Abstract
Objective:
To investigate the effect of optimal combination of evidence-based drug therapies including antihypertensive agents, lipid modifiers, and antithrombotic agents on risk of recurrent vascular events after stroke.
Methods:
We analyzed the database of a multicenter trial involving 3,680 recent noncardioembolic stroke patients aged 35 years or older and followed for 2 years. Patients were categorized by appropriateness level 0 to III depending on the number of drugs prescribed divided by the number of drugs potentially indicated for each patient (0 = none of the indicated medications prescribed and III = all indicated medications prescribed). Independent associations of medication appropriateness level with recurrent stroke (primary outcome), stroke/coronary heart disease/vascular death as major vascular events (secondary outcome), and death (tertiary outcome) were assessed.
Results:
The unadjusted rate of stroke declined with increasing medication appropriateness level (15.9% for level 0, 10.3% for level I, 8.6% for level II, and 7.3% for level III). Compared with level 0: the adjusted hazard ratio of stroke for level I was 0.51 (95% confidence interval, 0.21–1.25), level II 0.50 (0.23–1.09), and level III 0.39 (0.18–0.84); of stroke/coronary heart disease/vascular death for level I 0.60 (0.32–1.14), level II 0.45 (0.25–0.80), and level III 0.39 (0.22–0.69); and of death for level I 0.89 (0.30–2.64), level II 0.71 (0.26–1.93), and level III 0.35 (0.13–0.96).
Conclusions:
Optimal combination of secondary prevention medication classes after a recent noncardioembolic stroke is associated with a significantly lower risk of stroke, major vascular events, and death.
The greatest opportunity for decreased stroke incidence and mortality is through stroke prevention. Fortunately, several evidence-based treatments are available, which have been shown to improve stroke outcomes.1 Indeed, over the last decade there has been a documented decline in the stroke incidence and mortality in several developed nations around the world.2–5 This welcome development in the alleviation of stroke burden is thought to be mainly attributable to better treatment of traditional risk factors for stroke.6,7 However, a projected substantial rise in future overall incidence and costs of stroke8 means much more needs to be done to mitigate the societal burden of stroke. One proposed strategy to further enhance cardiovascular disease prevention moving forward is the systematic combination of individually proven secondary prevention drugs.9–11
Approximately 50% or fewer patients with an acute coronary syndrome received optimal combination therapy at the time of hospital discharge.12–15 Mounting evidence suggests that use of evidence-based combination medical treatments including antiplatelet agents, β-blockers, statins, and angiotensin-converting enzyme inhibitors are linked to a significantly lower risk of 6-month or 1-year mortality in patients with acute coronary syndrome.12,13,15 Underuse of optimal combination secondary preventive therapies is widespread in patients with prior vascular events, particularly those with previous ischemic stroke.16 Furthermore, little is known about the potential influence of optimal combination drug treatments on recurrent outcomes, especially for major clinical outcomes including recurrent stroke.
With this background, we investigated the relationship between the optimal combination of evidence-based single drug therapies and risk of recurrent stroke, major vascular events, and death, following the occurrence of a recent stroke.
METHODS
Patients and study.
We reviewed data from the Vitamin Intervention for Stroke Prevention (VISP) trial.17 VISP was a double-blind, randomized, controlled trial performed across multiple sites and countries including the United States, Canada, and Scotland. The study enrolled 3,680 patients aged 35 years or older to determine whether high doses of multivitamin (folic acid, pyridoxine, and cobalamin) given to lower total homocysteine levels would reduce the risk of recurrent stroke and major vascular events in patients with a recent (within the preceding 120 days) nondisabling, noncardioembolic stroke.17 Details of methods and main results of the trial have been reported previously. Demographic, clinical, and laboratory data were collected at baseline, with subsequent clinical and laboratory information obtained at follow-up visits of 6, 12, and 24 months. Serum lipid samples were obtained in the fasting state. For each eligible patient, presence of hypertension and diabetes was documented not only by history, but also if newly diagnosed at the first visit. We reviewed VISP data recorded on medication use, which was collected at every 6-month interval follow-up visit. We utilized secondary prevention drug information that was provided at the last follow-up visit (vs baseline visit approach) because (1) the number and type of secondary prevention medications among patients with symptomatic cardiovascular disease can vary during the postdischarge follow-up setting,18 and (2) our preliminary evaluation of the VISP data indicated that several hundred patients had their baseline prescriptions modified to include more therapeutic drug classes at the time of their second or third (and further) follow-up visit. Last follow-up visit was defined as the last documented study encounter that preceded either a vascular outcome event or end of the trial.
