Abstract
Background
Coronary artery disease has a high prevalence among lung transplant recipients and has historically been a contraindication to transplant at many institutions. In patients with mild-to-moderate coronary artery disease (Mod-CAD) undergoing lung transplant, outcomes are not well defined.
Methods
All patients who underwent pulmonary transplantation from January 1996 through November 2010 with pretransplant coronary angiogram were included in our study. Recipients of multivisceral, redo, and lobar lung transplants and those who underwent pretransplant coronary revascularization were excluded. Patients were grouped into Mod-CAD or no-coronary artery disease group (No-CAD). Primary end point was overall survival. Secondary end points were 30-day events and the need for posttransplant coronary revascularization.
Results
Approximately 539 patients were included in the study: 362 in the No-CAD, 177 in the Mod-CAD group. Patients with Mod-CAD were predominantly male, older, and had a higher body mass index. No difference in either perioperative morbidity and mortality (Mod-CAD, 4.2% vs. No-CAD 3.3%, P=0.705) or late overall mortality was shown between groups. Mod-CAD patients had a shorter hospitalization (median: 12 days vs. 14 days, P=0.009) and required a higher rate of late coronary revascularization procedures (PCI: Mod-CAD vs. No-CAD, 0.3% vs. 4.0%, P=0.0035; CABG: Mod-CAD vs. No-CAD, 0.3% vs. 2.3%, P=0.0411).
Conclusions
Mod-CAD does not appear to be associated with increased perioperative morbidity or decreased survival after transplant. Coronary artery disease may worsen and require coronary revascularization in patients with risk factors for disease progression. In these patients, close follow-up and screening for progression of coronary artery disease may help prevent late cardiac morbidity.
Keywords: Lung transplant, Coronary artery disease, Coronary artery bypass, Percutaneous coronary intervention
Coronary artery disease is a progressive and potentially lethal condition that is present in 60% of patients with advanced lung disease attributable to obstructive or restrictive lung processes (1). Although it is generally accepted that untreated, severe coronary artery disease represents an absolute contraindication to lung transplantation, no consensus has been reached on how to appropriately define eligibility for transplant in patients with asymptomatic, mild-to-moderate coronary artery disease (Mod-CAD) (2-4).
The hypothesis that native coronary artery disease in the setting of chronic immunosuppressive therapy demonstrates accelerated progression is now being challenged by a growing body of evidence suggesting that the use of newer immuno-suppressants and plaque stabilizing-agents alters the progression of atherosclerotic disease. Meanwhile, patient survival after any type of lung transplantation has improved minimally over the past 20 years to an average of 5.5 years, with only slightly over 20% of recipients surviving more than 10 years. It is therefore reasonable to postulate that progression of Mod-CAD may not significantly impact long-term survival after lung transplant. Nevertheless, concerns of increased early and late morbidity and mortality in these patients remain.
It has been the policy at our institution to perform lung transplants in carefully selected patients who had been diagnosed with Mod-CAD but were free from symptoms of myocardial ischemia. Our hypothesis is that the impact of Mod-CAD on short-term, as well as long-term, morbidity and mortality in lung transplant recipients is not clinically significant.
Choong et al. (2) have previously attempted to validate this hypothesis. Several authors have investigated the effects of CAD and coronary revascularization on lung transplantation outcomes (3, 5-8). Our study is the first one to investigate the impact of Mod-CAD on survival and morbidity after lung transplant by analyzing a large cohort of patients with a long-term, postoperative follow-up.
RESULTS
From January 1996 to November 2010, approximately 940 consecutive lung transplants were performed at our institution. Of these, 539 were included in the study (17 individuals had severe coronary artery disease on preoperative catheterization; 96 patients underwent a previous CABG, PCI, or CABG concomitant with lung transplant; 202 individuals had no preoperative coronary angiography; 78 patients had either undergone a multivisceral, lobar, or redo transplant and were therefore excluded from the study). When stratified by angiographic severity of coronary artery disease, our groups included 362 patients in the No-CAD and 177 in the Mod-CAD group.
