Abstract
Phenolic glycolipids (PGLs) are polyketide synthase-derived glycolipids unique to pathogenic mycobacteria. PGLs are found in several clinically relevant species, including various Mycobacterium tuberculosis strains, Mycobacterium leprae, and several nontuberculous mycobacterial pathogens, such as M. marinum. Multiple lines of investigation implicate PGLs in virulence, thus underscoring the relevance of a deep understanding of PGL biosynthesis. We report mutational and biochemical studies that interrogate the mechanism by which PGL biosynthetic intermediates (p-hydroxyphenylalkanoates) synthesized by the iterative polyketide synthase Pks15/1 are transferred to the noniterative polyketide synthase PpsA for acyl chain extension in M. marinum. Our findings support a model in which the transfer of the intermediates is dependent on a p-hydroxyphenylalkanoyl-AMP ligase (FadD29) acting as an intermediary between the iterative and the noniterative synthase systems. Our results also establish the p-hydroxyphenylalkanoate extension ability of PpsA, the first-acting enzyme of a multisubunit noniterative polyketide synthase system. Notably, this noniterative system is also loaded with fatty acids by a specific fatty acyl-AMP ligase (FadD26) for biosynthesis of phthiocerol dimycocerosates (PDIMs), which are nonglycosylated lipids structurally related to PGLs. To our knowledge, the partially overlapping PGL and PDIM biosynthetic pathways provide the first example of two distinct, pathway-dedicated acyl-AMP ligases loading the same type I polyketide synthase system with two alternate starter units to produce two structurally different families of metabolites. The studies reported here advance our understanding of the biosynthesis of an important group of mycobacterial glycolipids.
INTRODUCTION
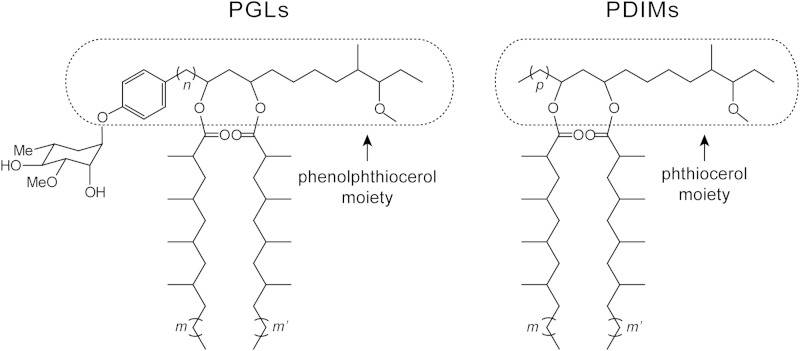
Mycobacterial infections are responsible for devastating morbidity and mortality worldwide (1–5). A critical player in the ability of the mycobacteria to produce disease is a formidable cell envelope believed to be responsible for the intrinsic resilience of the mycobacteria to inhospitable environments, antimicrobial agents, and host immune defenses (6–12). Among the unique components found in the cell envelope of several pathogenic mycobacteria are two structurally related families of glycosylated and nonglycosylated lipids commonly referred to as phenolic glycolipids (PGLs) and phthiocerol dimycocerosates (PDIMs), respectively (for a review, see reference 13). PGLs and PDIMs have unusual lipid scaffolds consisting of β-diol-containing, long-chain, aliphatic polyketides esterified with long-chain, multimethyl-branched fatty acids onto the diol functionality (Fig. 1). PGLs and PDIMs are found in several mycobacterial pathogens (e.g., Mycobacterium tuberculosis strains, Mycobacterium bovis, Mycobacterium leprae, and Mycobacterium marinum) and thought to be constituents of the characteristic mycobacterial outer membrane. Multiple lines of investigation have provided considerable support for the idea that PGLs and PDIMs are implicated in virulence via complex mechanisms of action that are not fully elucidated (14–32). These (glyco)lipids are also believed to strengthen the cell envelope permeability barrier (20, 33) and to increase the bacterium's intrinsic resistance to antimicrobial drugs (20, 22, 34).
FIG 1.
Representative structures of mycobacterial PGLs and PDIMs. The carbon chain variability and glycosyl unit represented are from main variants found in the opportunistic human pathogen M. marinum. m and m', 16 to 20; n, 16 to 22; p, 14 to 22.
The relevance of PGLs in mycobacterial biology underscores the importance of developing a comprehensive knowledge of the PGL biosynthetic pathway, which remains incompletely understood. Our previous studies of the PGL biosynthetic pathway have revealed a functional cooperation between the M. marinum proteins FadD22 (p-hydroxybenzoate-AMP ligase/initiation module) and Pks15/1 (iterative type I polyketide synthase [PKS]) for production of p-hydroxyphenylalkanoate (PHPA) intermediates required for PGL biosynthesis (35, 36). The PHPA intermediates synthesized by the M. marinum FadD22-Pks15/1 iterative system, which is conserved in PGL producers, are thought to be further extended to form the phenolphthiocerol moiety of PGLs (Fig. 1). The extension of the PHPA intermediates has been proposed to be carried out by the modular type I PKS system PpsABCDE, a noniterative synthase complex known to extend fatty acids to form the phthiocerol moiety of PDIMs in M. tuberculosis and conserved in PGL/PDIM producers (13, 16, 25, 37, 38) (Fig. 1). However, the mechanism by which the PHPA intermediates assembled by the FadD22-Pks15/1 system would be transferred to the PpsABCDE system for acyl chain extension remains to be experimentally investigated, and the ability of the PpsABCDE system to extend PHPA intermediates has yet to be validated.
Here, we report mutational and biochemical studies that interrogate the mechanism by which the PHPA intermediates are transferred to PpsA, the first-acting enzyme of the PpsABCDE system, and probe the ability of PpsA to extend these intermediates. The studies were conducted with M. marinum, a nontuberculous mycobacterial species that is the closest genetic relative of the M. tuberculosis complex, is often utilized to model aspects of M. tuberculosis biology, and offers greater experimental tractability than other PGL/PDIM producers (39–42). The findings of our studies support a mechanistic model in which the PHPA intermediates are activated and loaded onto the loading acyl carrier protein (ACPL) domain of M. marinum PpsA by a dedicated PHPA-AMP ligase. Our results also demonstrate that M. marinum PpsA is capable of extending PHPA intermediates. The conservation of the PGL biosynthetic genes across species (13, 43) (Fig. 2) and studies of members of the M. tuberculosis complex (44) strongly suggest that the mechanistic insights into PGL biosynthesis gained here are applicable to other PGL producers. Overall, these studies advance our understanding of the biosynthesis of an important group of mycobacterial cell envelope-associated glycolipids.
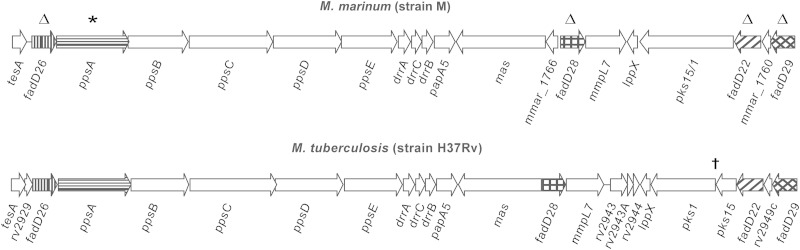
FIG 2.
Conservation of M. marinum and M. tuberculosis chromosomal loci involved in PGL-PDIM production. The five M. marinum genes targeted for mutational analysis (Δ, deletion; *, amino acid substitution) in this study and their respective orthologs in M. tuberculosis are highlighted with pattern-filled arrows. †, pks15/1 is essential for PGL production. The gene pks15/1 is disrupted (split into pks1 and pks15) by natural mutations in M. tuberculosis H37Rv and other Euro-American lineage strains, thus leading to PGL deficiency (18, 64). Additional genes implicated in PGL and/or PDIM production located downstream of fadD29 are not depicted. Adapted from reference 13 with permission of the publisher.
MATERIALS AND METHODS
Culturing conditions, recombinant DNA manipulations, and reagents.
M. marinum strain M (ATCC BAA-535) and its derivatives were cultured under standard conditions in Middlebrook 7H9 medium (Difco) supplemented with 10% ADN (5% bovine serum albumin [BSA], 2% dextrose, 0.85% NaCl) and 0.05% Tween 80 (supplemented 7H9) or on Middlebrook 7H11 agar (Difco) with ADN (supplemented 7H11) (45). The strains used in this study are listed in Table S1 in the supplemental material. Escherichia coli strains were cultured under standard conditions in Luria-Bertani (LB) medium (46). When required, kanamycin (30 μg ml−1), hygromycin (50 μg ml−1), sucrose (2%), and/or 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; 70 μg ml−1) were added to the growth media. DNA manipulations were carried out by standard methods and using E. coli DH5α (Invitrogen) as the primary cloning host (46). All PCR-generated DNA fragments used in plasmid constructions were sequenced to verify fidelity. The plasmids used in this study are listed in Table S2 in the supplemental material. Genomic DNA isolation and plasmid electroporation into mycobacteria were carried out as reported previously (45). Molecular biology reagents were obtained from Sigma, Invitrogen, Novagen, or Qiagen. The oligonucleotides used in this study are listed in Tables S3 and S4 in the supplemental material, and they were purchased from Integrated DNA Technologies, Inc. Solvents and nonradiolabeled chemicals were purchased from Sigma, Acros Organics, or Fisher Scientific. [1-14C]propionate (specific activity, 54 mCi mmol−1) and [carboxyl-14C]p-hydroxybenzoic acid (specific activity, 55 mCi mmol−1) were acquired from American Radiolabeled Chemicals, Inc. The compound 21-(4-hydroxyphenyl)henicosanoic acid (s-PHPA) was synthesized as described in the supplemental material.
