Abstract
Objective:
We aimed to explore factors associated with clinical evaluations for cognitive impairment among older residents of the United States.
Methods:
Two hundred ninety-seven of 845 subjects in the Aging, Demographics, and Memory Study (ADAMS), a nationally representative community-based cohort study, met criteria for dementia after a detailed in-person study examination. Informants for these subjects reported whether or not they had ever received a clinical cognitive evaluation outside of the context of ADAMS. Among subjects with dementia, we evaluated demographic, socioeconomic, and clinical factors associated with an informant-reported clinical cognitive evaluation using bivariate analyses and multivariable logistic regression.
Results:
Of the 297 participants with dementia in ADAMS, 55.2% (representing about 1.8 million elderly Americans in 2002) reported no history of a clinical cognitive evaluation by a physician. In a multivariable logistic regression model (n = 297) controlling for demographics, physical function measures, and dementia severity, marital status (odds ratio for currently married: 2.63 [95% confidence interval: 1.10–6.35]) was the only significant independent predictor of receiving a clinical cognitive evaluation among subjects with study-confirmed dementia.
Conclusions:
Many elderly individuals with dementia do not receive clinical cognitive evaluations. The likelihood of receiving a clinical cognitive evaluation in elderly individuals with dementia associates with certain patient-specific factors, particularly severity of cognitive impairment and current marital status.
More than 1 in 8 individuals in the United States over the age of 65 years has dementia.1 Only a fraction of these individuals, however, receive a clinical evaluation for cognitive concerns.2,3 In 2013, a US Preventive Services Task Force reported that there was insufficient evidence to support routine screening for dementia because of lack of published data to demonstrate that screening alters patient, family, and/or provider decision-making.4 Early identification and evaluation of affected individuals, however, may allow patients with dementia and their families to receive care earlier, which could contribute significantly to improved quality of life among patients and their caregivers, including more time spent at a milder disease stage, more time spent in the community, and less time spent in long-term care facilities.5
Unlike other common and disabling health conditions such as cancer or cardiovascular disease, there is no widely adopted clinical algorithm for the early recognition and evaluation of patients with suspected dementia. Although a number of published consensus guidelines exist,6,7 their use in clinical practice is inconsistent.8 Clinical cognitive evaluations occur infrequently and at variable rates in different clinical settings. The aim of the present study was to investigate the significance of demographic and socioeconomic factors in predicting an individual's likelihood of receiving a clinical cognitive evaluation for dementia. We used data from the Aging, Demographics, and Memory Study (ADAMS), a nationally representative cohort study of 856 individuals aged 70 years or older,9 to accomplish this objective. We hypothesized that lower socioeconomic status would be associated with a lower likelihood of receiving a clinical cognitive evaluation.
METHODS
Subjects.
Participants from ADAMS were sampled from the larger Health and Retirement Study (HRS), a nationally representative, community-based cohort study of individuals aged 51 years or older that began initially in 1992. In 2000 and 2002, a subsample of subjects 70 years or older were drawn from the HRS for ADAMS. This sample was stratified based on scoring levels of an abbreviated version of the modified Telephone Interview for Cognitive Status to identify strata that were likely to contain individuals with normal cognition, cognitive impairment not dementia (CIND), and dementia.9,10 Further details on the HRS and ADAMS sampling strategy are available on the HRS Web site (http://hrsonline.isr.umich.edu/). Of the 1,770 subjects identified as qualifying for ADAMS, 856 consented (56% of eligible living participants) and were assessed within 2 years with detailed neuropsychological testing, clinical examination, and informant interviews. Age, sex, and education were similar among those participants who consented to participate in ADAMS compared with those who did not participate. ADAMS participants were more likely to be African American and more likely to have scored in the normal range of cognitive testing at the prior HRS assessment compared with qualifying nonparticipants.9 We used previously established ADAMS sample selection weights to adjust the raw proportions seen in our sample to account for ADAMS selection probabilities and differential nonparticipation, leading to final results reflective of the US demographic and regional distribution of the elderly population in 2002.10 Our study—and the survey weighting applied to this sample—was restricted to the 844 individuals in ADAMS with complete responses to question items regarding community dementia evaluations for cognitive complaints.
Cognitive and demographic evaluation.