Standard protocol approvals, registrations, and patient consents.
The trial was approved by the ethics committee or institutional review board at each national or local site, and all participants provided written informed consent before enrollment.17
Secondary prevention medication classes.
There were 3 possible recommended medication classes for each patient: antihypertensive ([AH]: renin-angiotensin system modulator, calcium antagonist, β-blocker, diuretic, or/and α-blocker), antithrombotic ([AT]: antiplatelet or/and anticoagulation), and lipid modifier (LM) therapy (i.e., statin, ezetimibe, fenofibrate, niacin, or/and omega-3 fatty acids). Categorization of VISP patients by prescribed secondary prevention medications were as follows: none (n = 63); AH only (n = 103); LM only (n = 9); AT only (n = 355); AH + LM (n = 69); AH + AT (n = 1150); LM + AT (n = 263); and AH + LM + AT (n = 1,668). Expert consensus practice guidelines for secondary stroke prevention recommend the use of antiplatelet medication and statin therapy; both strategies have been linked to a proven reduction in the risk of recurrent stroke and other cardiovascular events (Class I, Level A; and Class I, Level B).1 All participants were considered to be eligible for AT medication and LM therapy. Subjects with established or newly diagnosed hypertension were considered to be eligible for AH medication based on Class I, Level A evidence.1 Initiation of AH for patients without known hypertension and with a blood pressure (BP) <140 mm Hg systolic and <90 mm Hg diastolic is of uncertain benefit (Class IIb, Level C evidence).1 With our main approach, evidence-based secondary prevention was defined using an appropriateness algorithm for various secondary prevention strategies based on a published study by Mukherjee et al.12 Composite appropriateness level was determined for each patient as follows: level 0, none of the indicated medications prescribed; level I, 1 medication prescribed even though 3 medications indicated; level II, 2 medications prescribed even though 3 medications indicated or 1 medication prescribed even though 2 medications indicated; and level III, all indicated medications were prescribed. If the patient did not have a diagnosis of hypertension and was prescribed both of the 2 other indicated medication classes (LM + AT), that patient's appropriateness was defined as level III. Using an alternate and simplified approach, we also separately examined the impact of prescribed number of secondary prevention medication classes on risk of outcome.
Assessment of outcomes.
The primary outcome for this analysis was ischemic stroke. Secondary outcome was a composite of ischemic stroke, coronary heart disease (CHD) including myocardial infarction, coronary revascularization, cardiac resuscitation, and fatal CHD, or vascular death as major vascular events. Tertiary outcome was death of any cause.
Statistical analysis.
Data were summarized as mean ± SD or number of subjects (percentage), as appropriate. Comparisons across the groups were examined using the χ2 test or Fisher exact test for categorical variables and 1-way analysis of variance, followed by the Dunnett test for multiple comparisons, for continuous variables. Subjects with no medication for secondary prevention were the referent group for purposes of comparison. Baseline demographic and clinical covariates were preselected based on previous studies of factors that influence vascular events after ischemic stroke. Backward elimination Cox proportional hazard regression analyses were performed to estimate the risk of outcome events on 2 years in the following ways: (1) after adjusting for the baseline covariates (p < 0.10) of age, sex, ethnicity, hypertension, diabetes, smoking, history of CHD, history of carotid endarterectomy, systolic BP, body mass index, and serum levels of low-density lipoprotein cholesterol, triglyceride, and high-density lipoprotein cholesterol (model I); and (2) after adjusting for model I plus stroke severity, history of stroke, and history of congestive heart failure that are linked to cardiovascular recurrence or death (model II). Impact of prescribed number of secondary prevention medication classes on risk of outcome was examined by Cox model after adjusting for baseline covariates (p < 0.10). Participants not having outcome events were censored at last follow-up examination, or last visit. Participants lost to follow-up during the course of the study were included in the Cox model until the last contact. The interaction between vitamin therapy (high vs low dose) and each medication class in predicting the risk of vascular events and death was assessed by including the appropriate interaction terms in the model. Results are given by hazard ratio (HR) and its 95% confidence interval (CI). All analyses were conducted using IBM SPSS version 22.0 (IBM Corp., Armonk, NY), and a probability value of <0.05 was considered statistically significant.
