to this study; and royalties from Wiley for his book Robust Methods in Biostatistics. G. Heller reports no disclosures relevant to the manuscript. K. Howard has received research support from the Australian Government National Health and Medical Research Council and the Australian Research Council. N. Allen has received research support from Parkinson's NSW. M. Latt has received research support from the Australian Government National Health and Medical Research Council. S. Murray and S. O'Rourke report no disclosures relevant to the manuscript. S. Paul has received research support from the Australian Government National Health and Medical Research Council and Parkinson's NSW. J. Song reports no disclosures. V. Fung receives a salary from NSW Health, has received research grants from the National Health and Medical Research Council of Australia, and is on advisory boards and/or has received travel grants from Abbott/AbbVie, Allergan, Boehringer-Ingelheim, Hospira, Ipsen, Lundbeck, Novartis, Parkinson's KinetiGraph, Solvay, and UCB. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Latt MD, Lord SR, Morris JG, Fung VS. Clinical and physiological assessments for elucidating falls risk in Parkinson's disease. Mov Disord 2009;24:1280–1289. [DOI] [PubMed] [Google Scholar]
- 2.Allen NE, Schwarzel AK, Canning CG. Recurrent falls in Parkinson's disease: a systematic review. Parkinsons Dis 2013;2013:906274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloem BR, Grimbergen YA, Cramer M. Prospective assessment of falls in Parkinson's disease. J Neurol 2001;248:950–958. [DOI] [PubMed] [Google Scholar]
- 4.Wielinski CL, Erickson-Davis C, Wichmann R, Walde-Douglas M, Parashos SA. Falls and injuries resulting from falls among patients with Parkinson's disease and other parkinsonian syndromes. Mov Disord 2005;20:410–415. [DOI] [PubMed] [Google Scholar]
- 5.Temlett JA, Thompson PD. Reasons for admission to hospital for Parkinson's disease. Intern Med J 2006;36:524–526. [DOI] [PubMed] [Google Scholar]
- 6.Sherrington C, Whitney JC, Lord SR, Herbert RD, Cumming RG, Close JCT. Effective exercise for the prevention of falls: a systematic review and meta-analysis. J Am Geriatr Soc 2008;56:2234–2243. [DOI] [PubMed] [Google Scholar]
- 7.Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2012;9:CD007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodwin VA, Richards SH, Henley W, Ewings P, Taylor AH, Campbell JL. An exercise intervention to prevent falls in people with Parkinson's disease: a pragmatic randomised controlled trial. J Neurol Neurosurg Psychiatry 2011;82:1232–1238. [DOI] [PubMed] [Google Scholar]
- 9.Ashburn A, Fazakarley L, Ballinger C, Pickering R, McLellan LD, Fitton C. A randomised controlled trial of a home-based exercise programme to reduce the risk of falling among people with Parkinson's disease. J Neurol Neurosurg Psychiatry 2007;78:678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Protas EJ, Mitchell K, Williams A, Qureshy H, Caroline K, Lai EC. Gait and step training to reduce falls in Parkinson's disease. NeuroRehabilitation 2005;20:183–190. [PubMed] [Google Scholar]
- 11.Li F, Harmer P, Fitzgerald K, et al. Tai chi and postural stability in patients with Parkinson's disease. N Engl J Med 2012;366:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smania N, Corato E, Tinazzi M, et al. Effect of balance training on postural instability in patients with idiopathic Parkinson's disease. Neurorehabil Neural Repair 2010;24:826–834. [DOI] [PubMed] [Google Scholar]
- 13.Tomlinson CL, Patel S, Meek C, et al. Physiotherapy intervention in Parkinson's disease: systematic review and meta-analysis. BMJ 2012;345:e5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dibble LE, Hale TF, Marcus RL, Droge J, Gerber JP, LaStayo PC. High-intensity resistance training amplifies muscle hypertrophy and functional gains in persons with Parkinson's disease. Mov Disord 2006;21:1444–1452. [DOI] [PubMed] [Google Scholar]
- 15.Nieuwboer A, Kwakkel G, Rochester L, et al. Cueing training in the home improves gait-related mobility in Parkinson's disease: the RESCUE trial. J Neurol Neurosurg Psychiatry 2007;78:134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen NE, Canning CG, Sherrington C, et al. The effects of an exercise program on fall risk factors in people with Parkinson's disease: a randomized controlled trial. Mov Disord 2010;25:1217–1225. [DOI] [PubMed] [Google Scholar]
- 17.Campbell AJ, Robertson MC, Gardner MM, Norton RN, Tilyard MW, Buchner DM. Randomised controlled trial of a general practice programme of home based exercise to prevent falls in elderly women. BMJ 1997;315:1065–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canning CG, Sherrington C, Lord SR, et al. Exercise therapy for prevention of falls in people with Parkinson's disease: a protocol for a randomised controlled trial and economic evaluation. BMC Neurol 2009;9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behrman AL, Light KE, Flynn SM, Thigpen MT. Is the Functional Reach Test useful for identifying falls risk among individuals with Parkinson's disease? Arch Phys Med Rehabil 2002;83:538–542. [DOI] [PubMed] [Google Scholar]