Pretransplant characteristics are compared in Table 1. Patients with Mod-CAD were predominantly male and were, on average, 6 years older than No-CAD patients. Furthermore, COPD was the primary indication for lung transplant in this group, whereas COPD and PF were equally represented diagnoses, followed by cystic fibrosis/bronchiectasis and pulmonary hypertension, in the No-CAD group. Body mass index was statistically significantly higher in patients with coronary artery disease. Ethnicity and lung allocation scores were similar in both groups. Interestingly, systemic hypertension and diabetes mellitus were virtually equally represented in the two study cohorts. Operative data were similar among the two study groups. Cardiopulmonary support was required in 39 cases (15.2%): 29 (16%) recipients in the No-CAD group and 10 (13.2%) recipients in the Mod-CAD, P=0.7321). Only 107 single lung transplants (19%) were performed (No-CAD: 65 (18%) vs. Mod-CAD: 42 (23.7%), P=0.1146). Graft ischemia time was not significantly different among groups (overall graft ischemia time, median (25th–75th): 6 hr (5–8), No-CAD: 6 (5–8), Mod-CAD: 6 (5–8), P=0.7932. In single lung transplant: 5 hr (4–6), No-CAD: 5 (4–6), Mod-CAD: 5 (4–6), P=0.7588. In bilateral lung transplant: 6 hr (6–8), No-CAD: 7 hr (6–8), Mod-CAD: 6 (5–8), P=0.8258).
TABLE 1.
Demographics and preoperative characteristics
| Characteristic | Total (n=539) | No CAD (n=362) | Mild/Mod CAD (n=177) | P |
|---|---|---|---|---|
| Age | <0.0001a | |||
| Median years, (25th–75th) | 57 (50–63) | 55 (47–61) | 61 (56–64) | |
| Min–Max (yr) | 23–78 | 23–77 | 39–78 | |
| Sex | <0.0001a | |||
| Male (%) | 311 (57.7) | 177 (48.9) | 134 (75.7) | |
| Race | 0.1213 | |||
| White (%) | 465 (86.3) | 303 (83.7) | 162 (91.5) | |
| AA (%) | 65 (12.1) | 51 (14.1) | 14 (7.9) | |
| Native Amer. (%) | 1 (0.2) | 1 (0.3) | 0 (0.0) | |
| Asian (%) | 2 (0.4) | 2 (0.6) | 0 (0.0) | |
| Other/NK (%) | 6 (1.1) | 5 (1.4) | 1 (0.6) | |
| Era of transplant | 0.8428 | |||
| 1995–1999 (%) | 107 (19.9) | 71 (19.6) | 36 (20.3) | |
| 2000–Present (%) | 432 (80.1) | 291 (80.4) | 141 (79.7) | |
| Diagnosis | 0.0011a | |||
| COPD (%) | 242 (44.9) | 151 (41.7) | 91 (51.4) | |
| PF (%) | 227 (42.1) | 149 (41.2) | 78 (44.1) | |
| CF/Bronchiectasis (%) | 44 (8.2) | 40 (11.0) | 4 (2.3) | |
| PH (%) | 7 (1.3) | 5 (1.4) | 2 (1.1) | |
| Other (%) | 19 (3.5) | 17 (4.7) | 2 (1.1) | |
| Comorbidities | ||||
| Diabetes (%) | 53 (9.8) | 39 (10.8) | 14 (7.9) | 0.2943 |
| Hypertension (%) | 135 (25.0) | 85 (23.5) | 50 (28.2) | 0.2303 |
| Hypercholesterolemia (%) | 36 (6.7) | 25 (6.9) | 11 (6.2) | 0.7627 |
| AFib (%) | 25 (4.6) | 16 (4.4) | 9 (5.1) | 0.7303 |
| BMI (recipient) (n=423) | <0.0001a | |||
| Median (range) | 25 (15–48) | 24 (15–48) | 25 (15–32) | |
| End match LAS (n=261) | 0.6473 | |||
| Median (range) | 40 (31–95) | 40 (31–95) | 39 (31–91) |
Indicates statistical significance.
AA, African American; Native Amer, Native American; NK, not known; COPD, chronic obstructive pulmonary disease; PF, pulmonary fibrosis; CF, cystic fibrosis; PH, pulmonary hypertension; AFib, atrial fibrillation; BMI, body mass index; LAS, lung allocation score (data available only from year 2005 on).