Construction of mycobacterial mutants.
The mutants were engineered using the p2NIL/pGOAL19-based flexible cassette method (47) as reported previously (34, 35, 48). A gene-specific mutagenesis cassette delivery vector (see below) was used to construct each mutant. Each vector was electroporated into M. marinum, and the transformants with a potential single crossover (blue colonies) were selected on supplemented 7H11 containing hygromycin, kanamycin, and X-Gal. Potential single-crossover-bearing clones were grown in antibiotic-free supplemented 7H9 and then plated for single colonies on supplemented 7H11 containing sucrose and X-Gal. White colonies that grew on sucrose plates were restreaked onto antibiotic-free and antibiotic-containing plates to identify drug-sensitive clones, a trait indicating a possible double-crossover event with consequent allelic replacement or reversion to wild type (wt). Gene deletions were confirmed by PCR. For PCR analysis, genomic DNA from mutant candidates was used as the template along with two independent mutant-specific primer pairs (see Table S3 in the supplemental material) to produce amplicons permitting differentiation between mutant and wt genotypes based on amplicon size (see Fig. S2 in the supplemental material). Nucleotide substitutions in the M. marinum ppsAS-to-A mutant were confirmed by DNA sequencing. The mutated region was PCR amplified with specific primers (see Table S3 in the supplemental material), and the resulting amplicon was sequenced (see Fig. S2 in the supplemental material).
Construction of mutagenesis cassette delivery vectors.
The following mutagenesis cassette delivery vectors were constructed: p2NILGOALc-ΔfadD22c, carrying a fadD22 (MMAR_1761) deletion cassette (ΔfadD22c); p2NILGOALc-ΔfadD26c, carrying a fadD26 (MMAR_1777) deletion cassette (ΔfadD26c); p2NILGOALc-ΔfadD28c, carrying a fadD28 (MMAR_1765) deletion cassette (ΔfadD28c); p2NILGOALc-ΔfadD29c, carrying a fadD29 (MMAR_1759) deletion cassette (ΔfadD29c); and p2NILGOALc-ppsAc, carrying a ppsA (MMAR_1776) mutagenesis cassette (ppsAc) (see Fig. S1 and Table S2 in the supplemental material). Each cassette was constructed by joining a 5′ arm and a 3′ arm using splicing by overlap extension (SOE) PCR (49). The primers and amplicon sizes are shown in Table S4 in the supplemental material. Each PCR-generated cassette was first cloned into pCR2.1-TOPO (Invitrogen), verified for sequence fidelity, and then subcloned into p2NIL (47). The cassettes ΔfadD22c, ΔfadD26c, ΔfadD28c, ΔfadD29c, and ppsAc were cloned into p2NIL as SalI-NotI, HindIII-KpnI, BamHI-NotI, HindIII-PmlI, and HindIII-HpaII fragments, respectively. Each resulting p2NIL-mutagenesis cassette construct and the plasmid pGOAL19 (47) were digested with PacI, and then the PacI fragment with the marker cassette (GOALc) of pGOAL19 was ligated to the p2NIL construct backbones to generate the final delivery vectors. The configuration of each final cassette was as follows: ΔfadD22c consisted of fadD22's 1,000-bp upstream segment plus fadD22's first 2 codons plus fadD22's last 2 coding codons plus stop codon plus 983-bp downstream segment; ΔfadD26c consisted of fadD26's 962-bp upstream segment plus fadD26's first 5 codons plus fadD26's last 3 coding codons plus stop codon plus 929-bp downstream segment; ΔfadD28c consisted of fadD28's 947-bp upstream segment plus fadD28's first 5 codons plus fadD28's last 4 coding codons plus stop codon plus 935-bp downstream segment; ΔfadD29c consisted of fadD29's 947-bp upstream segment plus fadD29's first 4 codons plus fadD29's last 5 coding codons plus stop codon plus 976-bp downstream segment; and ppsAc consisted of a 1,832-bp segment with Ser-to-Ala substitution mutations in the center.
Construction of pCP0 derivatives.
Plasmids pCP0-fadD22, pCP0-fadD26, pCP0-fadD28, pCP0-fadD29, and pCP0-ppsA, constitutively expressing fadD22, fadD26, fadD28, fadD29, and ppsA, respectively, from the mycobacterial hsp60 promoter were constructed using the expression vector pCP0 (34). DNA fragments each encompassing an M. marinum gene and its predicted ribosome-binding site were PCR generated using the primer pairs shown in Table S4 in the supplemental material. The fragments were first cloned into pCR2.1-TOPO, verified for sequence fidelity, and then subcloned into pCP0. The fadD22, fadD26, fadD28, fadD29, and ppsA fragments were cloned into pCP0 as HindIII-HpaI, EcoRI-HindIII, NheI-HindIII, EcoRI-HindIII, and HpaI-NheI inserts, respectively.
Analysis of PGLs and PDIMs.
Four-day-old cultures were diluted to an optical density at 595 nm (OD595) of 0.6 in supplemented 7H9 and loaded into 12-well plates (1 ml per well). [14C]propionate (which labels both PDIMs and PGLs) or [14C]p-hydroxybenzoic acid (which selectively labels PGLs) was added to each well (0.2 μCi ml−1), and the plates were incubated for 24 h (30°C, 170 rpm). After incubation, the OD595 of the cultures was measured in a plate reader (Beckman Coulter, Inc.) and the cells were harvested for apolar lipid fraction extraction with a biphasic mixture of methanolic saline and petroleum ether as reported previously (35, 50). Lipid extracts were subjected to radiometric thin-layer chromatography (radio-TLC) for analysis of 14C-labeled PGLs and 14C-labeled PDIMs as described earlier (35, 50). Developed TLC plates were exposed to phosphor screens, which were scanned using a Cyclone Plus Storage Phosphor System (PerkinElmer, Inc.).
Construction of pETDuet-PpsA.
M. marinum ppsA was assembled from three fragments: NT1 (5′-end fragment, 2,100 bp); NT2 (middle fragment, 2,000 bp); and NT3 (3′-end fragment, 809 bp). The fragments were PCR amplified from genomic DNA with fragment-specific primers (see Table S4 in the supplemental material) and independently cloned into pCR2.1-TOPO to create pCR2.1TOPO-PpsA-NT1, pCR2.1TOPO-PpsA-NT2, and pCR2.1TOPO-PpsA-NT3. The inserts were verified for sequence fidelity. Subsequently, the NT1 insert of pCR2.1TOPO-PpsA-NT1 was recovered as a BamHI-AscI fragment and subcloned into pETDuet-1 (Novagen) digested with the same enzymes to create pETDuet-PpsA-NT1. Then, the NT2 insert of pCR2.1TOPO-PpsA-NT2 was recovered as an SpeI-AscI fragment and subcloned into SpeI-AscI linearized pETDuet-PpsA-NT1, resulting in pETDuet-PpsA-NT1NT2. Finally, the NT3 insert of pCR2.1TOPO-PpsA-NT3 was recovered as an AscI-HindIII fragment and subcloned into pETDuet-PpsA-NT1NT2 digested with the same enzymes to generate pETDuet-PpsA. This final plasmid was introduced into E. coli BAP1 (51) for isopropyl β-d-1-thiogalactopyranoside (IPTG)-inducible production of N-terminally His6-tagged PpsA.
Construction of pCOLADuet-FadD29.
M. marinum fadD29 was PCR amplified from genomic DNA with gene-specific primers (see Table S4 in the supplemental material) and cloned into pCR2.1-TOPO to create pCR2.1TOPO-FadD29. The insert of pCR2.1TOPO-FadD29 was verified for sequence fidelity, recovered as an EcoRI-NotI fragment, and subcloned into pCOLADuet-1 (Novagen) linearized with EcoRI and NotI to generate pCOLADuet-FadD29. This final plasmid was introduced into E. coli BL21(DE3) (Stratagene) for IPTG-inducible production of N-terminally His6-tagged FadD29.
Overproduction and purification of PpsA.