- 20.Tiedemann A, Shimada H, Sherrington C, Murray S, Lord S. The comparative ability of eight functional mobility tests for predicting falls in community-dwelling older people. Age Ageing 2008;37:430–435. [DOI] [PubMed] [Google Scholar]
- 21.DeBolt LS, McCubbin JA. The effects of home-based resistance exercise on balance, power, and mobility in adults with multiple sclerosis. Arch Phys Med Rehabil 2004;85:290–297. [DOI] [PubMed] [Google Scholar]
- 22.Don't Fall for It: A Guide to Preventing Falls for Older People. Barton, ACT: Australian Government; 2008. [Google Scholar]
- 23.Gibson M, Andres B, Isaacs B, Radebaugh T, Worm-Peterson J. The prevention of falls in later life: a report of the Kellogg International Work Group on the prevention of falls by the elderly. Dan Med Bull 1987;34(suppl 4):1–24. [PubMed] [Google Scholar]
- 24.Lord SR, Menz HB, Tiedermann A. A physiological profile approach to falls risk assessment and prevention. Phys Ther 2003;83:237–252. [PubMed] [Google Scholar]
- 25.Lord SR, Menz HB, Tiedermann A. Exercise effect on dynamic stability in older women: a randomized controlled trial. Arch Phys Med Rehabil 1996;77:232–236. [DOI] [PubMed] [Google Scholar]
- 26.Onder G, Penninx BW, Ferrucci L, Fried LP, Guralnik JM, Pahor M. Measures of physical performance and risk for progressive and catastrophic disability: results from the Women's Health and Aging Study. J Gerontol A Biol Sci Med Sci 2005;60:74–79. [DOI] [PubMed] [Google Scholar]
- 27.Giladi N, Shabtai H, Simon ES, Biran S, Tal J, Korczyn AD. Construction of Freezing of Gait Questionnaire for patients with parkinsonism. Parkinsonism Relat Disord 2000;6:165–170. [DOI] [PubMed] [Google Scholar]
- 28.Yardley L, Beyer N, Hauer K, Kempen G, Piot-Ziegler C, Todd C. Development and initial validation of the Falls Efficacy Scale–International (FES-I). Age Ageing 2005;34:614–619. [DOI] [PubMed] [Google Scholar]
- 29.Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well being for individuals with Parkinson's disease. Qual Life Res 1995;4:241–248. [DOI] [PubMed] [Google Scholar]
- 30.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 1988;54:1063–1070. [DOI] [PubMed] [Google Scholar]
- 31.Stasinopoulous DM, Rigby RA. Generalized Additive Models for Location Scale and Shape (GAMLSS) in R. J Stat Softw 2007;23:1–46. [Google Scholar]
- 32.Robertson MC, Campbell AJ, Herbison P. Statistical analysis of efficacy in falls prevention trials. J Gerontol A Biol Sci Med Sci 2005;60:530–534. [DOI] [PubMed] [Google Scholar]
- 33.Shulman LM, Katzel LI, Ivey FM, et al. Randomized clinical trial of 3 types of physical exercise for patients with Parkinson disease. JAMA Neurol 2013;70:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allen NE, Sherrington C, Paul SS, Canning CG. Balance and falls in Parkinson's disease: a meta-analysis of the effect of exercise and motor training. Mov Disord 2011;26:1605–1615. [DOI] [PubMed] [Google Scholar]
- 35.Allen NE, Sherrington C, Suriyarachchi GD, Paul S, Song J, Canning CG. Exercise and motor training in people with Parkinson's disease: a systematic review of participant characteristics, intervention delivery, retention rates, adherence and adverse events in clinical trials. Parkinsons Dis 2012;2012:854328. 10.1155/2912/854328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paul SS, Sherrington C, Fung VS, Canning CG. Motor and cognitive impairments in Parkinson disease: relationships with specific balance and mobility tasks. Neurorehabil Neural Repair 2013;27:63–71. [DOI] [PubMed] [Google Scholar]
- 37.Mirelman A, Rochester L, Reelick M, et al. V-TIME: a treadmill training program augmented by virtual reality to decrease fall risk in older adults: study design of a randomized controlled trial. BMC Neurol 2013;13:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paul SS, Canning CG, Sherrington C, Lord SR, Close JC, Fung VS. Three simple clinical tests to accurately predict falls in people with Parkinson's disease. Mov Disord 2013;28:655–662. [DOI] [PubMed] [Google Scholar]
- 39.Chung KA, Lobb BM, Nutt JG, Horak FB. Effects of a central cholinesterase inhibitor on reducing falls in Parkinson disease. Neurology 2010;75:1263–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fahn S, Elton R. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent Development in Parkinson's Disease. Florham Park, NJ: Macmillan Health Care Information; 1987:153–163. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.