Abstract
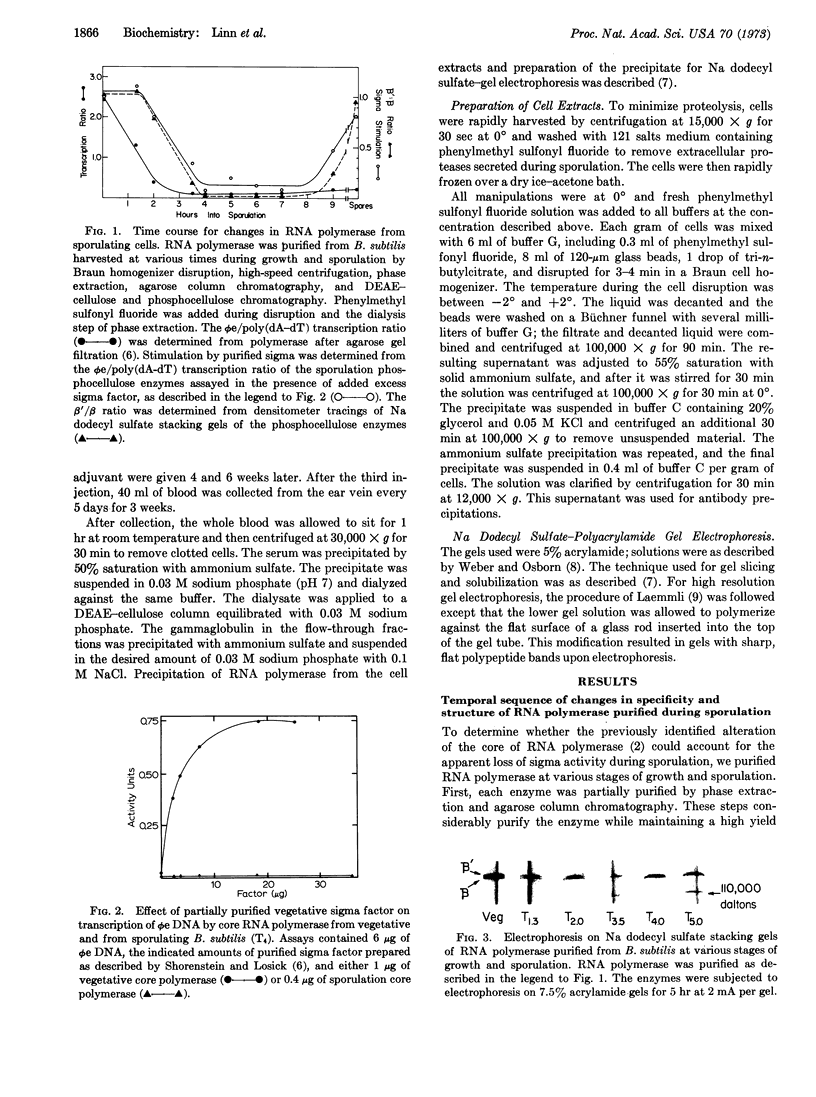
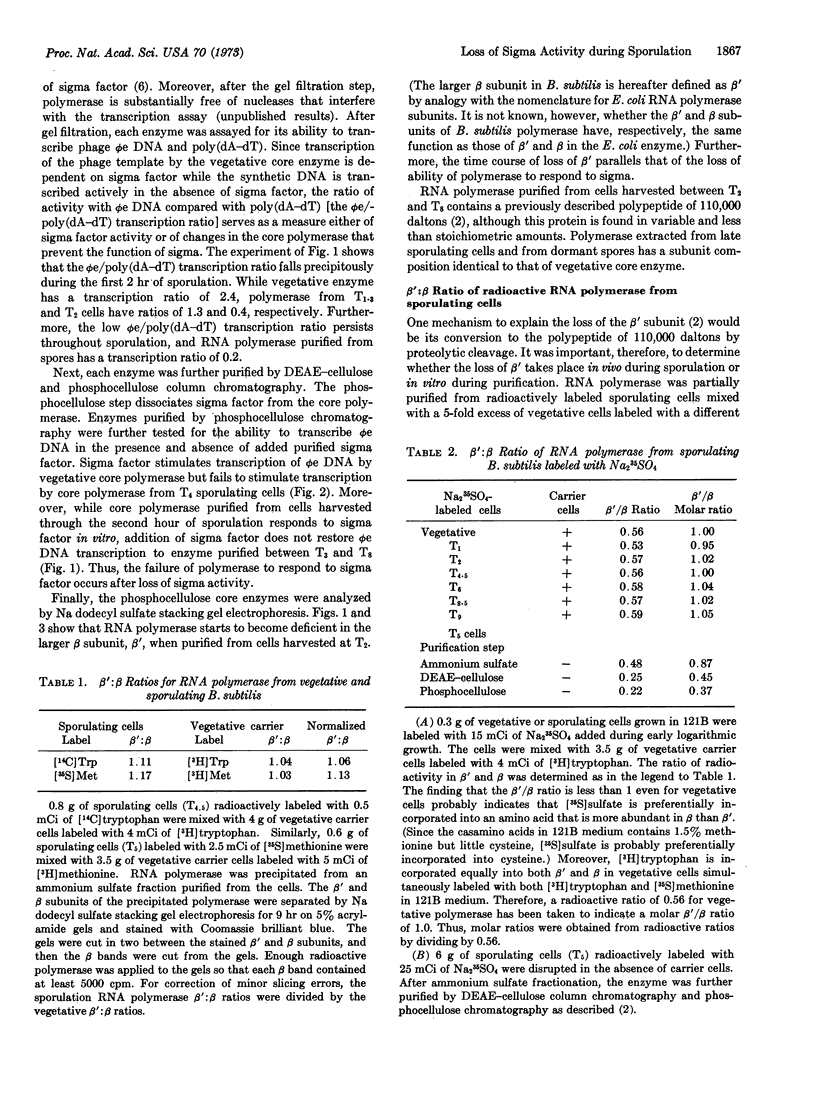
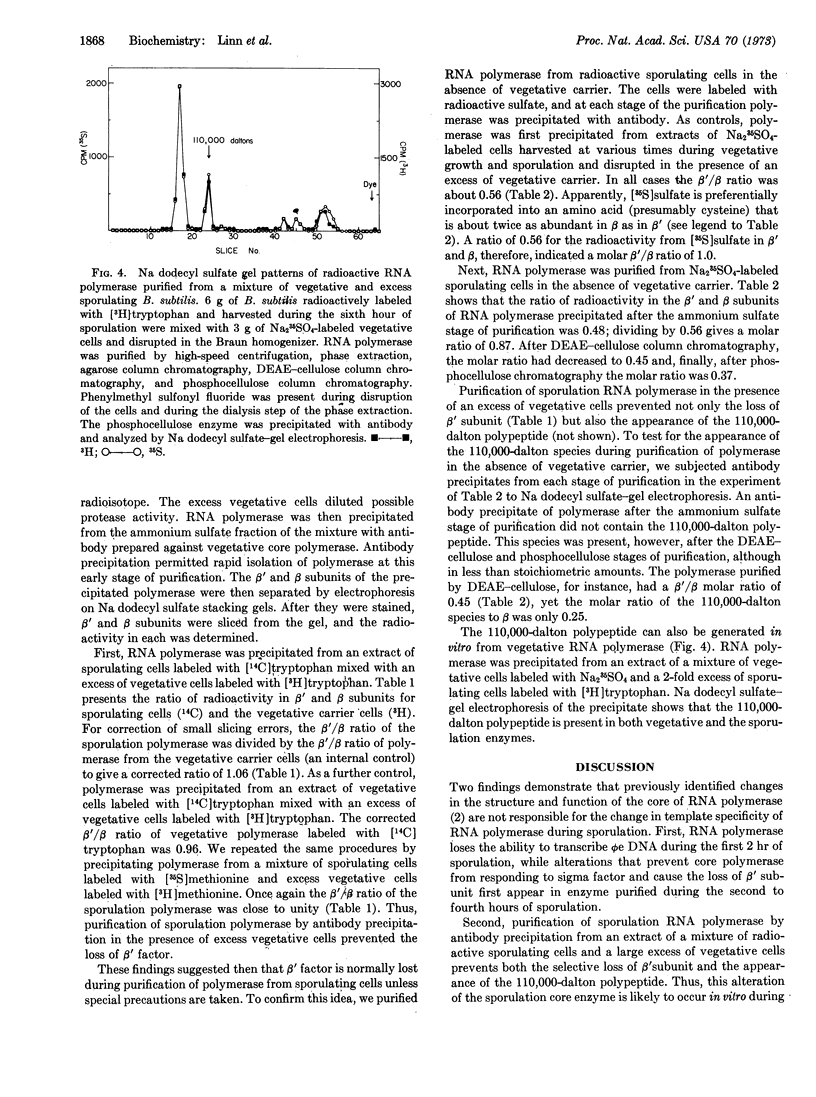
The activity of the sigma subunit of the RNA polymerase of Bacillus subtilis decreases markedly during the first 2 hr of sporulation. Moreover, sigma activity remains deficient throughout the sporulation process and in dormant spores. The time course of changes in RNA polymerase during sporulation indicates that alterations in the core of RNA polymerase occur after the loss of sigma activity. Core RNA polymerase purified after the second and before the ninth hour of sporulation fails to respond to vegetative sigma subunit in vitro and contains variable amounts of a 110,000-dalton polypeptide in place of the β′ subunit. Core RNA polymerase purified from dormant spores has a subunit structure indistinguishable from vegetative core enzyme.
RNA polymerase purified by antibody precipitation from an extract of a mixture of sporulating and excess vegetative cells separately labeled with two different radioisotopes contains β′ subunit and no 110,000-dalton polypeptide. However, RNA polymerase purified from sporulating bacteria in the absence of excess vegetative cells progressively loses the β′ subunit at each stage of purification even in the presence of the protease inhibitor, phenylmethyl sulfonyl fluoride. These findings suggest that the alteration of the β′ subunit is due to proteolysis in vitro.
Keywords: antibody precipitation, β subunits
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Greenleaf A. L., Linn T. G., Losick R. Isolation of a new RNA polymerase-binding protein from sporulating Bacillus subtilis. Proc Natl Acad Sci U S A. 1973 Feb;70(2):490–494. doi: 10.1073/pnas.70.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Losick R., Shorenstein R. G., Sonenshein A. L. Structural alteration of RNA polymerase during sporulation. Nature. 1970 Aug 29;227(5261):910–913. doi: 10.1038/227910a0. [DOI] [PubMed] [Google Scholar]
- Losick R., Sonenshein A. L. Change in the template specificity of RNA polymerase during sporulation of Bacillus subtilis. Nature. 1969 Oct 4;224(5214):35–37. doi: 10.1038/224035a0. [DOI] [PubMed] [Google Scholar]
- Maia J. C.C., Kerjan P., Szulmajster J. DNA-dependent RNA polymerase from vegetative cells and from spores of Bacillus subtilis. IV. Subunit composition. FEBS Lett. 1971 Mar 22;13(5):269–274. doi: 10.1016/0014-5793(71)80238-7. [DOI] [PubMed] [Google Scholar]
- Millet J., Kerjan P., Aubert J. P., Szulmajster J. Proteolytic conversion in vitro of B. subtilis vegetative RNA polymerase into the homologous spore enzyme. FEBS Lett. 1972 Jun 1;23(1):47–50. doi: 10.1016/0014-5793(72)80281-3. [DOI] [PubMed] [Google Scholar]
- Reysset G., Millet J. Characterization of an intracellular protease in B. subtillus during sporulation. Biochem Biophys Res Commun. 1972 Oct 17;49(2):328–334. doi: 10.1016/0006-291x(72)90414-7. [DOI] [PubMed] [Google Scholar]
- Sonenshein A. L., Roscoe D. H. The course of phage phi-e infection in sporulating cells of Bacillus subtilis strain 3610. Virology. 1969 Oct;39(2):265–275. doi: 10.1016/0042-6822(69)90047-6. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]