E. coli BAP1 carrying pETDuet-PpsA was cultured in LB broth supplemented with ampicillin (100 μg ml−1) at 37°C with orbital shaking (220 rpm) to an OD600 of 0.6. When the culture reached the target OD, the incubation temperature was reduced to 18°C, and PpsA expression was induced with IPTG (0.1 mM). After 20 h of additional incubation (18°C, 220 rpm), cells were harvested by centrifugation (6,000 × g, 20 min). The cell pellet was resuspended in lysis buffer (75 mM sodium phosphate [pH 7.5], 500 mM NaCl, 10% glycerol, 10 mM imidazole), and cells were disrupted using a high-pressure homogenizer (Avestin, Inc.). Cellular debris was removed from the lysate by centrifugation (1 h, 12,000 rpm, FX6100 rotor; Beckman Coulter Inc.) followed by subsequent filtration of the supernatant (0.45-μm filter). PpsA was purified from the clarified supernatant by Ni2+ column chromatography using Ni-nitrilotriacetic acid (Ni-NTA) Superflow resin according to the manufacturer's instructions (Qiagen). Proteins were eluted from the column using an imidazole gradient in lysis buffer run with an ÄKTA UPC10 fast protein liquid chromatography (FPLC) system (GE Healthcare). PpsA eluted at 125 mM imidazole. Fractions with the best purity were identified by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), pooled, concentrated using Amicon Ultra-15 centrifugal filter devices (50-kDa cutoff), and buffer exchanged into 100 mM sodium phosphate buffer (pH 7.2) using PD-10 desalting columns (GE Healthcare). Protein samples were aliquoted with 25% glycerol final concentration, flash-frozen in liquid nitrogen, and stored at −80°C (see Fig. S3 in the supplemental material). A typical yield was 14 mg liter−1.
Overproduction and purification of FadD29.
E. coli BL21(DE3) carrying pCOLADuet-FadD29 was cultured in LB broth containing kanamycin at 37°C with orbital shaking (220 rpm). When the culture reached an OD600 nm of 0.6, the incubation temperature was reduced to 30°C, and FadD29 production was induced with IPTG (0.1 mM). After 20 h of additional incubation (30°C, 220 rpm), cells were harvested by centrifugation and resuspended in lysis buffer (50 mM Tris-HCl, 500 mM NaCl [pH 7.5], 10 mM imidazole). Cell disruption and removal of cellular debris from the lysate were carried out as noted above. FadD29 was purified from the clarified supernatant by Ni2+ column chromatography. Proteins were eluted from the column using an imidazole gradient in lysis buffer run with an ÄKTA UPC10 FPLC system. FadD29 eluted at 140 mM imidazole. Fractions with the best purity were identified by SDS-PAGE, pooled, concentrated using Amicon Ultra-15 centrifugal filter devices (30-kDa cutoff), and buffer exchanged into 50 mM Tris-HCl–300 mM NaCl (pH 7.8) using PD-10 desalting columns. Purified proteins were aliquoted with 25% glycerol (final concentration), flash-frozen in liquid nitrogen, and stored at −80°C (see Fig. S3 in the supplemental material). A typical yield was 6.3 mg liter−1.
In vitro FadD29-PpsA reconstituted system.
The standard complete reaction mixture (250 μl) contained 0.5 μM FadD29, 5.0 μM PpsA, 2.5 μM s-PHPA, 100 mM sodium phosphate buffer (pH 7.2), 1 mM tris(2-carboxyethyl)phosphine (TCEP), 0.5 mM MgCl2, 0.5 mM ATP, 10% glycerol, 0.1 mM malonyl coenzyme A (malonyl-CoA) thioester, and 1 mM NADPH. After incubation (30°C, 2.5 h), the reaction was quenched by the addition of 50 μl of 1 M NaOH, and then the mixture was incubated at 65°C for 20 min to release the covalently bound products from PpsA. Following the alkaline hydrolysis, the reaction mixture was acidified with 50 μl of 2 M HCl, and the reaction products were extracted with ethyl acetate (750 μl × 2). The recovered organic layer was evaporated to dryness. The dried residual material was dissolved in 50 μl of dichloromethane and analyzed by high-resolution liquid chromatography-mass spectrometry (LC-MS) as described below. Control reaction mixtures lacking selected components were also set up, treated, and analyzed in the same manner as the complete reaction mixture.
Liquid chromatography-mass spectrometry instrumentation and analysis.
Mass spectral data were collected on an Agilent Technologies G6550A iFunnel high-resolution quadrupole time of flight (Q-TOF) mass spectrometer attached to an Agilent Technologies 1290 ultrahigh-performance liquid chromatography (UHPLC) system. Samples were ionized using Agilent's dual-sprayer Jet Stream electrospray ionization source (Dual AJS ESI) with the analysis performed in negative mode. Chromatography was performed on an Agilent Technologies Poroshell 120 EC-C8 column (2.1 mm by 75 mm, 2.7 μm) using water containing 0.1% formic acid (solvent A) and methanol containing 0.1% formic acid (solvent B) at a flow rate of 350 μl min−1. The UHPLC gradient was 75% solvent B (0 min) to 100% solvent B (15 min), and the analysis was stopped after 20 min. The column was equilibrated under the starting conditions for 10 min and held at 45°C for the entire analysis. The UHPLC stream was diverted to waste for the first 1.5 min of the analysis. Mass spectrometer parameters for the MS were as follows: 225°C drying gas temperature; 17-liters min−1 drying gas flow; 35-lb/in2 nebulizer pressure; 200°C sheath gas temperature; 12-liters min−1 sheath gas flow; 3,500-V capillary voltage; 2,000-V nozzle voltage; and 365-V fragmentor. Data were collected with the instrument set to low mass range (100 to 1,700 m/z) and extended dynamic range conditions (2 GHz mode) at 2 spectra per second. Both centroid and profile data were stored (1.5 to 20 min) with a threshold of 300 counts for MS mode. The reference masses 112.985587 m/z (trifluoroacetate ion) and 966.000725 m/z (HP 921 reference compound + formate ion) were infused into the spray chamber through the second sprayer using an Agilent binary pump. A minimum height of 5,000 counts and a 100 ppm window were used. The instrument was controlled with Agilent MassHunter Workstation Acquisition Software B.05.00. The data were analyzed using Agilent MassHunter Workstation Qualitative Analysis Software B.05.00.
RESULTS AND DISCUSSION
Possible pathways for Pks15/1-to-PpsA PHPA intermediate transfer in M. marinum.
Previous studies have shown that the conserved modular (noniterative) type I PKS system PpsABCDE encoded in the PGL/PDIM biosynthetic gene cluster of M. tuberculosis (Fig. 2) extends fatty acids to form the phthiocerol moiety of PDIMs (Fig. 1). The fatty acids are activated and loaded by the conserved fatty acyl-AMP ligase FadD26 (Fig. 2) onto the loading acyl carrier protein (ACPL) domain of PpsA, the first-acting enzyme of the PpsABCDE system (37, 52). The PpsABCDE system is proposed to also extend pathway-dedicated PHPA intermediates synthesized by the conserved iterative PKS system FadD22-Pks15/1 (Fig. 2) to form the phenolphthiocerol moiety of PGLs (36, 38) (Fig. 1). The mechanism by which PHPA intermediates synthesized by the iterative FadD22-Pks15/1 system would be transferred to the noniterative PpsABCDE system for chain extension has not been experimentally interrogated.
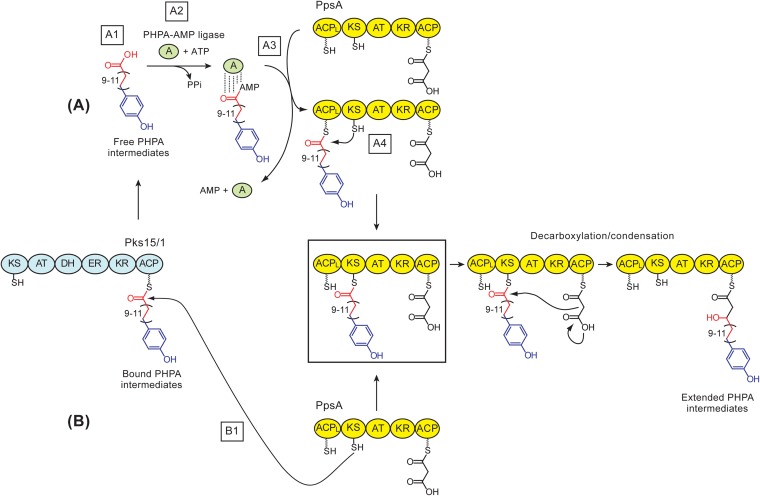
We hypothesized two possible pathways by which this PHPA intermediate transfer could take place in M. marinum (Fig. 3). In one of these pathways (Fig. 3, pathway A), the intermediates would be released first from the phosphopantetheinyl (P-pant) group of the acyl carrier protein (ACP) domain of Pks15/1 (step A1) and subsequently activated (step A2) and loaded (step A3) by a dedicated PHPA-AMP ligase onto the P-pant group of the ACPL domain of PpsA. The PHPA intermediates bound to the ACPL would be subsequently captured by the ketosynthase (KS) domain (step A4) to generate the loaded PpsA (Fig. 3, boxed acyl-PpsA species) ready for KS domain-dependent decarboxylation/condensation. The Pks15/1-to-PpsA PHPA intermediate transfer mechanism represented in pathway A (Fig. 3) emerges from analogy to the fatty acyl-AMP ligase-dependent mechanism of fatty acid activation and loading onto the ACPL domain of PpsA during PDIM biosynthesis in M. tuberculosis (52). Pathway A is further supported by recent studies in the M. tuberculosis complex, leading to the proposal that the conserved fatty acyl-AMP ligase FadD29 (Fig. 2) loads PHPAs onto PpsA (44), yet this idea remains to be experimentally validated.