RESULTS
Subject characteristics by secondary prevention medication class.
In 3,680 participants in the VISP trial, mean age was 66.3 ± 10.8 years, 37.5% were women, and 79.5% were white. Eighty-one percent received AH, 54.6% LM, and 93.4% AT at last follow-up visits. Overall, 51.0% of total subjects received optimal therapy (appropriateness level III). Baseline demographic and clinical characteristics by appropriateness level are provided in table 1. Compared with those with level 0, subjects receiving optimal therapy were more likely to be older, had higher systolic BP and body mass index, and higher serum levels of total cholesterol, low-density lipoprotein cholesterol, and triglycerides, had more frequencies of hypertension and diabetes, and more histories of CHD (defined as history of myocardial infarction, angina, coronary angioplasty/stenting, or coronary artery bypass graft surgery) and carotid endarterectomy, whereas serum levels of high-density lipoprotein cholesterol and frequencies of nonwhite and smoker were lower.
Table 1.
Baseline characteristics of study subjects after recent noncardioembolic stroke by evidence-based secondary prevention levelsa
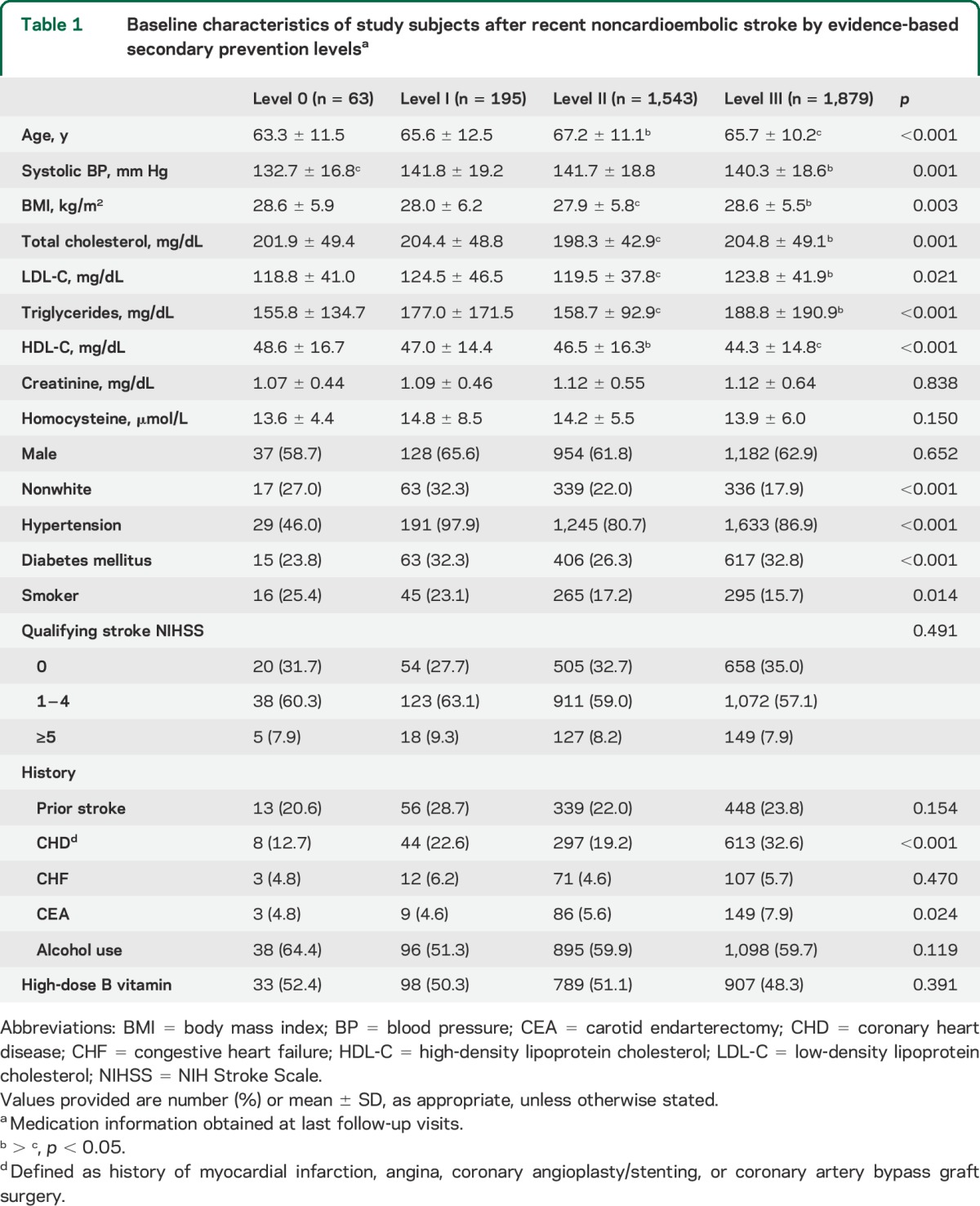
Effect of evidence-based secondary prevention medication on vascular outcomes by appropriateness level.
During an average of 20 months of follow-up, a total of 300 (8.2%) incident stroke and 619 (16.8%) stroke/CHD/vascular deaths, and 216 (5.6%) all-cause deaths were recorded. Results of the adjusted associations between secondary prevention levels and vascular outcomes appear in table 2 and figure 1. The adjusted HR for stroke for level III was 0.37 (95% CI, 0.17–0.82; p = 0.014) vs level 0, and this association remained stable (0.39, 0.18–0.84) after further adjustment for cardiovascular risk factors shown in model II (figure 1A). When compared with level 