All subjects received evaluations at their place of residence with multidomain neuropsychological testing, the Mini-Mental State Examination (MMSE),11 and a brief videotaped assessment capturing portions of the cognitive and neurologic examination.9 These data were supplemented by information from reliable informants, most of whom were either spouses (n = 293) or children (n = 317). Information collected from a knowledgeable informant included a chronological history of cognitive symptoms, medical comorbidities, current medications, current neuropsychiatric symptoms, measures of severity of cognitive and functional impairment, and family history of memory problems. The informant also completed the Dementia Severity Rating Scale (DSRS),12 which assesses informant-perceived, subject-specific cognitive impairment in multiple domains including decision-making, social and community activity, personal care, and mobility.
All informants were asked, “Has [name] ever seen a doctor for any of the memory problems we have discussed?” If no memory problems were endorsed, the informant was instead asked, “Has [name] ever seen a doctor for any concerns with her/his memory or thinking?” If the informant answered yes to either of these questions, they were asked to provide the name, location, and medical specialty of the doctor. They were also asked, “What did the doctor say was the cause of the memory trouble?” Data from these specific questions, directed toward informants alone, were used to determine whether or not a subject had received a clinical cognitive evaluation (CCE) outside of the context of the study for all 856 ADAMS subjects. Subject self-report, medical record review, and claims data were not used in our outcome assessments. Of the 308 ADAMS subjects with study-confirmed dementia, 11 subjects were missing informant-derived data on whether or not they had previously received a CCE. These 11 subjects were excluded from analysis, yielding a final sample size of 297.
Demographic information included sex, education (years), and marital status. Age was treated as a continuous variable for the logistic regression analysis but is displayed in 3 strata in table 1. Race was categorized as Caucasian, African American, or other/don't know. Subjects were grouped into geographic categories by their place of residence within the United States: Northeast, Midwest, South, and West. Household net worth was derived from HRS-linked data taken from prior waves proximate to the ADAMS assessment and were grouped into quartiles.13