FIG 3.
Two possible mechanisms for transfer of p-hydroxyphenylalkanoate (PHPA) intermediates to PpsA in M. marinum. (A) PHPA-AMP ligase-dependent model. The model includes the release of PHPAs thioesterified to Pks15/1's ACP domain (A1), activation of free PHPAs by a PHPA-AMP ligase (A2), PHPA-AMP ligase-dependent loading of PHPAs onto PpsA's ACPL domain (A3), and capture of the ACPL domain-bound PHPAs by PpsA's KS domain to yield the fully loaded PpsA (boxed) ready for KS domain-dependent decarboxylation/condensation. (B) Direct KS domain capture model. In this model, the fully loaded PpsA (boxed) is generated directly by the capture of Pks15/1-bound PHPAs by PpsA's KS domain (B1), thus bypassing the need for steps A1 through A4. Decarboxylation/condensation leads to extension of PHPAs by a 2-carbon unit via the first noniterative extension cycle in the formation of phenolphthiocerols. In the scheme, the depicted carbon chain variability in the PHPA-Pks15/1 thioester intermediate is that expected during synthesis of M. marinum PGLs. Adenylated PHPAs are shown bound to the PHPA-AMP ligase via noncovalent linkages (by analogy to other acyl adenylating enzymes). PpsA's C-terminal ACP domain is shown loaded (AT domain dependent) with the malonyl-CoA-derived extender unit. Thiol groups of the phosphopantetheinyl group in the ACP domains and the catalytic Cys in the KS domain are depicted. The hydroxyl group in the extended PHPAs generated from the keto group by action of the KR domain of PpsA is shown. Sections of the PHPA intermediates are color coded based on origin: blue, derived from a p-hydroxybenzoic acid starter unit; red, derived from malonyl extender units via iterative extension cycles; black, section derived from a malonyl extender unit via a noniterative extension cycle. Domain abbreviations: A, adenylation; ACP, acyl carrier protein; ACPL, loading acyl carrier protein; AT, acyltransferase; DH dehydratase; ER, enoylreductase; KR, ketoreductase; KS, ketosynthase.
Notably, pathway A (Fig. 3) requires free PHPA intermediates (step A1), yet sequence analysis of Pks15/1 orthologs does not reveal the presence of a possible thioesterase domain that would conveniently catalyze the release of the PHPA intermediates thioesterified to the P-pant group of the ACP domain of the synthase (13, 36). This does not rule out, however, the possibility that the PHPA intermediates are released without the assistance of an external (self-standing) thioesterase or by the action of one. In the latter option, the thioesterase could be TesA, which is encoded in the PGL/PDIM biosynthetic gene cluster (Fig. 2) and was recently shown to be required for PGL and PDIM production in M. marinum (22, 34).
In the second possible pathway for Pks15/1-to-PpsA PHPA intermediate transfer in M. marinum (Fig. 3, pathway B), the PHPAs thioesterified to the P-pant group of the ACP domain of Pks15/1 would be directly captured by the KS domain of PpsA (step B1), thus bypassing the need for steps A1 to A4. This direct Pks15/1-to-PpsA chain translocation would “skip” the ACPL domain of PpsA. Domain skipping has in fact been demonstrated in a few PKS systems (53–55). Moreover, this direct-capture pathway would have adaptive value because it would not require ATP for PHPA intermediate activation, an essential step in pathway A (step A2). This pathway would also obviate the need to off-load the PHPA intermediates from the ACP domain of Pks15/1 (pathway A, step A1).
A functional ACPL domain in PpsA is required for production of both PGLs and PDIMs in M. marinum.
The ACPL domain of PpsA requires phosphopantetheinylation of the Ser residue embedded in the P-pant group attachment site motif (NCBI Conserved Domains Database [CDD] no. pfam00550/smart00823) of the domain to become functional (56–58). Phosphopantetheinylation of the ACPL domain introduces the P-pant group onto which the fatty acids are loaded with the assistance of the fatty acyl-AMP ligase FadD26 to form the fatty acyl-ACPL domain thioester intermediate required for PDIM biosynthesis in M. tuberculosis (37, 52). Formation of the analogous PHPA-ACPL domain thioester intermediate would be required for PGL production in M. marinum if the Pks15/1-to-PpsA PHPA intermediate transfer takes place by the PHPA-AMP ligase-dependent pathway outlined in Fig. 3 (pathway A). On the other hand, formation of the PHPA-ACPL domain intermediate would not be needed for PGL production if the intermediate transfer proceeds via direct capture by the KS domain of PpsA as depicted in Fig. 3 (pathway B). With these considerations in mind, we probed the essentiality of the P-pant group attachment site of the ACPL domain of PpsA for PGL production by mutational analysis in M. marinum. To this end, we engineered an unmarked, site-directed mutant (M. marinum ppsAS-to-A) with a Ser-to-Ala substitution that eliminated the phosphopantetheinylation site (Ser43) in the ACPL domain of the synthase. We identified Ser43 as the phosphopantetheinylation target in the P-pant group attachment site sequence motif of the ACPL domain of PpsA by sequence analysis (not shown). The M. marinum ppsAS-to-A mutant carried also a Ser42-to-Ala substitution. Ser42 (adjacent to Ser43) was replaced in case it could become a surrogate phosphopantetheinylation target in the absence of Ser43, a potentially confounding scenario.
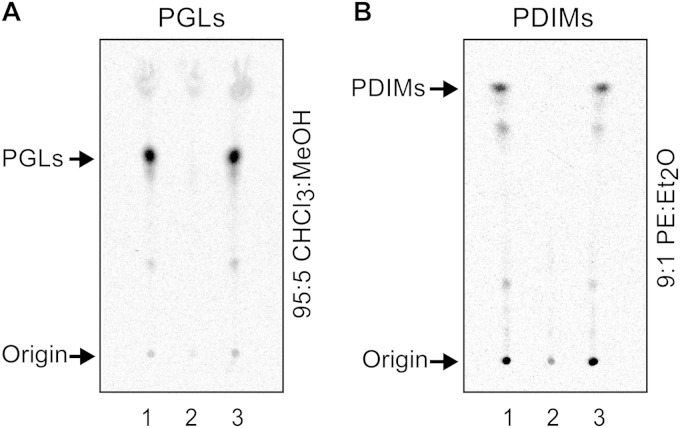
Evaluation of PGL production in the M. marinum ppsAS-to-A mutant by radiometric thin-layer chromatography (radio-TLC) analysis revealed that the strain was PGL deficient (Fig. 4A, cf. lanes 1 and 2). Radio-TLC analysis revealed that the mutant strain was also unable to produce PDIMs (Fig. 4B, cf. lanes 1 and 2), a result in line with previous biochemical studies on PDIM biosynthesis in M. tuberculosis (37, 52). Introduction of the plasmid pCP0-ppsA (expressing M. marinum ppsA) into M. marinum ppsAS-to-A restored the capacity of the mutant to produce both PGLs (Fig. 4A, cf. lanes 2 and 3) and PDIMs (Fig. 4B, cf. lanes 2 and 3). Overall, the findings of the mutational analysis in M. marinum are in line with the idea that the Pks15/1-to-PpsA PHPA intermediate transfer takes place by the PHPA-AMP ligase-dependent pathway outlined in pathway A of Fig. 3. The results also suggest that direct capture of the PHPA intermediates thioesterified to the ACP domain of Pks15/1 by the KS domain of PpsA (Fig. 3, pathway B) is not a transfer mechanism of physiological relevance in M. marinum.
FIG 4.
Inactivation of PpsA's ACPL domain leads to a PGL− PDIM− phenotype in M. marinum. Radio-TLC analysis of 14C-labeled PGLs (A) and 14C-PDIMs (B) from M. marinum wt + pCP0 (lane 1), M. marinum ppsAS-A + pCP0 (lane 2), and M. marinum ppsAS-A + pCP0-ppsA (lane 3). The wild-type (wt) and mutant M. marinum strains carried the vector pCP0 so they could be cultured in the same kanamycin-containing medium used for the complemented M. marinum ppsAS-A + pCP0-ppsA strain. TLC solvent systems used are indicated. CHCl3, chloroform; MeOH, methanol; PE, petroleum ether; Et2O, diethyl ether.
Mutational analysis in M. marinum identifies FadD29 as a PHPA-AMP ligase candidate.