0, risk of major vascular events (stroke/CHD/vascular death) was lower in the level II group (0.44, 0.25–0.78; p = 0.005) and in the level III group (0.39, 0.22–0.68; p = 0.001), and these associations remained constant after further adjustment (0.45, 0.25–0.80 for level II; 0.39, 0.22–0.69 for level III; figure 1B). The adjusted HR for all-cause death was lower in the level III group (0.36, 0.13–0.99; p = 0.047) vs level 0, and this association also remained similar (0.35, 0.13–0.96) in model II (figure 2). Table e-1 on the Neurology® Web site at Neurology.org provides the impact of simple number of prevention drug classes on recurrent vascular risk and mortality, revealing a significant protective effect of triple class therapy on stroke (0.37, 0.16–0.90), major vascular events (0.34, 0.18–0.62), and all-cause death (0.25, 0.09–0.74). For major vascular events, dual class therapy including AH + LM (0.40, 0.18–0.90), AH + AT (0.39, 0.21–0.72), or LM + AT (0.36, 0.18–0.73) was also more beneficial than monotherapy including AH/LM (0.44, 0.21–0.93) or AT (0.59, 0.31–1.12). AT was associated with lesser risk of stroke (0.38, 0.18–0.79) and major vascular events (0.52, 0.30–0.91) but these associations lost significance after adjusting for multiple covariates. The adjusted HRs of covariates included in the multivariable Cox model (model II) appear in table e-2. Among them, independent predictors of all the primary, secondary, and tertiary outcome events were diabetes and history of stroke. The interaction effect between vitamin dose and each secondary prevention medication level on risk of stroke/major vascular events/all-cause death was not significant (high-dose B vitamin × level I, p = 0.208/p = 0.494/p = 0.712; high-dose B vitamin × level II, p = 0.384/p = 0.438/p = 0.925; and high-dose B vitamin × level III, p = 0.173/p = 0.258/p = 0.799; data not shown).