Table 1.
Demographic information of subjects with dementia
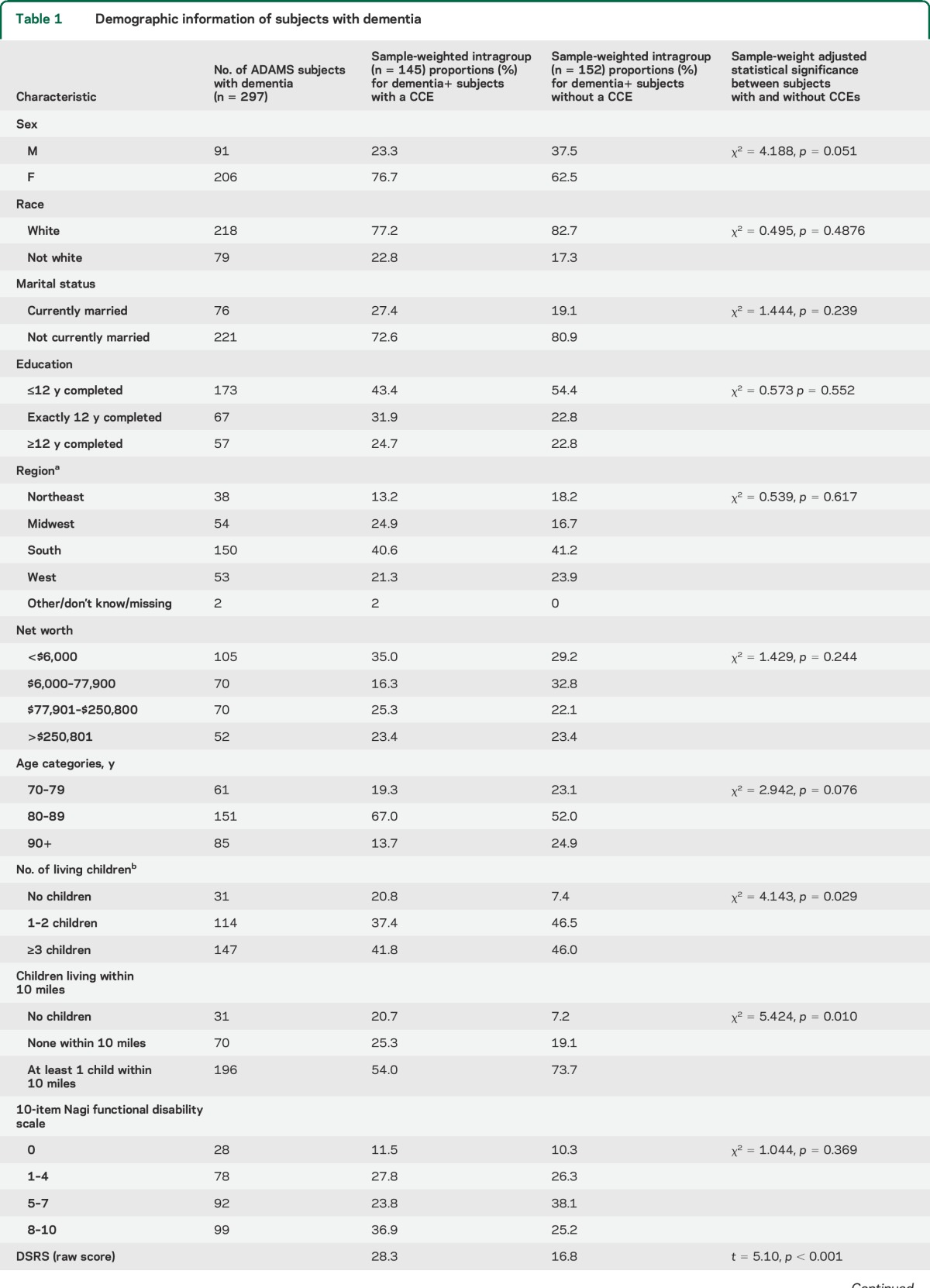
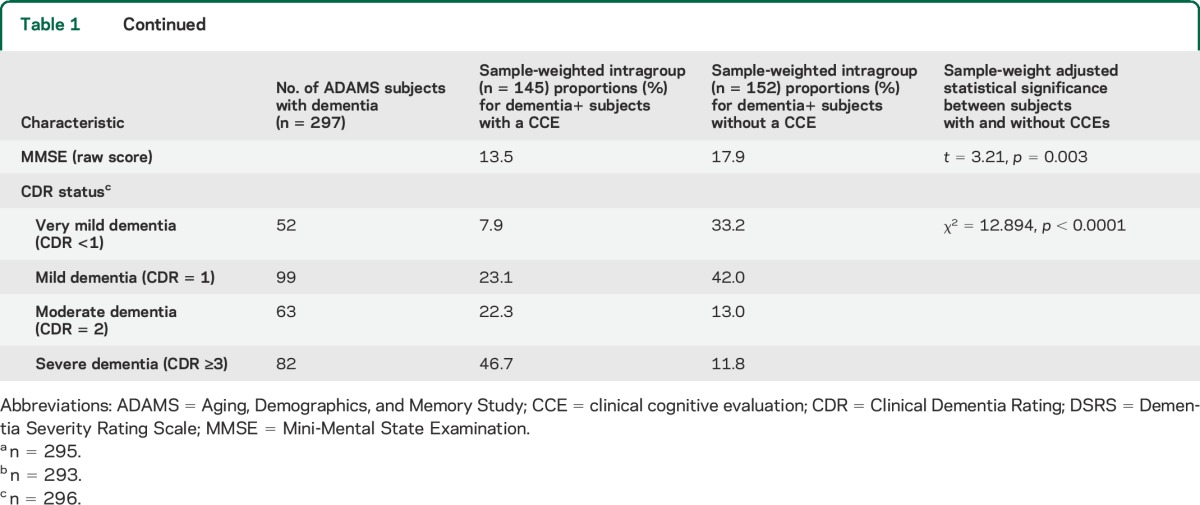
A consensus panel of experts including neuropsychologists, neurologists, geropsychiatrists, and internists reviewed clinical data to determine the presence or absence of dementia and its cause. A consensus diagnosis of dementia was based on guidelines from DSM-III-R and DSM-IV14,15; diagnoses of Alzheimer disease and other causes of dementia were based on published consensus criteria.16–19 Specific diagnostic criteria for CIND in this cohort are described elsewhere.10 A Clinical Dementia Rating (CDR) score was calculated at this time.20
Standard protocol approvals, registrations, and patient consents.
The study was approved by the institutional review board of the University of Michigan. Written informed consent was obtained from all subjects.
Statistical analyses.
Using the ADAMS sample weights, we computed estimates for the national prevalence of CCEs among all ADAMS participants and among individuals with dementia, aged 70 years or older. Weight-adjusted demographic features of subjects who met study consensus criteria for the presence of dementia were explored using descriptive statistics, χ2 testing, and pooled 2-sample t tests. Given that previous studies in different cohorts have suggested that demographic,21 socioeconomic,22 family-specific,23 and clinical24 factors may be associated with health care resource utilization in elderly individuals, we constructed a multivariable logistic regression model (n = 297) to explore the combined effects of these variables on predicting the likelihood of receiving a CCE among those subjects with study-confirmed dementia. Because the proximity of living children can influence trends in caregiving and resource utilization for age-related conditions,25 this variable was include as well. Nagi disability scores were introduced into the model to account for the possibility that individuals with greater health-related disability might be seen more frequently by health care providers, thereby increasing their relative odds for receiving a CCE.