The mechanistic model proposed in pathway A (Fig. 3) includes an acyl-AMP ligase competent to activate and load the PHPA intermediates onto the ACPL domain of M. marinum PpsA (steps A2 and A3, respectively). Aside from the p-hydroxybenzoic acid-specific adenylation domain of M. marinum FadD22 (35, 36), there are three conserved acyl-AMP ligases encoded in the PGL/PDIM biosynthetic gene cluster, i.e., FadD26, FadD28, and FadD29 (13, 16, 25, 37, 43, 52) (Fig. 2). Recent studies in the M. tuberculosis complex have led to the proposal that FadD29 adenylates and loads PHPAs onto PpsA in M. tuberculosis (44), but the idea has not been experimentally explored.
To seek further support for the mechanistic model proposed in pathway A (Fig. 3), we undertook a systematic mutational analysis to conclusively establish the involvement of fadD22, fadD26, fadD28, and fadD29 in PGL and PDIM production in M. marinum and inform the identification of a PHPA-AMP ligase candidate in a nontuberculous mycobacterial species. To our knowledge, the involvement of M. marinum fadD22 and M. marinum fadD29 in the production of PDIMs and PGLs has not been probed by mutational analysis. M. marinum mutants with a transposon insertion in the promoter region of fadD26 or in fadD28 were recently shown to have defects in PGL and PDIM production, but potentially confounding polar effects produced by the transposon upstream of fadD26 on other genes of the pathway (ppsA to papA5 genes [Fig. 2]) preclude unequivocal gene-to-function assignment for fadD26 (20). To conclusively probe the involvement of the four M. marinum acyl-AMP ligases encoded in the PGL/PDIM biosynthetic gene cluster in PGL and PDIM production, we engineered four mutants (M. marinum ΔfadD22, M. marinum ΔfadD26, M. marinum ΔfadD28, and M. marinum ΔfadD29), each with an unmarked, in-frame deletion in one of the four acyl-AMP ligase genes. We then examined the ability of the M. marinum mutants to produce PGLs and PDIMs by radio-TLC analysis.
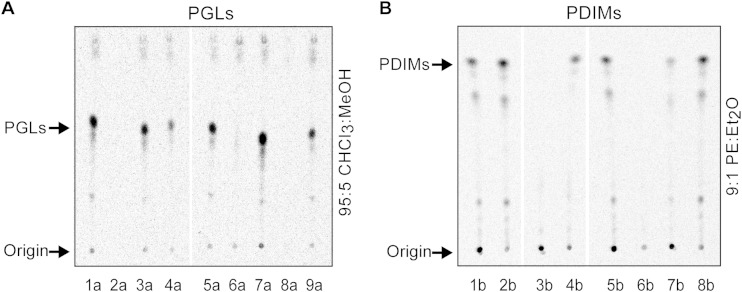
The analysis of the PGL and PDIM production capacity of the fadD gene mutants revealed that deletion of fadD28 led to a PGL− PDIM− phenotype (Fig. 5, cf. lanes 5a and 6a and cf. lanes 5b and 6b), deletion of fadD26 produced selective loss of PDIMs (Fig. 5, cf. lanes 1a and 4a and cf. lanes 1b and 3b), and deletion of fadD22 and fadD29 led to selective loss of PGLs (Fig. 5, cf. lanes 1a and 2a and cf. lanes 1b and 2b for fadD22 and cf. lanes 5a and 8a and cf. lanes 5b and 8b for fadD29). We also constructed and analyzed four corresponding genetic complementation control strains (M. marinum ΔfadD22 + pCP0-fadD22, M. marinum ΔfadD26 + pCP0-fadD26, M. marinum ΔfadD28 + pCP0-fadD28, and M. marinum ΔfadD29 + pCP0-fadD29). Each of these control strains carried a pCP0-based plasmid expressing the specific fadD gene deleted from the genome of the host strain. Radio-TLC analysis demonstrated that episomal expression of the fadD gene reasonably restored (fully or partially) the PGL and/or PDIM production capacity of each of the M. marinum mutants (Fig. 5, lanes 3a, 7a, 9a, 4b, and 7b). The complementation controls indicate that none of the deletions exerted a confounding polar effect preventing the functional assignment of the fadD genes. Overall, the findings of the mutational analysis of fadD genes conclusively demonstrate the specific roles of fadD22, fadD26, fadD28, and fadD29 in PGL and/or PDIM production in M. marinum and, in conjunction with the available biochemical information establishing the function of the p-hydroxybenzoic acid-adenylating FadD22 protein (35, 36, 44), point at M. marinum FadD29 as the likely PHPA-specific AMP ligase involved in PHPA intermediate activation and loading onto PpsA in M. marinum. The conclusions supported by our mutational analysis in the nontuberculous mycobacterial species M. marinum parallel those derived from experiments in the M. tuberculosis complex (35, 44, 59).
FIG 5.
Mutational analysis points at M. marinum FadD29 as a PHPA-AMP ligase candidate. Radio-TLC analysis of 14C-labeled PGLs (A) and 14C-PDIMs (B) from M. marinum wt + pCP0 (lanes 1a, 5a, 1b, 5b), M. marinum ΔfadD22 + pCP0 (lanes 2a and 2b), M. marinum ΔfadD22 + pCP0-fadD22 (lane 3a), M. marinum ΔfadD26 + pCP0 (lanes 4a and 3b), M. marinum ΔfadD26 + pCP0-fadD26 (lane 4b), M. marinum ΔfadD28 + pCP0 (lanes 6a and 6b), M. marinum ΔfadD28 + pCP0-fadD28 (lanes 7a and 7b), M. marinum ΔfadD29 + pCP0 (lanes 8a and 8b), and M. marinum ΔfadD29 + pCP0-fadD29 (lane 9a). The wild-type (wt) and mutant M. marinum strains carried the vector pCP0 so they could be cultured in the same kanamycin-containing medium used for the complemented strains. The TLC solvent systems used are as described for Fig. 4.
An in vitro-reconstituted M. marinum FadD29-PpsA system displays PHPA intermediate activation, transfer, extension, and reduction capacity.
Our mutational analysis in M. marinum supports the view that the Pks15/1-to-PpsA PHPA intermediate transfer takes place via the PHPA-AMP ligase-dependent pathway outlined in Fig. 3 (pathway A) and that FadD29 activates and loads PHPAs onto PpsA in M. marinum. We sought to further probe this mechanistic model by evaluating the ability of M. marinum FadD29 and M. marinum PpsA to functionally cooperate in vitro to produce an expected PHPA extended product. In these experiments, we utilized purified M. marinum FadD29 and PpsA proteins recombinantly produced in E. coli. Since PpsA was needed in its phosphopantetheinylated form, the synthase was coexpressed with the phosphopantetheinyl transferase Sfp to increase posttranslational modification stoichiometry. Sfp is a robust enzyme from Bacillus subtilis (60) that we have previously used to phosphopantetheinylate recombinant M. marinum FadD22 and Pks15/1 proteins (35, 36).
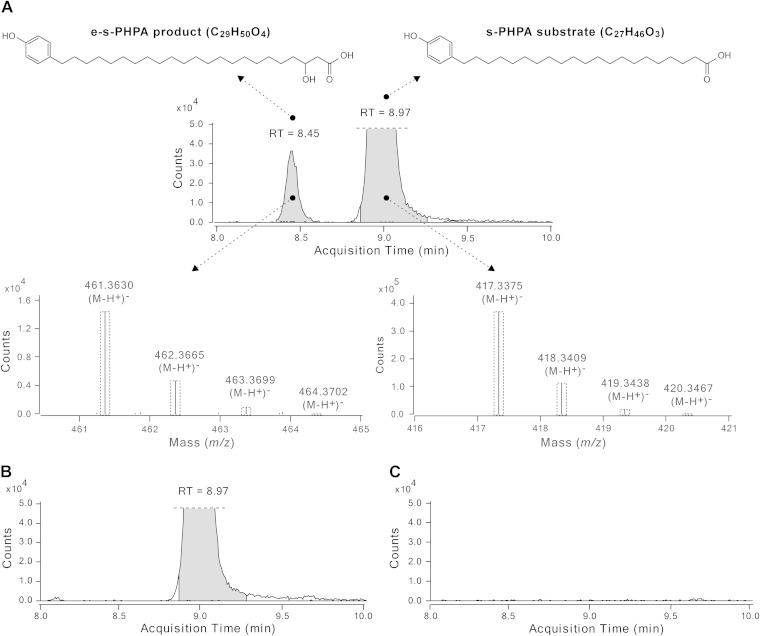
We sought to use a predicted physiological PHPA substrate in these in vitro experiments. To this end, we used the synthetic PHPA substrate 21-(4-hydroxyphenyl)henicosanoic acid (s-PHPA; C27H46O3), which was synthesized by a novel seven-step procedure (described in Fig. S4 in the supplemental material). The s-PHPA substrate corresponds to the released form of one of the most abundant PHPA intermediates produced by the M. marinum FadD22-Pks15/1 system in vitro and in vivo (36). By the canonical polyketide biosynthetic pathway predicted for PpsA based on its domain composition (13, 37) (Fig. 3), the s-PHPA substrate would be expected to be extended by two carbons via a KS domain-dependent decarboxylative Claisen condensation and to undergo ketoreductase (KR) domain-dependent β-keto group reduction to form the corresponding C29 acyl-PpsA thioester intermediate (Fig. 3). The competency of an in vitro M. marinum FadD29-PpsA system to catalyze the formation of this predicted s-PHPA-derived product was investigated by high-resolution LC-MS analysis of the extracted reaction product chemically released from the synthase by alkaline hydrolysis. Representative results of these studies are depicted in Fig. 6 and Table 1.