Table 2.
Effect of evidence-based secondary prevention medication levels on vascular outcomes and all-cause mortality at 2 years in populations after recent noncardioembolic stroke

Figure 1. Adjusted effect of drug treatment on stroke (A) and major vascular events (B) after noncardioembolic stroke.
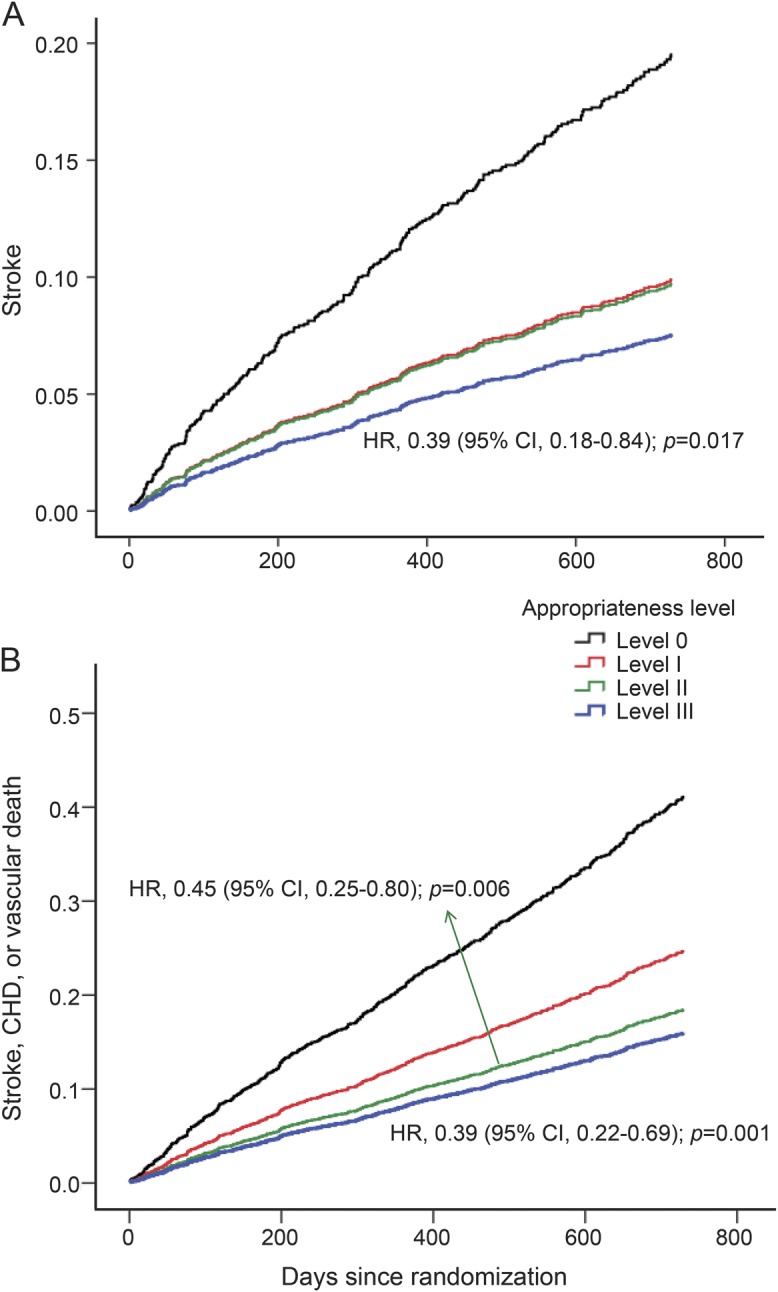
Secondary prevention medication classes were categorized by appropriateness level strata (0 to III) depending on the number of the drugs prescribed divided by the number of the drugs potentially indicated for each patient (0 = none of the indicated medications prescribed, and III = all indicated medications prescribed). CHD = coronary heart disease; CI = confidence interval; HR = hazard ratio.