Clinical outcomes are illustrated in Table 2. In patients with Mod-CAD, total postoperative length of stay was significantly shorter compared with No-CAD patients. However, there was no difference in ICU length of stay (LOS), perioperative events, or 30-day mortality in the two study groups. When unadjusted logistic regression analysis for 30-day mortality was performed, preoperative diagnosis of primary pulmonary hypertension (OR 15.73, 95% CI 1.99–92.10, P=0.0131) and transplantation occurring before the year 2000 (OR 6.07, 95% CI 2.39–16.07, P=0.0002) were determined to be risk factors for perioperative mortality. Age, sex, ethnicity, preexisting comorbidities (diabetes mellitus, atrial fibrillation), and the diagnosis of pulmonary fibrosis were found to have no effect on perioperative mortality. During follow-up posttransplant, recipients with Mod-CAD underwent coronary revascularization at a higher rate than patients without coronary artery disease (Table 2). All CABGs occurred after 5 years from transplant. The 5-year PCI event rate for patients with Mod-CAD was 2.6% (0% in the No-CAD group). Descriptions of preoperative angiograms of patients who underwent postoperative revascularization are reported in Table 3. Seven (70%) patients with preoperative Mod-CAD that required CABG and/or PCI after transplant had multiple vessel disease and in-series lesions at their baseline angiogram. Furthermore, virtually all patients who underwent posttransplant revascularization had left main stem (LMA) and/or left anterior descending artery (LAD) associated with right coronary artery (RCA) disease, preoperatively as an expression of diffuse coronary artery disease. None of those patients had perioperative ischemic events. Among the recipients with Mod-CAD who underwent post-operative coronary revascularization (PCI or CABG), five developed diabetes mellitus, eight developed systemic arterial hypertension, and five developed hypercholesterolemia in the interval period between transplant and PCI/CABG.
TABLE 2.
Clinical outcomes
| Characteristic | Total (n=539) | No CAD (n=362) | Mild/Mod CAD (n=177) | P |
|---|---|---|---|---|
| Postop-Op LOS (d)b | 13 (0, 249) | 14 (0, 249) | 12 (1, 180) | 0.0094c |
| Postop ICU LOS (d)a | 3 (0, 197) | 3 (0, 197) | 3 (0, 172) | 0.1837 |
| Postop Vent (d)a | 1 (0, 144) | 1 (0, 144) | 1 (0, 56) | 0.6198 |
| Mortality (30 d) | 19 (3.5%) | 12 (3.3%) | 7 (4.2%) | 0.7052 |
| Perioperative Events | ||||
| MI | 7 (1.3%) | 4 (1.1%) | 3 (1.7%) | 0.5700 |
| CVA | 20 (3.7%) | 14 (3.9%) | 6 (3.4%) | 0.7830 |
| Dialysis | 39 (7.2%) | 31 (8.6%) | 8 (4.5%) | 0.0888 |
| Readmission (30 d) | 238 (44.7%) | 153 (43.1%) | 85 (48.0%) | 0.2818 |
| DVT | 6 (1.1%) | 6 (1.7%) | 0 (0.0%) | 0.0850 |
| PE | 21 (3.9%) | 14 (3.9%) | 7 (4.0%) | 0.9607 |
| ARE | 68 (12.6%) | 46 (12.7%) | 22 (12.4%) | 0.9273 |
| Tracheostomy | 93 (17.3%) | 67 (18.5%) | 26 (14.7) | 02705 |
| Eeeding tube | 182 (33.8%) | 129 (35.6%) | 53 (29.9) | 0.1894 |
| Postop AEib | 178 (33.0%) | 113 (31.2%) | 65 (36.7) | 0.2017 |
| Perioperative cardiac events | 225 (41.7%) | 149 (41.2%) | 76 (42.9%) | 0.6943 |
| Any adverse event (perioperative) | 430 (79.8%) | 288 (79.6%) | 142 (80.2%) | 0.8561 |
| PCI during follow-up | 8 (1.5%) | 1 (0.3%) | 7 (4.0%) | 0.0035c |
| CABG during follow-up | 5 (0.9%) | 1 (0.3%) | 4 (2.3%) | 0.0411c |
| Follow-up time (mo)a | 48 (0, 180) | 36 (0, 180) | 48 (0,180) | 0.0166c |
Continuous variables are displayed as median (range). Categorical variables are displayed as number (percent).