RESULTS
After applying sample weights to the ADAMS cohort, CCEs were reported in 1.2% of individuals with normal cognition, 5.3% of individuals characterized as having CIND, and in 44.8% of individuals with study-confirmed dementia. In total, 8.1% of all subjects in the sample weighted-adjusted ADAMS cohort reported a CCE. These data suggest that 55.2% of Americans over the age of 70 with dementia, equating to approximately 1.8 million elderly adults, would not report having a CCE. Subjects with dementia had a mean age of 84.3 years (SE: 49, range: 70–110). The mean education level was 10.3 years (SE: 31, range: 0–17 years). The median net worth was $35,200 with a range from −$17,000 to $6,040,000. Additional demographic factors for these 297 subjects are presented in table 1.
Informants for the subjects with study-confirmed dementia reported knowing the subjects on average for 50.3 years (SD 17.7; n = 289). Among informants for the entire ADAMS cohort (n = 856), 427 reported living with the subject, 135 reported seeing or talking with the subject daily, and 147 reported seeing or talking with the subject several times a week. Only 10 reported seeing the subject less than once per month.
The most common community physician diagnoses applied to subjects and their families were dementia (31.6%) and Alzheimer disease (28.8%), followed by strokes or TIAs (17.3%), don't know (8.9%), other causes (8.3%), and normal aging (2.3%). Family medicine and internal medicine physicians performed the majority (n = 85; 60.5%) of the CCEs, followed by neurologists (n = 30; 21.5%) and psychiatrists (n = 11; 8.7%).
Among subjects with study-confirmed dementia, those with a history of a CCE did not differ significantly in race, education, marital status, level of functional disability, or net worth from subjects with dementia without a reported CCE (table 1). There were nonsignificant trends toward younger age and female sex for those receiving a CCE. Participants with study-confirmed dementia who received CCEs were more likely to have fewer living children and were less likely to have a child living within close proximity. Subjects who reported a history of a CCE were more impaired on the MMSE, DSRS, and CDR.
Multivariable logistic regression was used among ADAMS subjects with dementia (n = 297) to understand the combined effects of these factors in predicting CCEs (table 2). Among all demographic factors, only currently being married (p = 0.031) was associated with an increased likelihood of receiving a CCE. There was a nonsignificant associative trend between likelihood of receiving a CCE and nonwhite race (p = 0.076) and a trend of lower likelihood of CCE seen with age between 80 and 89 (p = 0.08). Higher CDR scores also predicted a greater likelihood of CCE.