FIG 6.
Extension of a synthetic PHPA substrate by the M. marinum FadD29-PpsA in vitro system. Extracted ion chromatogram and isotopic distributions for the synthetic PHPA substrate (s-PHPA) and the extended s-PHPA product (e-s-PHPA) in the complete reaction mixture (A) and extracted ion chromatograms from two representative negative-control reaction mixtures, i.e., one containing no malonyl-CoA (B) and the other no s-PHPA (C). The retention time (RT) is indicated in the chromatograms. The dashed-line boxes in the observed isotopic distributions indicate the calculated relative species abundance, which is in agreement with the experimental data.
TABLE 1.
LC-MS analysis of e-s-PHPA formation
| Compound | Formula | Calculated [(M-H+)]− m/z | Reaction mixture | Experimental [(M-H+)]− m/z | Retention time (min) |
|---|---|---|---|---|---|
| s-PHPA | C27H46O3 | 417.3374 | Complete | 417.3375 | 8.97 |
| No Mal-CoA | 417.3374 | 8.97 | |||
| No NADPH | 417.3373 | 9.01 | |||
| No ATP | 417.3374 | 8.97 | |||
| No s-PHPA | NDa | ||||
| No FadD29 | 417.3373 | 9.06 | |||
| No PpsA | 417.3373 | 9.07 | |||
| e-s-PHPA | C29H50O4 | 461.3636 | Complete | 461.3630b | 8.45 |
| No Mal-CoA | ND | ||||
| No NADPH | ND | ||||
| No ATP | ND | ||||
| No s-PHPA | ND | ||||
| No FadD29 | ND | ||||
| No PpsA | ND |
ND, not detected.
ppm = −1.37. Replicates rendered comparable experimental [(M-H+)]− mass data, e.g., 461.3633 (ppm = −0.83), 461.3632 (ppm = −1.07).
Gratifyingly, in vitro incubation of FadD29 and PpsA in the presence of s-PHPA substrate, malonyl-CoA (extender unit donor), ATP (for s-PHPA activation), and NADPH (for β-keto group reduction) led to the formation of a distinct s-PHPA-derived extended product (e-s-PHPA). The experimental mass for the e-s-PHPA product matched that calculated for 3-hydroxy-23-(4-hydroxyphenyl)tricosanoic acid (C29H50O4; calculated m/z [M-H+]− of 461.3636; experimental m/z [M-H+]− of 461.3630), which is the expected released product after s-PHPA extension and reduction by PpsA (Fig. 6A; Table 1). Conversely, the e-s-PHPA product was not detected in control reaction mixtures lacking a single enzyme (FadD29 or PpsA) or a single substrate (malonyl-CoA, NADPH, ATP, or s-PHPA) (Table 1). The unreacted s-PHPA substrate was also detected in the complete reaction mixture (C27H46O3; calculated m/z [M-H+]− of 417.3374; experimental m/z [M-H+]− of 417.3375) (Fig. 6A; Table 1). As expected, the s-PHPA substrate was detected by LC-MS in all control reaction mixtures lacking one of the enzymes or a substrate other than s-PHPA (e.g., malonyl-CoA [Fig. 6B]) but not in the control reaction mixture in which s-PHPA was omitted (Fig. 6C). Formation of the e-s-PHPA product was not observed in reaction mixtures in which FadD29 was replaced by recombinant M. marinum FadD26, although FadD26 was able to load a model fatty acid substrate (dodecanoic acid) on PpsA in vitro (not shown). The competency of M. marinum FadD26 to load PpsA with a fatty acyl starter unit is in line with the in vitro activity previously demonstrated for M. tuberculosis FadD26 (37, 52). The inability of M. marinum FadD26 to replace M. marinum FadD29 in vitro is consistent with our finding that M. marinum ΔfadD29 is PGL deficient despite having fadD26 (Fig. 5A). Altogether, the results with the reconstituted M. marinum FadD29-PpsA system demonstrate that M. marinum FadD29 and M. marinum PpsA cooperate in vitro to produce an expected PHPA extended product and support the genetic studies identifying FadD29 as the PHPA-AMP ligase required for PGL biosynthesis in M. marinum. Overall, the M. marinum FadD29-PpsA in vitro system appears to be competent to catalyze PHPA activation, loading, extension, and reduction in line with the model proposed in Fig. 3. The M. marinum FadD29-PpsA in vitro system along with the M. marinum FadD22-Pks15/1 in vitro system for biosynthesis of PHPAs that we have developed previously (36) lays a valuable foundation for further mechanistic analysis of the enzymatic machinery involved in PGL biosynthesis in M. marinum.
The M. marinum FadD29-PpsA functional partnership established by our in vitro studies represents the first acyl-AMP ligase and type I PKS partnership for acyl starter unit activation and PKS loading established in nontuberculous mycobacteria. Three analogous partnerships have been demonstrated in M. tuberculosis. These are the FadD26-PpsA partnership noted above for PDIM production (37, 52), a FadD32-Pks13 partnership that takes place during mycolic acid biosynthesis (52, 61, 62), and a FadD30-Pks6 partnership believed to be required for production of novel polar lipids (52, 63). To our knowledge, however, the partially overlapping PGL/PDIM biosynthetic pathways provide the first example of two distinct acyl-AMP ligases (i.e., FadD29 and FadD26) loading the same type I PKS (i.e., PpsA) with two alternate starter units (i.e., PHPAs and fatty acids). This bimodal loading strategy allows the bacterium to use the PpsABCDE megasynthase system to generate two structurally different products. Interestingly, recent host-pathogen interaction studies suggest that M. marinum PGLs and M. marinum PDIMs have different roles within a complex immune evasion mechanism (15). It will be interesting to investigate whether the FadD29-PpsA versus FadD26-PpsA alternative partnership is utilized by the pathogen as a control point to modulate the relative abundance of PGLs and PDIMs in the cell.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by NIH grant R15AI105884 awarded to L.E.N.Q. We are grateful for the endowment support from Carol and Larry Zicklin and acknowledge the support from instrumentation grant NSF-CHE-MRI 1228921.
We thank Yasmin Chen (L.E.N.Q. laboratory) for assistance with mutant constructions. We are grateful to Chaitan Khosla (Stanford University) for providing E. coli BAP1.
This paper is dedicated to the memory of our wonderful colleague Clifford E. Soll, who recently passed away.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.02546-14.