“Perioperative” indicates prior to discharge or up until 30 days if discharged before hospital day 30.
Log-rank P value.
LOS, length of stay; ICU, intensive care unit; Vent, mechanical ventilation; MI, myocardial infarction; CVA, cerebrovascular accident; DVT, deep vein thrombosis; PE, pulmonary embolism; ARF, acute renal failure; AFib, atrial fibrillation; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting.
TABLE 3.
Postoperative myocardial revascularization
| Patients N, Group |
Preoperative angio |
Ischemic event |
Postevent angio |
Time after transplant (d) |
Event | Alive | Cause of death |
Follow-up time (d)a |
|---|---|---|---|---|---|---|---|---|
| 1, Non-CAD | Normal | Dyspnea | RCA, CX, LAD >70% | 3218 | CABG | Yes | — | 4636 |
| 2, Non-CAD | Normal | Dyspnea | 70% LAD, 30% RCA, 20% 0M2 | 2085 | CABG | No | NK | 2182 |
| 3, CAD | 50% LM, 50% LAD | Angina 7: | 5% RCA, 75% LAD, 75% Cx, 50% L | M 2271 | CABG | No | Cancer | 2775 |
| 4, CAD | 25% LAD | Palpitations | 80% RCA, 75% CX, 80% LAD | 3305 | CABG | No | NK | 4573 |
| 1st event: dyspnea | 50% RCA, 95% Cx, 75% LAD; | 1st event: 2107 | 1st event: CABG | |||||
| 5, CAD | 25% RCA, 50% LAD | 2nd event: angina | 80% Cx | 2nd event: 3531 | 2nd event: PCI (Stent) | No | Pneumonia | 3614 |
| 6, CAD | 25% LAD, 25%RCA, 50% Cx | Angina | 40% LAD, 30% RCA, 90% Cx | 1048 | PCI (Stent) | Yes | — | 1534 |
| 7, CAD | 50% LAD, 25% Cx, 20% RCA | STEMI-cardiogenic shock | 100%LAD, 70% Cx, 20% RCA | 780 | PCI (Stent) | No | Cardiogenic Shock | 784 |
| 8, CAD | 50% LAD | Angina | 80% LAD | 1364 | PCI (Stent) | Yes | — | 2739 |
| 9, CAD | 25% Cx, 25% LAD | NSTEMl | 25% Cx, 70% LAD, 30% RCA | 2382 | CABG | Yes | — | 3179 |
| 10, CAD | 25% RCA, 25% Cx, 25% LAD | Preop Cath | 100% RCA, 25% Cx, 75% LAD | 1835 | PCI (Stent) | Yes | — | 3987 |
| 11, CAD | 25% RCA, 25% Cx, 25% LAD | Angina | 25% RCA, 50% Cx, 75% LAD | 3207 | PCI (Stent) | No | NK | 3552 |
| 12. CAD | 50% RCA | Positive preop stress test | 95% RCA, 25% LAD, 95% Cx | 1912 | PCI (stent) | No | Cancer | 2217 |
Date of death or September 1, 2012, whichever date came first.
Angio, coronary angiography; LM, left main; LAD, left anterior descending coronary artery; Cx, circumflex coronary artery; RCA, right coronary artery; CABG, coronary aortic bypass grafting; PCI, percutaneous coronary intervention; STEMl, ST segment elevation myocardial infarction; NSTEMI, non-ST segment elevation myocardial infarction; Preop Cath, preoperative cardiac catheterization.