Table 2.
Multivariable logistic regression model (n = 297)
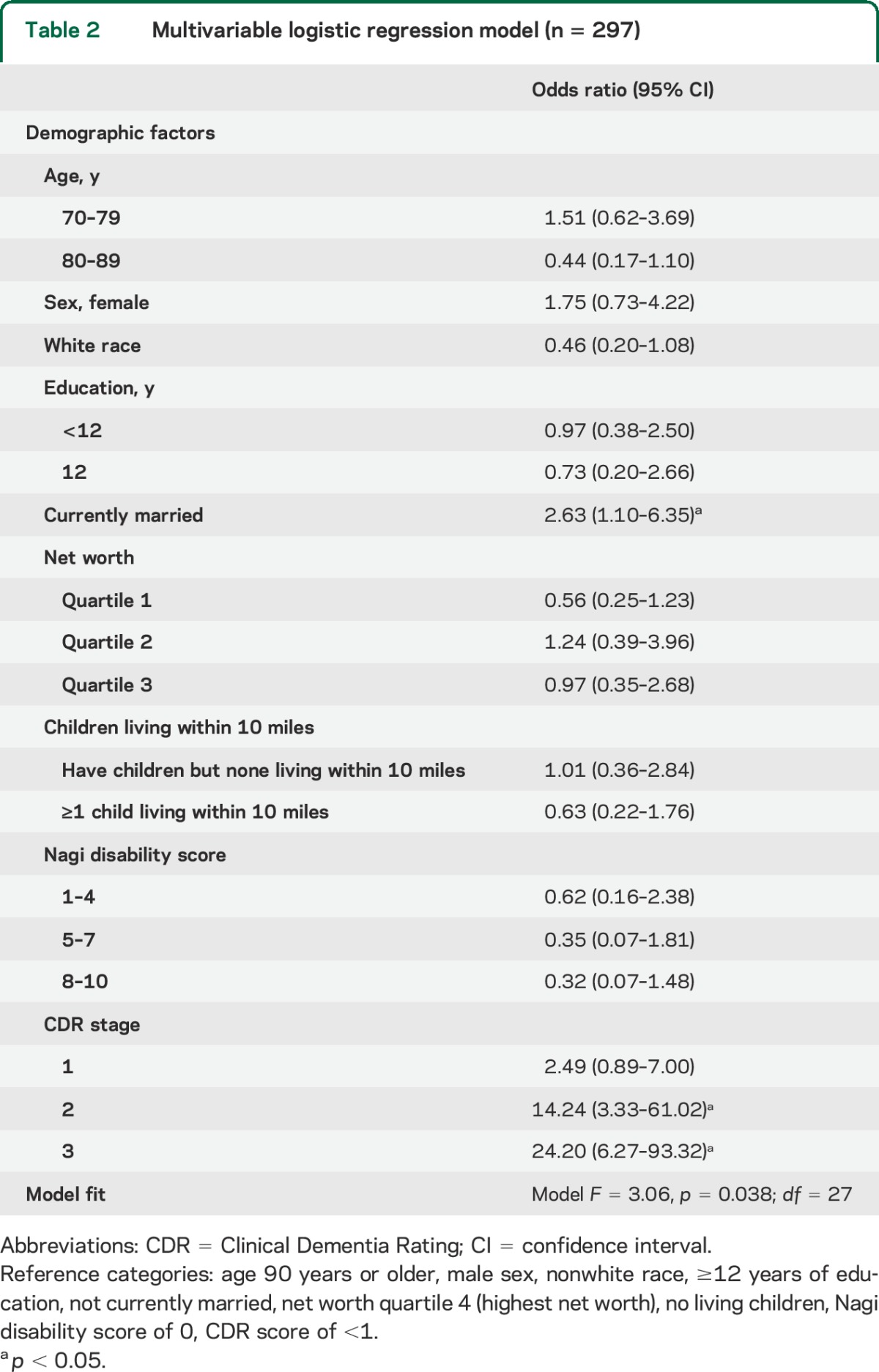
Previous literature has suggested that the effect of age may differ between racial categories in its influence on accepting or declining a community cognitive evaluation.21 In an exploratory analysis, we did not find a significant interaction between “age 70–79 and race category” (95% confidence interval [CI]: 0.83, 34.79) and “age 80–89 and race category” (95% CI: 0.88, 27.90). Marital status remained significant (95% CI: 1.34, 6.77) in this exploratory model.
DISCUSSION
This study provides insight into the current status of clinical cognitive evaluations on a national scale. Only a small minority of community-dwelling individuals older than 70 years receives a clinical cognitive evaluation for thinking and memory concerns. Remarkably, only approximately 5% of those 70 years or older with CIND and less than half of those with frank dementia report receiving a CCE. As expected, more severe dementia as indicated by performance on the CDR, DSRS, and MMSE was associated with an increased likelihood of receiving a CCE. The low rate of cognitive assessment in our cohort cannot be attributed solely to primary physicians, or to family members. Both are likely to contribute. Although we hypothesized that lower socioeconomic status would be associated with a lower rate of receiving CCEs, we found no differences in net worth in subjects with dementia with and without CCEs.
Our findings are consistent with previous studies that suggest dementia may be markedly underrecognized even among elderly patients who receive regular primary care.3 This trend is likely driven by multiple factors including physician-specific factors, patient and family factors, and broader societal- and systems-based practices. Physician-reported barriers to making a dementia diagnosis include poor recognition of dementia symptoms, physician appointment time constraints, and therapeutic pessimism that may lead to a lower prioritization of cognitive complaints and subsequent diagnostic evaluation.26 Brief, office-based cognitive instruments may also have variable sensitivity in different patient populations making recognition of dementia more challenging.27
Our results are also consistent with a previous community-based study of individuals over the age of 65 in which 47.7% of individuals screening positive for dementia declined further cognitive assessment.21 Older age and African American race were noted to be risk factors for declining a dementia evaluation in that study although neither showed a significant association with CCEs in our study. This may relate to differences in cohort characteristics.