Figure 2. Adjusted effect of drug treatment on all-cause death after noncardioembolic stroke.
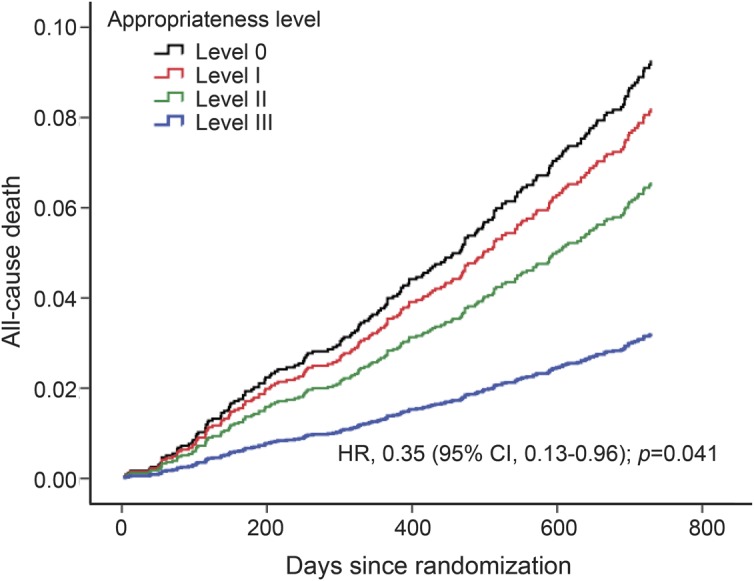
Secondary prevention medication classes were categorized by appropriateness level strata (0 to III) depending on the number of drugs prescribed divided by the number of drugs potentially indicated for each patient. CI = confidence interval; HR = hazard ratio.
DISCUSSION
We observed that optimal combination of evidence-based secondary prevention medication classes was significantly associated with lower rates of stroke, major vascular events, and all-cause death after a recent noncardioembolic stroke. To our knowledge, this is the first study to investigate the effect of combinations of individually proven medication classes on future clinical events among stroke patients. Only optimal prescription, which comprised a combination of 2 to 3 distinct drug classes, was related to substantive and significant relative risk reductions in stroke (61%), major vascular events (61%), and all-cause death (65%), when compared with least optimal prescription, which was use of none of these agent classes. Of note, major vascular events were also reduced by 55% in those with next-highest optimal level of prescription (level II).
The aforementioned favorable results for optimal prescription were roughly similar to what we found when we classified prescriptions by simple number of medication classes: triple AH + LM + AT therapy showed significant lesser risk of stroke by 63%, major vascular events by 66%, and all-cause death by 75%. In accord with the above findings for level II prescription, major vascular events were also reduced by 60% to 64% in those prescribed any dual combination of the 3 drug classes. It also bears mentioning that while patients in the level III prescription group had fewer vascular events and risk of dying over the 2-year period, they were at relatively higher risk of further cardiovascular events because of their greater risk factor burden and comorbidities when compared with the level 0 group. Furthermore, despite this being a clinical trial, the proportion of patients who received level III therapy was 51%; this is compatible with the proportion of patients receiving similar therapy in observational studies of patients with cardiac disease,12–15 which ranges from 46% to 52%.