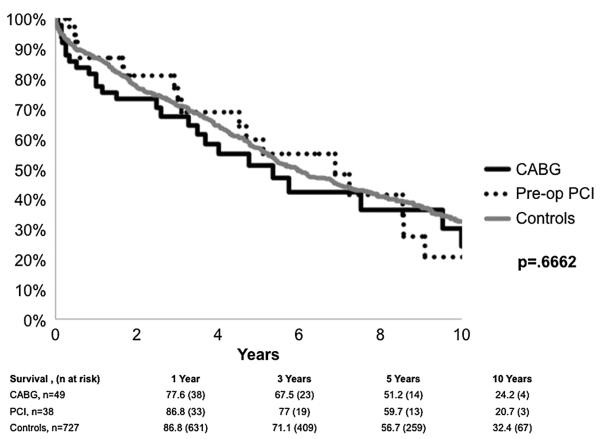
Overall unadjusted survival after transplant is illustrated in Figure 1. Survival among patients with and without CAD was similar. Results of a Cox survival model indicate that the diagnosis of cystic fibrosis/bronchiectasis (HR: 0.58; 95% CI, 0.32–1.00, P=0.048), as compared to COPD, and a more recent era of transplant (after year 2000) (HR,1.92; 95% CI, 1.45–2.55, P<0.001) were associated with a lower risk of postoperative death.
FIGURE 1.
Logistic regression analysis for the composite end point including all postoperative events (Table 4) shows an association with higher risk for older age (per year increase; OR, 1.04; 95% CI, 1.02–1.07, P<0.001), pretransplant atrial fibrillation (OR, 4.98; 95% CI, 1.94–14.71, P<0.001), and a reduced risk for increasing era of transplant (OR, 0.31; 95% CI, 0.18–0.52, P<0.001). Female sex, ethnicity, type of pulmonary disease leading to transplant and preoperative Mod-CAD seemed to have no impact on recorded postoperative adverse events.
TABLE 4.
Multivariate logistic regression for postoperative cardiac (left columns) and any postoperative adverse (right columns) events
| Description | OR cardiac ev | 95% CI cardiac ev | P cardiac ev | OR any ev | 95% CI any ev | P any ev |
|---|---|---|---|---|---|---|
| CAD | 0.79 | 0.53–1.19 | 0.2672 | 1.14 | 0.69–1.93 | 0.6053 |
| Age (per year increase) | 1.04 | 1.02–1.07 | 0.0013a | 1.02 | 0.99–1.05 | 0.2117 |
| Era of transplant (before 2000) | 0.93 | 0.57–1.51 | 0.7672 | 0.28 | 0.17–0.47 | <0.0001a |
| Female sex | 0.76 | 0.51–1.11 | 0.1548 | 1.56 | 0.97–2.54 | 0.0699 |
| Black ethnicity (white ethnicity as reference) | 0.74 | 0.39–1.38 | 0.3516 | 0.83 | 0.41–1.75 | 0.6107 |
| Preexisting comorb | ||||||
| Diabetes | 0.75 | 0.38–1.47 | 0.4101 | 1.17 | 0.48–3.30 | 0.7461 |
| AFib | 5.07 | 1.98–14.916 | 0.0005a | 5.11 | 1.02–93.12 | 0.0473a |
| Hypertension | 1.11 | 0.72–1.70 | 0.6408 | 0.76 | 0.44–1.32 | 0.3241 |
| Hypcreolesterolemia | 1.49 | 0.72–3.13 | 0.2846 | 0.78 | 0.32–2.1 | 0.6021 |
| Diagnosis category (COPD as reference) | ||||||
| PF | 1.55 | 1.03–2.36 | 0.0373a | 1.75 | 1.03–2.99 | 0.0373a |
| CF/Bronchiectasis | 1.12 | 0.47–2.55 | 0.7963 | 2.28 | 0.85–6.70 | 0.1037 |
| PH | 6.07 | 1.13–46.87 | 0.0353a | (b) | (b) | (b) |
| Other | 2.18 | 0.76–6.19 | 0.1429 | 3.28 | 0.77–24.30 | 0.1156 |
Statistical significance. AFib, atrial fibrillation; COPD, chronic obstructive pulmonary disease; PF, pulmonary fibrosis; CF, cystic fibrosis; PH, pulmonary hypertension.
All patients had events.
Ev, events.