Being married was associated with a greater likelihood of receiving a CCE. There are several potential explanations for this finding. Family detection of cognitive change is likely to be higher in married couples, and spouses may feel more comfortable than children in raising their concerns with a health care provider. A previous study has demonstrated that informants who live with a cognitively impaired family member more accurately describe memory impairments than informants who live separately.28 Marriage may be associated with increased health care utilization although prior studies show mixed findings regarding this possibility.23,29 It is also possible that unmarried elderly patients may be more reluctant to divulge cognitive concerns to their physician out of concern for the impact such a disclosure might have on their overall autonomy. Our bivariate and multivariable analyses suggest that the number and proximity of living children is no substitute for a spousal caregiver when it comes to seeking medical care for cognitive impairment in a loved one.
A limitation of our study design is the reliance on the report of informants who may have variable degrees of interaction with a given study participant. Asking informants to think back to a remote physician encounter also introduces the possibility of recall bias. Similar to other cohort studies,30 we used both passive (informant report) and active (ADAMS evaluation) case ascertainment methods. It should be noted, however, that informant report of a CCE was not corroborated through a review of patient records and hence represents a potential limitation of our study design. Nevertheless, these informant recollections reflect real-world understanding of medical explanations given for a family member's cognitive decline and remain likely to inform family decision-making. We did not collect detailed information from physician records regarding the exact clinical presenting signs and symptoms that led to CCEs. It is not known to what degree the specific elements and details of these community evaluations altered the postevaluation likelihood of receiving a physician diagnosis of dementia. For this reason, we cannot draw inferences about the quality of evaluations conducted and their association with patient-specific factors.
In the appropriate clinical context, CCEs offer significant health care utility including improved short-term cognitive and functional outcomes,31,32 reduced long-term nursing home placement,33 and overall cost-effective care.34 A lower likelihood of receiving a CCE may also adversely affect the control of contributing coexisting medical illnesses such as diabetes and hypertension, and likely leads to the underdiagnosis of remediable factors associated with cognitive problems, thus leading to potentially preventable disability. Early recognition and evaluation of dementias in affected individuals may encourage family members to take a more active role in direct caregiving including (1) helping with day-to-day tasks such as ensuring correct medication administration, (2) preventing poly-pharmacy and other iatrogenic measures that increase the risk of delirium, and (3) in directing discussions about long-term goals of care including the need for other routine but potentially challenging diagnostic and therapeutic interventions such as screening colonoscopy and/or cardiac stress testing.
In January 2011, Medicare coverage under the Affordable Care Act expanded to include an annual wellness visit that requires a screening to assess cognition. The Alzheimer's Association has published guidelines for these annual wellness visits and a list of suggested cognitive screening tools for health care providers to consider.35 Routine screening protocols aimed at making early diagnoses of dementing conditions also carry the potential risk of false-positive diagnoses that are likely to harm quality of life. The risk of false-positive diagnoses must be weighed carefully given that there is no convincing evidence to date that routine screening for dementia improves aggregate health outcomes.4 The efficacy of dementia screening measures, however, is likely to be stronger if applied to a sample known to be at risk of cognitive decline. Our study did not explore the specific utility of CCEs in different clinical settings and hence we cannot draw more definitive conclusions about screening protocols for dementia, the pros and cons of which are well-reviewed elsewhere.36,37
Despite the aforementioned limitations, our results show trends seen in a large, nationally representative sample and reflect real-world practices. Improved understanding of the variability seen in current care models may lead to more effective and equitable use of emerging dementia resources. Our results, however, suggest that future studies directed toward investigating the perceived utility of cognitive evaluations among patients, families, and health care providers are needed to improve the utilization of clinical cognitive evaluations and the aggregate care of older individuals at risk of dementing diseases.
GLOSSARY
- ADAMS
Aging, Demographics, and Memory Study
- CCE
clinical cognitive evaluation
- CDR
Clinical Dementia Rating
- CI
confidence interval
- CIND
cognitive impairment not dementia
- DSM-III-R
Diagnostic and Statistical Manual of Mental Disorders (Third Edition Revised)
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition)
- DSRS
Dementia Severity Rating Scale
- HRS
Health and Retirement Study
- MMSE
Mini-Mental State Examination
AUTHOR CONTRIBUTIONS
Vikas Kotagal: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, statistical analysis. Kenneth M. Langa: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data. Brenda L. Plassman: drafting/revising the manuscript, accepts responsibility for conduct of research and will give final approval, acquisition of data. Gwenith G. Fisher: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data, study supervision. Bruno J. Giordani: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data. Robert B. Wallace: analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data, obtaining funding. James R. Burke: drafting/revising the manuscript, accepts responsibility for conduct of research and will give final approval, acquisition of data. David C. Steffens: drafting/revising the manuscript, accepts responsibility for conduct of research and will give final approval. Mohammed Kabeto: analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data, statistical analysis. Roger L. Albin: drafting/revising the manuscript, accepts responsibility for conduct of research and will give final approval. Norman L. Foster: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data.