REFERENCES
- 1.Dye C, Williams BG. 2010. The population dynamics and control of tuberculosis. Science 328:856–861. doi: 10.1126/science.1185449. [DOI] [PubMed] [Google Scholar]
- 2.Gopinath K, Singh S. 2010. Non-tuberculous mycobacteria in TB-endemic countries: are we neglecting the danger? PLoS Negl Trop Dis 4:e615. doi: 10.1371/journal.pntd.0000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL. 2006. The continuing challenges of leprosy. Clin Microbiol Rev 19:338–381. doi: 10.1128/CMR.19.2.338-381.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. 2014. Global tuberculosis report. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 5.Rodrigues LC, Lockwood D. 2011. Leprosy now: epidemiology, progress, challenges, and research gaps. Lancet Infect Dis 11:464–470. doi: 10.1016/S1473-3099(11)70006-8. [DOI] [PubMed] [Google Scholar]
- 6.Brennan PJ, Nikaido H. 1995. The envelope of mycobacteria. Annu Rev Biochem 64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- 7.Crick DC, Quadri LE, Brennan PJ. 2008. Biochemistry of the cell envelope of Mycobacterium tuberculosis, p 1–20. In Kaufmann SHE, Rubin R (ed), Handbook of tuberculosis: molecular biology and biochemistry. Wiley-VCH Verlag GmbH & Co. KgaA, Weinheim, Germany. [Google Scholar]
- 8.Minnikin DE, Kremer L, Dover LG, Besra GS. 2002. The methyl-branched fortifications of Mycobacterium tuberculosis. Chem Biol 9:545–553. doi: 10.1016/S1074-5521(02)00142-4. [DOI] [PubMed] [Google Scholar]
- 9.Niederweis M, Danilchanka O, Huff J, Hoffmann C, Engelhardt H. 2010. Mycobacterial outer membranes: in search of proteins. Trends Microbiol 18:109–116. doi: 10.1016/j.tim.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daffé M. 2008. The global architecture of the mycobacterial cell envelope, p 3–11. In Daffé M, Reyrat JM (ed), The mycobacterial cell envelope. ASM Press, Washington, DC. [Google Scholar]
- 11.Angala SK, Belardinelli JM, Huc-Claustre E, Wheat WH, Jackson M. 2014. The cell envelope glycoconjugates of Mycobacterium tuberculosis. Crit Rev Biochem Mol Biol 49:361–399. doi: 10.3109/10409238.2014.925420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neyrolles O, Guilhot C. 2011. Recent advances in deciphering the contribution of Mycobacterium tuberculosis lipids to pathogenesis. Tuberculosis (Edinb) 91:187–195. doi: 10.1016/j.tube.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Onwueme KC, Vos CJ, Zurita J, Ferreras JA, Quadri LE. 2005. The dimycocerosate ester polyketide virulence factors of mycobacteria. Prog Lipid Res 44:259–302. doi: 10.1016/j.plipres.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Astarie-Dequeker C, Le Guyader L, Malaga W, Seaphanh FK, Chalut C, Lopez A, Guilhot C. 2009. Phthiocerol dimycocerosates of M. tuberculosis participate in macrophage invasion by inducing changes in the organization of plasma membrane lipids. PLoS Pathog 5:e1000289. doi: 10.1371/journal.ppat.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cambier CJ, Takaki KK, Larson RP, Hernandez RE, Tobin DM, Urdahl KB, Cosma CL, Ramakrishnan L. 2014. Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature 505:218–222. doi: 10.1038/nature12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox JS, Chen B, McNeil M, Jacobs WR Jr. 1999. Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature 402:79–83. doi: 10.1038/47042. [DOI] [PubMed] [Google Scholar]
- 17.Passemar C, Arbues A, Malaga W, Mercier I, Moreau F, Lepourry L, Neyrolles O, Guilhot C, Astarie-Dequeker C. 2014. Multiple deletions in the polyketide synthase gene repertoire of Mycobacterium tuberculosis reveal functional overlap of cell envelope lipids in host-pathogen interactions. Cell Microbiol 16:195–213. doi: 10.1111/cmi.12214. [DOI] [PubMed] [Google Scholar]
- 18.Reed MB, Domenech P, Manca C, Su H, Barczak AK, Kreiswirth BN, Kaplan G, Barry CE III. 2004. A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature 431:84–87. doi: 10.1038/nature02837. [DOI] [PubMed] [Google Scholar]
- 19.Tsenova L, Ellison E, Harbacheuski R, Moreira AL, Kurepina N, Reed MB, Mathema B, Barry CE III, Kaplan G. 2005. Virulence of selected Mycobacterium tuberculosis clinical isolates in the rabbit model of meningitis is dependent on phenolic glycolipid produced by the bacilli. J Infect Dis 192:98–106. doi: 10.1086/430614. [DOI] [PubMed] [Google Scholar]
- 20.Yu J, Tran V, Li M, Huang X, Niu C, Wang D, Zhu J, Wang J, Gao Q, Liu J. 2012. Both phthiocerol dimycocerosates and phenolic glycolipids are required for virulence of Mycobacterium marinum. Infect Immun 80:1381–1389. doi: 10.1128/IAI.06370-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tabouret G, Astarie-Dequeker C, Demangel C, Malaga W, Constant P, Ray A, Honore N, Bello NF, Perez E, Daffe M, Guilhot C. 2010. Mycobacterium leprae phenolglycolipid-1 expressed by engineered M. bovis BCG modulates early interaction with human phagocytes. PLoS Pathog 6:e1001159. doi: 10.1371/journal.ppat.1001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alibaud L, Rombouts Y, Trivelli X, Burguiere A, Cirillo SL, Cirillo JD, Dubremetz JF, Guerardel Y, Lutfalla G, Kremer L. 2011. A Mycobacterium marinum TesA mutant defective for major cell wall-associated lipids is highly attenuated in Dictyostelium discoideum and zebrafish embryos. Mol Microbiol 80:919–934. doi: 10.1111/j.1365-2958.2011.07618.x. [DOI] [PubMed] [Google Scholar]
- 23.Brodin P, Poquet Y, Levillain F, Peguillet I, Larrouy-Maumus G, Gilleron M, Ewann F, Christophe T, Fenistein D, Jang J, Jang MS, Park SJ, Rauzier J, Carralot JP, Shrimpton R, Genovesio A, Gonzalo-Asensio JA, Puzo G, Martin C, Brosch R, Stewart GR, Gicquel B, Neyrolles O. 2010. High content phenotypic cell-based visual screen identifies Mycobacterium tuberculosis acyltrehalose-containing glycolipids involved in phagosome remodeling. PLoS Pathog 6:e1001100. doi: 10.1371/journal.ppat.1001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins DM, Skou B, White S, Bassett S, Collins L, For R, Hurr K, Hotter G, de Lisle GW. 2005. Generation of attenuated Mycobacterium bovis strains by signature-tagged mutagenesis for discovery of novel vaccine candidates. Infect Immun 73:2379–2386. doi: 10.1128/IAI.73.4.2379-2386.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camacho LR, Ensergueix D, Perez E, Gicquel B, Guilhot C. 1999. Identification of a virulence gene cluster of Mycobacterium tuberculosis by signature-tagged transposon mutagenesis. Mol Microbiol 34:257–267. doi: 10.1046/j.1365-2958.1999.01593.x. [DOI] [PubMed] [Google Scholar]
- 26.Murry JP, Pandey AK, Sassetti CM, Rubin EJ. 2009. Phthiocerol dimycocerosate transport is required for resisting interferon-gamma-independent immunity. J Infect Dis 200:774–782. doi: 10.1086/605128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng V, Zanazzi G, Timpl R, Talts JF, Salzer JL, Brennan PJ, Rambukkana A. 2000. Role of the cell wall phenolic glycolipid-1 in the peripheral nerve predilection of Mycobacterium leprae. Cell 103:511–524. doi: 10.1016/S0092-8674(00)00142-2. [DOI] [PubMed] [Google Scholar]
- 28.Rambukkana A, Zanazzi G, Tapinos N, Salzer JL. 2002. Contact-dependent demyelination by Mycobacterium leprae in the absence of immune cells. Science 296:927–931. doi: 10.1126/science.1067631. [DOI] [PubMed] [Google Scholar]
- 29.Ruley KM, Ansede JH, Pritchett CL, Talaat AM, Reimschuessel R, Trucksis M. 2004. Identification of Mycobacterium marinum virulence genes using signature-tagged mutagenesis and the goldfish model of mycobacterial pathogenesis. FEMS Microbiol Lett 232:75–81. doi: 10.1016/S0378-1097(04)00017-5. [DOI] [PubMed] [Google Scholar]
- 30.Robinson N, Kolter T, Wolke M, Rybniker J, Hartmann P, Plum G. 2008. Mycobacterial phenolic glycolipid inhibits phagosome maturation and subverts the pro-inflammatory cytokine response. Traffic 9:1936–1947. doi: 10.1111/j.1600-0854.2008.00804.x. [DOI] [PubMed] [Google Scholar]
- 31.Sinsimer D, Huet G, Manca C, Tsenova L, Koo MS, Kurepina N, Kana B, Mathema B, Marras SA, Kreiswirth BN, Guilhot C, Kaplan G. 2008. The phenolic glycolipid of Mycobacterium tuberculosis differentially modulates the early host cytokine response but does not in itself confer hypervirulence. Infect Immun 76:3027–3036. doi: 10.1128/IAI.01663-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rousseau C, Winter N, Pivert E, Bordat Y, Neyrolles O, Ave P, Huerre M, Gicquel B, Jackson M. 2004. Production of phthiocerol dimycocerosates protects Mycobacterium tuberculosis from the cidal activity of reactive nitrogen intermediates produced by macrophages and modulates the early immune response to infection. Cell Microbiol 6:277–287. doi: 10.1046/j.1462-5822.2004.00368.x. [DOI] [PubMed] [Google Scholar]