DISCUSSION
Lung transplantation is indicated in patients with end-stage pulmonary disease who are otherwise free from concomitant life-threatening comorbidities (4, 9). Coronary artery disease has historically been a contraindication to lung transplant at most institutions in an effort to decrease the risk of postoperative morbidity and mortality for eligible recipients and to derive the most benefit from available donor allografts (2, 3, 10–13). However, many patients referred for lung transplantation have a history of smoking, are men older than 50 years, and have one or more cardio-vascular risk factors (hypertension, diabetes mellitus, and hypercholesterolemia). It is therefore not surprising that a high prevalence of Mod-CAD is found in these individuals (3, 10, 14). We perform coronary angiography in selected patients as part of the pretransplant evaluation. Over the past 20 years, patients with asymptomatic Mod-CAD that were free from other disqualifying comorbidities have not been excluded from lung transplantation at our institution. The rationale for this approach is the expectation that the median survival of a lung transplant recipient is generally reported to be between 5 and 6 years, which is a relatively short interval for significant progression of Mod-CAD (4). Previous studies have also demonstrated acceptable outcomes after lung transplantation in patients with varying degrees of coronary artery disease (2, 3, 5–7).
The present study is the first to analyze a large patient population and to report true long-term outcomes. Our study found that 16% of our transplant recipients who had preoperative coronary angiography had noncritical coronary artery disease. This was not associated with increased perioperative morbidity and mortality or decreased long-term survival. Our results also demonstrate that long-term survival in recipients with Mod-CAD after lung transplant is comparable to a control group with no preoperative angiographic evidence of coronary artery disease. These findings are aligned with previous reports that support offering lung transplant to patients with Mod-CAD (2). The only significant difference with what previously reported is that among the recipients with Mod-CAD, seven (6%) progressed to develop symptomatic coronary artery disease as early as 26 months after transplant and required myocardial revascularization. This seems to be lower than what has been previously reported by Choong et al. (2) where 3 patients (18%) with moderate preoperative CAD developed late symptoms of myocardial ischemia requiring coronary revascularization with no effect on mortality. A smaller patient population sampled at an earlier era (before year 2000) in the study by Choong at al. may in part account for the differences reported. Our data show that transplantation in more recent times (after the year 2000) was associated with better results perhaps because of improved medical care and experience in the management of transplant recipients. Our study showed, indeed, that in patients with longer follow-up times and most commonly greater than the average graft survival reported in the literature (4), the incidence of cardiac events and need for coronary revascularization becomes significant in patients with pretransplant coronary artery disease. Also in these patients, several in-series coronary lesions were often found within the same artery at the time of baseline cardiac catheterization. We therefore believe that careful patient selection and medical management of subclinical coronary artery disease with aspirin and statins that is in line with standard of care may allow satisfactory postoperative outcomes in this patient cohort. Whenever possible, we prefer to not prescribe beta-blocking agents in an effort to avoid bronchoconstrictive sequelae in the pulmonary graft. Close posttransplant follow-up is imperative; we routinely screen recipients with preoperative Mod-CAD with yearly stress test for progression of coronary artery disease. We strive to correct risk factors for progression of CAD (diabetes mellitus, hypertension, and obesity) in all our recipients. We treat severe coronary artery disease in patients with no other disqualifying comorbidities with either pretransplant PCI or concurrent CABG at the time of transplant, if their left ventricular ejection fraction is normal and there is suitable coronary anatomy for revascularization (8).
Our study underscores the importance of preoperative coronary angiography as part of the pretransplant workup in selected patients and supports the use of the traditional cutoff of 40 years of age for requiring coronary angiography.
There are several limitations in our study. It is retrospective in nature, based on the experience of a single, large transplant center, and subject to the limitations of retrospective review. Compliance with medical treatment for coronary artery disease before and after lung transplant could not be assessed. Surrogates for long-term outcomes were limited to the need for myocardial revascularization and overall survival. Nonetheless, we believe that completeness and length of follow-up provides valuable insight into the natural history of this patient population. Furthermore, it suggests that the presence of noncritical coronary artery disease at the time of transplant allows for achievement of satisfactory outcomes and should therefore not be a criterion of exclusion from transplantation. Close recipient follow-up is imperative to maximize patient safety and outcomes.