STUDY FUNDING
The Health and Retirement Study is conducted by the Institute for Social Research at the University of Michigan, with funding from the National Institute on Aging (U01 AG009740). Additional support was provided by the University of Utah Center for Alzheimer's Care, Imaging and Research.
DISCLOSURE
V. Kotagal: research funding through the American Academy of Neurology Clinical Research Training Fellowship and the Blue Cross Blue Shield of Michigan Foundation. K. Langa and B. Plassman report no disclosures relevant to the manuscript. G. Fisher: supported by NIH/NIA (R01 AG027010, U01 AG009740, and R37 R37 AG007137). B. Giordani, R. Wallace, and J. Burke report no disclosures relevant to the manuscript. D. Steffens: research support from the National Institute of Mental Health. M. Kabeto reports no disclosures relevant to the manuscript. R. Albin: research support from the NIH and the VA. Dr. Albin has received compensation for expert witness testimony in litigation regarding dopamine agonist–induced impulse control disorders. Dr. Albin serves on the editorial boards of Neurology®, Experimental Neurology, and Neurobiology of Disease and has served on the Data Safety and Monitoring Boards for the QE3 and HORIZON trials. N. Foster: receives a salary from the University of Utah as a faculty member through reimbursed clinical services, and for administrative and teaching activities. He provides unpaid services to the Alzheimer's Association, the American Academy of Neurology, the Society of Nuclear Medicine and Molecular Imaging, as a member of the Utah State Plan Task Force, the Working Interdisciplinary Network of Guardianship Stakeholders, and a number of other community organizations. Dr. Foster has received personal compensation from Bristol-Myers Squibb, GE Healthcare, the National Association for Continuing Education, and Sanofi for consulting activities. Dr. Foster has received research support for clinical trials from GE Healthcare, the Center for Health Improvement, Merck, and Lilly. Dr. Foster also has received research support within the past year from the NIH, and the Veterans Affairs Office of Rural Health. He is CEO and co-owner of Proactive Memory Services, Inc., a University of Utah for-profit startup company developing a mobile application to improve the quality of care for cognitive concerns. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the Aging, Demographics, and Memory Study. Neuroepidemiology 2007;29:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chodosh J, Petitti DB, Elliott M, et al. Physician recognition of cognitive impairment: evaluating the need for improvement. J Am Geriatr Soc 2004;52:1051–1059. [DOI] [PubMed] [Google Scholar]
- 3.Callahan CM, Hendrie HC, Tierney WM. Documentation and evaluation of cognitive impairment in elderly primary care patients. Ann Intern Med 1995;122:422–429. [DOI] [PubMed] [Google Scholar]
- 4.Lin JS, O'Connor E, Rossom RC, et al. Screening for Cognitive Impairment in Older Adults: An Evidence Update for the U.S. Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality (US); 2013. [PubMed] [Google Scholar]
- 5.Budd D, Burns LC, Guo Z, L'Italien G, Lapuerta P. Impact of early intervention and disease modification in patients with predementia Alzheimer's disease: a Markov model simulation. Clinicoecon Outcomes Res 2011;3:189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001;56:1143–1153. [DOI] [PubMed] [Google Scholar]
- 7.Segal-Gidan F, Cherry D, Jones R, Williams B, Hewett L, Chodosh J. Alzheimer's disease management guideline: update 2008. Alzheimers Dement 2011;7:e51–e59. [DOI] [PubMed] [Google Scholar]
- 8.Rosen CS, Chow HC, Greenbaum MA, et al. How well are clinicians following dementia practice guidelines? Alzheimer Dis Assoc Disord 2002;16:15–23. [DOI] [PubMed] [Google Scholar]
- 9.Langa KM, Plassman BL, Wallace RB, et al. The Aging, Demographics, and Memory Study: study design and methods. Neuroepidemiology 2005;25:181–191. [DOI] [PubMed] [Google Scholar]
- 10.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med 2008;148:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grigoletto F, Zappala G, Anderson DW, Lebowitz BD. Norms for the Mini-Mental State Examination in a healthy population. Neurology 1999;53:315–320. [DOI] [PubMed] [Google Scholar]
- 12.Clark CM, Ewbank DC. Performance of the Dementia Severity Rating Scale: a caregiver questionnaire for rating severity in Alzheimer disease. Alzheimer Dis Assoc Disord 1996;10:31–39. [PubMed] [Google Scholar]