- 33.Camacho LR, Constant P, Raynaud C, Laneelle MA, Triccas JA, Gicquel B, Daffe M, Guilhot C. 2001. Analysis of the phthiocerol dimycocerosate locus of Mycobacterium tuberculosis. Evidence that this lipid is involved in the cell wall permeability barrier. J Biol Chem 276:19845–19854. doi: 10.1074/jbc.M100662200. [DOI] [PubMed] [Google Scholar]
- 34.Chavadi SS, Edupuganti UR, Vergnolle O, Fatima I, Singh SM, Soll CE, Quadri LE. 2011. Inactivation of tesA reduces cell wall lipid production and increases drug susceptibility in mycobacteria. J Biol Chem 286:24616–24625. doi: 10.1074/jbc.M111.247601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferreras JA, Stirrett KL, Lu X, Ryu JS, Soll CE, Tan DS, Quadri LE. 2008. Mycobacterial phenolic glycolipid virulence factor biosynthesis: mechanism and small-molecule inhibition of polyketide chain initiation. Chem Biol 15:51–61. doi: 10.1016/j.chembiol.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He W, Soll CE, Chavadi SS, Zhang G, Warren JD, Quadri LE. 2009. Cooperation between a coenzyme A-independent stand-alone initiation module and an iterative type I polyketide synthase during synthesis of mycobacterial phenolic glycolipids. J Am Chem Soc 131:16744–16750. doi: 10.1021/ja904792q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trivedi OA, Arora P, Vats A, Ansari MZ, Tickoo R, Sridharan V, Mohanty D, Gokhale RS. 2005. Dissecting the mechanism and assembly of a complex virulence mycobacterial lipid. Mol Cell 17:631–643. doi: 10.1016/j.molcel.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Azad AK, Sirakova TD, Fernandes ND, Kolattukudy PE. 1997. Gene knockout reveals a novel gene cluster for the synthesis of a class of cell wall lipids unique to pathogenic mycobacteria. J Biol Chem 272:16741–16745. doi: 10.1074/jbc.272.27.16741. [DOI] [PubMed] [Google Scholar]
- 39.Tobin DM, Ramakrishnan L. 2008. Comparative pathogenesis of Mycobacterium marinum and Mycobacterium tuberculosis. Cell Microbiol 10:1027–1039. doi: 10.1111/j.1462-5822.2008.01133.x. [DOI] [PubMed] [Google Scholar]
- 40.Cosma CL, Humbert O, Ramakrishnan L. 2004. Superinfecting mycobacteria home to established tuberculous granulomas. Nat Immunol 5:828–835. doi: 10.1038/ni1091. [DOI] [PubMed] [Google Scholar]
- 41.Stamm LM, Brown EJ. 2004. Mycobacterium marinum: the generalization and specialization of a pathogenic mycobacterium. Microbes Infect 6:1418–1428. doi: 10.1016/j.micinf.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Stinear TP, Seemann T, Harrison PF, Jenkin GA, Davies JK, Johnson PD, Abdellah Z, Arrowsmith C, Chillingworth T, Churcher C, Clarke K, Cronin A, Davis P, Goodhead I, Holroyd N, Jagels K, Lord A, Moule S, Mungall K, Norbertczak H, Quail MA, Rabbinowitsch E, Walker D, White B, Whitehead S, Small PL, Brosch R, Ramakrishnan L, Fischbach MA, Parkhill J, Cole ST. 2008. Insights from the complete genome sequence of Mycobacterium marinum on the evolution of Mycobacterium tuberculosis. Genome Res 18:729–741. doi: 10.1101/gr.075069.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quadri LE. 2014. Biosynthesis of mycobacterial lipids by polyketide synthases and beyond. Crit Rev Biochem Mol Biol 49:179–211. doi: 10.3109/10409238.2014.896859. [DOI] [PubMed] [Google Scholar]
- 44.Simeone R, Leger M, Constant P, Malaga W, Marrakchi H, Daffe M, Guilhot C, Chalut C. 2010. Delineation of the roles of FadD22, FadD26 and FadD29 in the biosynthesis of phthiocerol dimycocerosates and related compounds in Mycobacterium tuberculosis. FEBS J 277:2715–2725. doi: 10.1111/j.1742-4658.2010.07688.x. [DOI] [PubMed] [Google Scholar]
- 45.Parish T, Stoker NG. 1998. Mycobacteria protocols. In Walker JM. (ed), Methods in molecular biology, vol 101 Humana Press, Totowa, NJ. [Google Scholar]
- 46.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Press, Cold Spring Harbor, NY. [Google Scholar]
- 47.Parish T, Stoker NG. 2000. Use of a flexible cassette method to generate a double unmarked Mycobacterium tuberculosis tlyA plcABC mutant by gene replacement. Microbiology 146:1969–1975. [DOI] [PubMed] [Google Scholar]
- 48.Onwueme KC, Ferreras JA, Buglino J, Lima CD, Quadri LE. 2004. Mycobacterial polyketide-associated proteins are acyltransferases: proof of principle with Mycobacterium tuberculosis PapA5. Proc Natl Acad Sci U S A 101:4608–4613. doi: 10.1073/pnas.0306928101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- 50.Chavadi S, Onwueme K, Edupuganti U, Jerome J, Chatterjee D, Soll C, Quadri L. 2012. The mycobacterial acyltransferase PapA5 is required for biosynthesis of cell wall-associated phenolic glycolipids. Microbiology 158:1379–1387. doi: 10.1099/mic.0.057869-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pfeifer BA, Admiraal SJ, Gramajo H, Cane DE, Khosla C. 2001. Biosynthesis of complex polyketides in a metabolically engineered strain of E. coli. Science 291:1790–1792. doi: 10.1126/science.1058092. [DOI] [PubMed] [Google Scholar]
- 52.Trivedi OA, Arora P, Sridharan V, Tickoo R, Mohanty D, Gokhale RS. 2004. Enzymic activation and transfer of fatty acids as acyl-adenylates in mycobacteria. Nature 428:441–445. doi: 10.1038/nature02384. [DOI] [PubMed] [Google Scholar]
- 53.Beck BJ, Yoon YJ, Reynolds KA, Sherman DH. 2002. The hidden steps of domain skipping: macrolactone ring size determination in the pikromycin modular polyketide synthase. Chem Biol 9:575–583. doi: 10.1016/S1074-5521(02)00146-1. [DOI] [PubMed] [Google Scholar]
- 54.Tang GL, Cheng YQ, Shen B. 2006. Polyketide chain skipping mechanism in the biosynthesis of the hybrid nonribosomal peptide-polyketide antitumor antibiotic leinamycin in Streptomyces atroolivaceus S-140. J Nat Prod 69:387–393. doi: 10.1021/np050467t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang L, Ward S, Chung L, Carney JR, Li Y, Reid R, Katz L. 2004. Elucidating the mechanism of cis double bond formation in epothilone biosynthesis. J Am Chem Soc 126:46–47. doi: 10.1021/ja030503f. [DOI] [PubMed] [Google Scholar]
- 56.Fischbach MA, Walsh CT. 2006. Assembly-line enzymology for polyketide and nonribosomal peptide antibiotics: logic, machinery, and mechanisms. Chem Rev 106:3468–3496. doi: 10.1021/cr0503097. [DOI] [PubMed] [Google Scholar]
- 57.Walsh CT, Gehring AM, Weinreb PH, Quadri LE, Flugel RS. 1997. Post-translational modification of polyketide and nonribosomal peptide synthases. Curr Opin Chem Biol 1:309–315. doi: 10.1016/S1367-5931(97)80067-1. [DOI] [PubMed] [Google Scholar]
- 58.Lai JR, Koglin A, Walsh CT. 2006. Carrier protein structure and recognition in polyketide and nonribosomal peptide biosynthesis. Biochemistry 45:14869–14879. doi: 10.1021/bi061979p. [DOI] [PubMed] [Google Scholar]
- 59.Fitzmaurice AM, Kolattukudy PE. 1998. An acyl-CoA synthase (acoas) gene adjacent to the mycocerosic acid synthase (mas) locus is necessary for mycocerosyl lipid synthesis in Mycobacterium tuberculosis var. bovis BCG. J Biol Chem 273:8033–8039. doi: 10.1074/jbc.273.14.8033. [DOI] [PubMed] [Google Scholar]
- 60.Quadri LEN, Weinreb PH, Lei M, Nakano MM, Zuber P, Walsh CT. 1998. Characterization of Sfp, a Bacillus subtilis phosphopantetheinyl transferase for peptidyl carrier protein domains in peptide synthetases. Biochemistry 37:1585–1595. doi: 10.1021/bi9719861. [DOI] [PubMed] [Google Scholar]
- 61.Gavalda S, Leger M, van der Rest B, Stella A, Bardou F, Montrozier H, Chalut C, Burlet-Schiltz O, Marrakchi H, Daffé M, Quemard A. 2009. The Pks13/FadD32 crosstalk for the biosynthesis of mycolic acids in Mycobacterium tuberculosis. J Biol Chem 284:19255–19264. doi: 10.1074/jbc.M109.006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leger M, Gavalda S, Guillet V, van der Rest B, Slama N, Montrozier H, Mourey L, Quemard A, Daffé M, Marrakchi H. 2009. The dual function of the Mycobacterium tuberculosis FadD32 required for mycolic acid biosynthesis. Chem Biol 16:510–519. doi: 10.1016/j.chembiol.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 63.Waddell SJ, Chung GA, Gibson KJ, Everett MJ, Minnikin DE, Besra GS, Butcher PD. 2005. Inactivation of polyketide synthase and related genes results in the loss of complex lipids in Mycobacterium tuberculosis H37Rv. Lett Appl Microbiol 40:201–206. doi: 10.1111/j.1472-765X.2005.01659.x. [DOI] [PubMed] [Google Scholar]
- 64.Constant P, Perez E, Malaga W, Laneelle MA, Saurel O, Daffe M, Guilhot C. 2002. Role of the pks15/1 gene in the biosynthesis of phenolglycolipids in the M. tuberculosis complex: evidence that all strains synthesize glycosylated p-hydroxybenzoic methyl esters and that strains devoid of phenolglycolipids harbor a frameshift mutation in the pks15/1 gene. J Biol Chem 277:38148–38158. doi: 10.1074/jbc.M206538200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.