MATERIALS AND METHODS
The study population consisted of all patients who underwent pulmonary transplantation at our institution from January 1996 through November 2010 and that had also undergone pretransplant coronary angiogram. Patients with a history of myocardial revascularization (percutaneous coronary intervention (PCI) and/or coronary artery bypass grafting (CABG)) either preceding or during lung transplant, redo transplants, and lobar and multivisceral transplants (heart, liver, and kidney) were excluded from the study. Patients who did not have preoperative coronary angiography were also excluded from this analysis.
Two study groups were identified for comparison: asymptomatic patients with documented evidence of Mod-CAD by coronary angiogram that was not intervened upon at the time of transplant (Mod-CAD group) and patients without angiographic evidence of CAD (No-CAD group). Coronary artery disease was graded based on the degree of coronary stenosis and coronary disease burden. Coronary artery disease was classified as mild to moderate in the case of single or multivessel CAD with less than 70% stenoses (<50% stenosis, if the left main coronary artery is involved). To select patients with Mod-CAD, we chose a cutoff of coronary stenosis of less than 70% (<50% stenosis, if left main coronary artery) to include all recipients whose coronary lesions were not severe enough to warrant percutaneous (PCI) or surgical (CABG) revascularization before or at the time of transplant (3, 15). In our clinical practice, the decision to transplant patients with Mod-CAD is unaffected by the number of diseased vessels. We, as others before, consider severe CAD in case of a presence of a coronary artery stenosis of 70% or greater (≥50%, if left main stem is involved) (3). All the patients included in our study had normal ejection fraction.
Patient data were obtained from the Duke Enterprise Data Unified Content Explorer, a Duke University enterprise data warehouse that provides investigators with clinical information collected as a byproduct of patient care (16). These data were supplemented and validated with manual chart review and with data obtained from United Network for Organ Sharing site-specific reports and the Duke Database for Cardiovascular Disease. Survival data were cross-referenced with the Social Security Death Index. Data review was approved, and individual consent was waived by the Duke University School of Medicine institutional review board.
Our primary end point was overall survival (early, within 30 days from the time of transplant and late, after the first postoperative month). Secondary end points were 30-day cardiac and noncardiac events and need for coronary revascularization during follow-up (PCI and/or CABG). Postoperative 30-day cardiac events included mortality, myocardial infarction, cerebrovascular accident, atrial fibrillation, cardiac arrests, deep vein thrombosis, and pulmonary embolism. Noncardiac postoperative events at 30 days included: sepsis or septic shock, dialysis, acute renal failure (defined as normal creatinine at the time of surgery and greater or equal to 3 post-operatively), need for tracheostomy and/or feeding tube placement, UTI or other infectious complications, and readmission.
Statistical analysis was performed using standard methodology and SAS 9.3 and JMP Pro 10.0 (SAS Institute, Cary, NC). Percentages were reported for categorical variables and mean (± standard deviation) or median (interquartile range) for continuous measures depending on the distribution. Pearson chi-square test was used to test for differences in categorical variables across strata, whereas Kruskal-Wallis testing was used for continuous variables. Kaplan-Meier analysis of long-term survival with log-rank testing for statistical significance was conducted. An unadjusted logistic regression model was used to assess 30-day end points. A multivariable logistic regression was used to assess postoperative cardiac events and postoperative all adverse events in the adjusted setting. Adjusted time to death was assessed by means of a multivariable Cox proportional hazards model. Kaplan-Meier methods and the log-rank test were used for assessment of long-term revascularization rate comparisons.
As part of the pretransplant workup, we routinely perform coronary catheterization in all patients aged 40 or older or in those patients with suspected coronary artery disease or a history suggestive of the same. Indications for lung transplant (single vs. double), surgical techniques, and postoperative care have been previously described (4, 9). Because of institutional policy, double lung transplant was the preferred procedure. All donor lungs were procured using the same techniques, and an extracellular preservation solution, such as Celsior (Genzyme Corp Cambridge, MA) or Perfadex (Medisan, Uppsala, Sweden), with antegrade and retrograde flushing was used during procurement.