- 13.Soldo BJ, Hurd MD, Rodgers WL, Wallace RB. Asset and health dynamics among the oldest old: an overview of the AHEAD Study. J Gerontol B Psychol Sci Soc Sci 1997;52:1–20. [DOI] [PubMed] [Google Scholar]
- 14.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-III-R. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 15.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 16.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 17.Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology 1993;43:250–260. [DOI] [PubMed] [Google Scholar]
- 18.Clinical and neuropathological criteria for frontotemporal dementia. The Lund and Manchester Groups. J Neurol Neurosurg Psychiatry 1994;57:416–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. Neurology 1996;47:1113–1124. [DOI] [PubMed] [Google Scholar]
- 20.Morris JC. Clinical Dementia Rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr 1997;9(suppl 1):173–176. [DOI] [PubMed] [Google Scholar]
- 21.Boustani M, Perkins AJ, Fox C, et al. Who refuses the diagnostic assessment for dementia in primary care? Int J Geriatr Psychiatry 2006;21:556–563. [DOI] [PubMed] [Google Scholar]
- 22.Zivin K, Ratliff S, Heisler MM, Langa KM, Piette JD. Factors influencing cost-related nonadherence to medication in older adults: a conceptually based approach. Value Health 2010;13:338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dryden R, Williams B, McCowan C, Themessl-Huber M. What do we know about who does and does not attend general health checks? Findings from a narrative scoping review. BMC Public Health 2012;12:723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stephens CE, Newcomer R, Blegen M, Miller B, Harrington C. Emergency department use by nursing home residents: effect of severity of cognitive impairment. Gerontologist 2012;52:383–393. [DOI] [PubMed] [Google Scholar]
- 25.Brown HS, III, Herrera AP, Angel JL. Opportunity costs associated with caring for older Mexican-Americans. J Cross Cult Gerontol 2013;28:375–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boise L, Camicioli R, Morgan DL, Rose JH, Congleton L. Diagnosing dementia: perspectives of primary care physicians. Gerontologist 1999;39:457–464. [DOI] [PubMed] [Google Scholar]
- 27.Wood RY, Giuliano KK, Bignell CU, Pritham WW. Assessing cognitive ability in research: use of MMSE with minority populations and elderly adults with low education levels. J Gerontol Nurs 2006;32:45–54. [DOI] [PubMed] [Google Scholar]
- 28.Ready RE, Ott BR, Grace J. Validity of informant reports about AD and MCI patients' memory. Alzheimer Dis Assoc Disord 2004;18:11–16. [DOI] [PubMed] [Google Scholar]
- 29.Joung IM, van der Meer JB, Mackenbach JP. Marital status and health care utilization. Int J Epidemiol 1995;24:569–575. [DOI] [PubMed] [Google Scholar]
- 30.Knopman DS, Petersen RC, Rocca WA, Larson EB, Ganguli M. Passive case-finding for Alzheimer's disease and dementia in two U.S. communities. Alzheimers Dement 2011;7:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gauthier S, Lopez OL, Waldemar G, et al. Effects of donepezil on activities of daily living: integrated analysis of patient data from studies in mild, moderate and severe Alzheimer's disease. Int Psychogeriatr 2010;22:973–983. [DOI] [PubMed] [Google Scholar]
- 32.Birks J, Harvey RJ. Donepezil for dementia due to Alzheimer's disease. Cochrane Database Syst Rev 2006;CD001190. [DOI] [PubMed] [Google Scholar]
- 33.Mittelman MS, Ferris SH, Shulman E, Steinberg G, Levin B. A family intervention to delay nursing home placement of patients with Alzheimer disease: a randomized controlled trial. JAMA 1996;276:1725–1731. [PubMed] [Google Scholar]
- 34.Weimer DL, Sager MA. Early identification and treatment of Alzheimer's disease: social and fiscal outcomes. Alzheimers Dement 2009;5:215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cordell CB, Borson S, Boustani M, et al. Alzheimer's Association recommendations for operationalizing the detection of cognitive impairment during the Medicare Annual Wellness Visit in a primary care setting. Alzheimers Dement 2013;9:141–150. [DOI] [PubMed] [Google Scholar]
- 36.Fox C, Lafortune L, Boustani M, Brayne C. The pros and cons of early diagnosis in dementia. Br J Gen Pract 2013;63:e510–e512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borson S, Frank L, Bayley PJ, et al. Improving dementia care: the role of screening and detection of cognitive impairment. Alzheimers Dement 2013;9:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]