Findings from a systematic review indicated that treatment with AH by itself after an ischemic or hemorrhagic stroke is associated with significant reductions in recurrent strokes (relative risk, 0.76; 95% CI, 0.63–0.92) and major vascular events (0.79, 0.66–0.95),19 but similar to our current study (table e-1), a beneficial role for AH on reduction of ischemic stroke alone did not achieve statistical significance.19 Whereas the Perindopril Protection Against Recurrent Stroke Study20 trial showed the benefits of BP-lowering in both hypertensive (relative risk reduction, 32%; 95% CI, 17–44) and nonhypertensive (relative risk reduction, 27%; 95% CI, 8–42) patients, diagnosis of hypertension was based on older definitions (presence of baseline hypertension in the trial was defined as ≥160/90 mm Hg). Therefore, with this caveat and the lack of dedicated clinical trial evidence specifically examining the role of AH for recent stroke patients without known hypertension and with a systolic BP <140 mm Hg and diastolic BP <90 mm Hg, use of AH in this patient population is of uncertain benefit.1
In the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study, high-dose atorvastatin decreased the risk of stroke (5-year absolute risk reduction, 2.2%; HR = 0.84; 95% CI, 0.71–0.99) and major vascular events (5-year absolute risk reduction, 3.5%; 0.80, 0.69–0.92).21 Differences in study design limit direct comparisons between the Aggressive Reduction in Cholesterol Levels trial and VISP, but the combination of LM therapy (mostly statin treatment) with AH, AT, or AH + AT appears even more beneficial for the reduction of major vascular events (table e-1).
This study has several limitations. First, because patients in clinical practice are at an increased overall cardiovascular risk than those in clinical trials,22 we cannot necessarily extrapolate our findings to the overall stroke patient population. Second, the choice of lipid-lowering therapy or AT was at the clinician's discretion, and statins or antiplatelet agents were not distinguished LM/AT such that the individual contributions, dose of statins or antiplatelet agents to outcome in this study could not be analyzed. Third, because stroke prevention may be determined by not just BP-lowering effects, but also by the extent of BP variability,23 the influence of various AH agents, which have differing impact on BP variability, may need to be factored into the design of future studies of this topic. Finally, for secondary prevention drug information, we utilized the database at the last follow-up visits (vs at discharge), which may have influenced our results. However, published studies among patients with cardiac disease on this topic (i.e., optimal combination secondary prevention therapy vs outcome) were focused on treatments before discharge from the hospital and generally had a single outcome determination time point (6 months or 1 year) and endpoint (mortality).12–15,24 Furthermore, in most of these cardiac studies, outcome information was obtained by health system record review, phone call interview, or patient questionnaire without an organized follow-up visit system, which made it difficult to obtain drug information during the regular follow-up interval. Strengths of our study include the rigorous procedures of the prospective VISP trial design with a relatively long-term period (2 years),17 and several different clinical outcomes of major significance to stroke patients and their caregivers.
In this study, optimal combination prescription of individually proven secondary prevention medication classes including AH, LM, and AT was associated with a significant and independent lower risk of stroke, major vascular events, and all-cause death in recent noncardioembolic stroke patients over a 2-year follow-up period. However, optimal combination medication itself should not be considered as an absolute secondary prevention, but as an integral part of a comprehensive recurrent stroke prevention strategy that includes efforts to reduce smoking, increase physical activity, and control diet.11 While future studies are certainly warranted to confirm or disconfirm our findings, clinicians may want to ensure that for eligible recent stroke patients, all appropriate secondary prevention drug classes are prescribed and maintained.
Supplementary Material
GLOSSARY
- AH
antihypertensive
- AT
antithrombotic
- BP
blood pressure
- CHD
coronary heart disease
- CI
confidence interval
- HR
hazard ratio
- LM
lipid modifier
- VISP
Vitamin Intervention for Stroke Prevention
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Jong-Ho Park: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data, statistical analysis. Bruce Ovbiagele: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, study supervision.