Acknowledgments
This work was supported by research funds from the Department of Surgery as well as the Division of Cardiothoracic Surgery, Duke University.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Reed RM, Eberlein M, Girgis RE, et al. Coronary artery disease is under-diagnosed and under-treated in advanced lung disease. Am J Med. 2012;125:1228. doi: 10.1016/j.amjmed.2012.05.018. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choong CK, Meyers BF, Guthrie TJ, et al. Does the presence of pre-operative mild or moderate coronary artery disease affect the outcomes of lung transplantation? Ann Thorac Surg. 2006;82:1038. doi: 10.1016/j.athoracsur.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 3.Sherman W, Rabkin DG, Ross D, et al. Lung transplantation and coronary artery disease. Ann Thorac Surg. 2011;92:303. doi: 10.1016/j.athoracsur.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 4.Christie JD, Edwards LB, Kucheryavaya AY, et al. The registry of the international society for heart and lung transplantation: 29th adult lung and heart-lung transplant report-2012. J Heart Lung Transplant. 2012;31:1073. doi: 10.1016/j.healun.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Patel VS, Palmer SM, Messier RH, et al. Clinical outcome after coronary artery revascularization and lung transplantation. Ann Thorac Surg. 2003;75:372. doi: 10.1016/s0003-4975(02)04639-8. discussion 377. [DOI] [PubMed] [Google Scholar]
- 6.Lee R, Meyers BF, Sundt TM, et al. Concomitant coronary artery revascularization to allow successful lung transplantation in selected patients with coronary artery disease. J Thoracic Cardiovasc Surg. 2002;124:1250. doi: 10.1067/mtc.2002.125651. [DOI] [PubMed] [Google Scholar]
- 7.La Francesca S, Shennib H. Coronary artery revascularization followed by single-lung transplantation in a patient with combined end-stage idiopathic pulmonary fibrosis and left main coronary artery stenosis. Tex Heart Inst J. 1995;22:189. [PMC free article] [PubMed] [Google Scholar]
- 8.Castleberry AW, Osho AA, et al. Coronary revascularization in lung transplant recipients with concomitant coronary artery disease. Am J Transplant. 2013:2978. doi: 10.1111/ajt.12435. JM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau CL, Palmer SM, D’Amico TA, et al. Lung transplantation at Duke University Medical Center. Clin Transpl. 1998:327. [PubMed] [Google Scholar]
- 10.Leibowitz DW, Caputo AL, Shapiro GC, et al. Coronary angiography in smokers undergoing evaluation for lung transplantation: is routine use justified? J Heart Lung Transplant. 1994;13:701. [PubMed] [Google Scholar]
- 11.Kaza AK, Dietz JF, Kern JA, et al. Coronary risk stratification in patients with end-stage lung disease. J Heart Lung Transplant. 2002;21:334. doi: 10.1016/s1053-2498(01)00387-4. [DOI] [PubMed] [Google Scholar]
- 12.Henzlova MJ, Padilla ML, Freilich A, et al. Dobutamine thallium 201 perfusion imaging in candidates for lung transplantation. J Heart Lung Transplant. 1995;14:251. [PubMed] [Google Scholar]
- 13.Thaik CM, Semigran MJ, Ginns L, et al. Evaluation of ischemic heart disease in potential lung transplant recipients. J Heart Lung Transplant. 1995;14:257. [PubMed] [Google Scholar]
- 14.Snell GI, Richardson M, Griffiths AP, et al. Coronary artery disease in potential lung transplant recipients 9 50 years old: the role of coronary intervention. Chest. 1999;116:874. doi: 10.1378/chest.116.4.874. [DOI] [PubMed] [Google Scholar]
- 15.Patel MR, Dehmer GJ, Hirshfeld JW, et al. ACCF/SCAI/STS/AATS/ AHA/ASNC/HFSA/SCCT 2012 Appropriate use criteria for coronary revascularization focused update: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, American Society of Nuclear Cardiology, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2012;59:857. doi: 10.1016/j.jacc.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Horvath MM, Winfield S, Evans S, et al. The DEDUCE Guided Query tool: providing simplified access to clinical data for research and quality improvement. J Biomed Inform. 2011;44:266. doi: 10.1016/j.jbi.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]