STUDY FUNDING
Dr. Ovbiagele is supported by award number U01 NS079179 from the National Institute of Neurological Disorders and Stroke.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:2160–2236. [DOI] [PubMed] [Google Scholar]
- 2.Hall MJ, Levant S, DeFrances CJ. Hospitalization for stroke in US hospitals, 1989–2009. NCHS Data Brief 2012;95:1–8. [PubMed] [Google Scholar]
- 3.Rosengren A, Giang KW, Lappas G, Jern C, Toren K, Bjorck L. Twenty-four-year trends in the incidence of ischemic stroke in Sweden from 1987 to 2010. Stroke 2013;44:2388–2393. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Rudd AG, Wolfe CD. Trends and survival between ethnic groups after stroke: the South London Stroke Register. Stroke 2013;44:380–387. [DOI] [PubMed] [Google Scholar]
- 5.Ovbiagele B. National sex-specific trends in hospital-based stroke rates. J Stroke Cerebrovasc Dis 2011;20:537–540. [DOI] [PubMed] [Google Scholar]
- 6.Gu Q, Burt VL, Dillon CF, Yoon S. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: the National Health and Nutrition Examination Survey, 2001 to 2010. Circulation 2012;126:2105–2114. [DOI] [PubMed] [Google Scholar]
- 7.Lackland DT, Roccella EJ, Deutsch AF, et al. Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke 2014;45:315–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ovbiagele B, Goldstein LB, Higashida RT, et al. Forecasting the future of stroke in the United States: a policy statement from the American Heart Association and American Stroke Association. Stroke 2013;44:2361–2375. [DOI] [PubMed] [Google Scholar]
- 9.Wald NJ, Law MR. A strategy to reduce cardiovascular disease by more than 80%. BMJ 2003;326:1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Combination Pharmacotherapy and Public Health Research Working Group. Combination pharmacotherapy for cardiovascular disease. Ann Intern Med 2005;143:593–599. [DOI] [PubMed] [Google Scholar]
- 11.Lonn E, Bosch J, Teo KK, Pais P, Xavier D, Yusuf S. The polypill in the prevention of cardiovascular diseases: key concepts, current status, challenges, and future directions. Circulation 2010;122:2078–2088. [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee D, Fang J, Chetcuti S, Moscucci M, Kline-Rogers E, Eagle KA. Impact of combination evidence-based medical therapy on mortality in patients with acute coronary syndromes. Circulation 2004;109:745–749. [DOI] [PubMed] [Google Scholar]
- 13.Danchin N, Cambou JP, Hanania G, et al. Impact of combined secondary prevention therapy after myocardial infarction: data from a nationwide French registry. Am Heart J 2005;150:1147–1153. [DOI] [PubMed] [Google Scholar]
- 14.Yan AT, Yan RT, Tan M, et al. Optimal medical therapy at discharge in patients with acute coronary syndromes: temporal changes, characteristics, and 1-year outcome. Am Heart J 2007;154:1108–1115. [DOI] [PubMed] [Google Scholar]
- 15.Bramlage P, Messer C, Bitterlich N, et al. The effect of optimal medical therapy on 1-year mortality after acute myocardial infarction. Heart 2010;96:604–609. [DOI] [PubMed] [Google Scholar]
- 16.Bejot Y, Zeller M, Lorgis L, et al. Secondary prevention in patients with vascular disease: a population based study on the underuse of recommended medications. J Neurol Neurosurg Psychiatry 2013;84:348–353. [DOI] [PubMed] [Google Scholar]
- 17.Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA 2004;291:565–575. [DOI] [PubMed] [Google Scholar]
- 18.Bushnell CD, Olson DM, Zhao X, et al. Secondary preventive medication persistence and adherence 1 year after stroke. Neurology 2011;77:1182–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke 2003;34:2741–2748. [DOI] [PubMed] [Google Scholar]
- 20.PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001;358:1033–1041. [DOI] [PubMed] [Google Scholar]
- 21.Amarenco P, Bogousslavsky J, Callahan A, III, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006;355:549–559. [DOI] [PubMed] [Google Scholar]
- 22.Fox KA, Dabbous OH, Goldberg RJ, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). BMJ 2006;333:1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothwell PM, Howard SC, Dolan E, et al. Effects of beta blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol 2010;9:469–480. [DOI] [PubMed] [Google Scholar]
- 24.Mukherjee D, Fang J, Kline-Rogers E, Otten R, Eagle KA. Impact of combination evidence based medical treatment in patients with acute coronary syndromes in various TIMI risk groups. Heart 2005;91:381–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


