Abstract
The Dietary Patterns Methods Project (DPMP) was initiated in 2012 to strengthen research evidence on dietary indices, dietary patterns, and health for upcoming revisions of the Dietary Guidelines for Americans, given that the lack of consistent methodology has impeded development of consistent and reliable conclusions. DPMP investigators developed research questions and a standardized approach to index-based dietary analysis. This article presents a synthesis of findings across the cohorts. Standardized analyses were conducted in the NIH-AARP Diet and Health Study, the Multiethnic Cohort, and the Women’s Health Initiative Observational Study (WHI-OS). Healthy Eating Index 2010, Alternative Healthy Eating Index 2010 (AHEI-2010), alternate Mediterranean Diet, and Dietary Approaches to Stop Hypertension (DASH) scores were examined across cohorts for correlations between pairs of indices; concordant classifications into index score quintiles; associations with all-cause, cardiovascular disease (CVD), and cancer mortality with the use of Cox proportional hazards models; and dietary intake of foods and nutrients corresponding to index quintiles. Across all cohorts in women and men, there was a high degree of correlation and consistent classifications between index pairs. Higher diet quality (top quintile) was significantly and consistently associated with an 11–28% reduced risk of death due to all causes, CVD, and cancer compared with the lowest quintile, independent of known confounders. This was true for all diet index–mortality associations, with the exception of AHEI-2010 and cancer mortality in WHI-OS women. In all cohorts, survival benefit was greater with a higher-quality diet, and relatively small intake differences distinguished the index quintiles. The reductions in mortality risk started at relatively lower levels of diet quality. Higher scores on each of the indices, signifying higher diet quality, were associated with marked reductions in mortality. Thus, the DPMP findings suggest that all 4 indices capture the essential components of a healthy diet.
Keywords: dietary guidance, dietary quality, dietary indices, mortality, cohorts, epidemiology
Introduction
The concept of healthy eating patterns has been adopted by the Dietary Guidelines for Americans over time, as a growing body of research has emerged on their health benefits (1–4). Every 5 y, the USDA and Department of Health and Human Services update the Dietary Guidelines to reflect the latest scientific evidence from a range of studies including those from epidemiologic research. The Dietary Guidelines Advisory Committee informs this process and has been supported since 2010 by the USDA Nutrition Evidence Library, which conducts systematic reviews of the nutrition and health literature to inform federal policy and programs (5). In the context of one such review on dietary patterns initiated in 2011, gaps in the needed scientific data hampered the committee’s ability to draw firm conclusions about the health benefits (or risks) of dietary patterns. The main issue of concern was a lack of consistent methods used in dietary patterns research, which severely limited the ability to compare and synthesize findings.
In response, 4 research groups collaborated to create the Dietary Patterns Methods Project (DPMP)10 with the explicit goal of conducting standardized and parallel analyses on the prospective association of select dietary patterns as characterized by dietary quality indices and mortality outcomes in 3 large cohort studies in the United States, including the NIH-AARP Diet and Health Study (AARP study), the Multiethnic Cohort (MEC), and the Women’s Health Initiative Observational Study (WHI-OS). DPMP investigators considered a broad range of dietary indices and selected 4 with particular relevance for dietary guidance that had been commonly used in US populations: the Healthy Eating Index 2010 (HEI-2010), the Alternative Healthy Eating Index 2010 (AHEI-2010), the alternate Mediterranean Diet (aMED) score, and the Dietary Approaches to Stop Hypertension (DASH) score (6–9). The DPMP group developed a protocol for standardized methodologic approaches, which were subsequently applied to and replicated in each of the 3 cohorts, thereby producing 3 parallel evaluations of the prospective associations of the 4 dietary quality indices with mortality from all causes, cardiovascular disease (CVD), and cancer in 3 study populations (10–12).
In addition to strengthening the dietary pattern evidence base by publishing cohort-specific analyses, a synthesis of the findings across cohorts was envisioned (10–12). Keeping in mind the broader goal of informing dietary guidance, the DPMP collectively developed the following sequence of a priori research questions for this synthesis article:
How do the 3 cohorts included in the DPMP differ in demographic, socioeconomic, and lifestyle characteristics and all-cause, CVD, and cancer mortality risks?
What is the magnitude of correlations between the HEI-2010, AHEI-2010, aMED, and DASH scores within and across the cohorts? What proportions of the cohorts are ranked in a similar manner between pairs of dietary quality index scores?
Is higher dietary quality, as characterized by 4 dietary quality indices, consistently associated with lower all-cause, CVD, and cancer mortality in all cohorts? If so, at what rank (i.e., quintile) of index score does the mortality benefit begin?
How does diet quality, as characterized by the 4 indices, relate to absolute intake amounts of food groups, foods, beverages, and nutrients across the cohorts? What conclusions can be drawn relative to mortality benefits?
Thus, the purpose of this article is to address the above research questions by comparing and synthesizing the findings from all cohorts and arriving at generalized conclusions. We also offer a discussion considering what insights the DPMP might offer regarding the importance of unique components of the dietary quality indices, healthy ways to eat, and how much of an improvement in diet might be needed to improve health. In this process, we present findings not included in the cohort-specific publications and reference results from those existing publications (10–12). We did not conduct a pooled data analysis or a meta-analysis of the cohorts (13–15) because each study was sufficiently large to generate statistically significant findings for clinically meaningful effect sizes.
Methods
Overview of the DPMP.
The DPMP was initiated in the summer of 2012 as a collaboration of 4 research groups, one of which, the National Cancer Institute, provided leadership for the overall project; the 3 other groups were the University of Hawaii Cancer Center, the Fred Hutchinson Cancer Research Center, and the University of South Carolina (10–12, 16). The 3 large cohort studies selected for the DPMP had an adequate number of CVD and cancer deaths (i.e., ≥5000 deaths for each outcome), sufficient follow-up time (i.e., ≥10 y), detailed ascertainment of causes of death, and dietary assessment with a comprehensive FFQ. They also supported linkage to the MyPyramid Equivalents Database (MPED) (17) and included an internal dietary validation substudy in which 24-h dietary recalls or dietary records were collected (18–21).
All 3 cohorts were initiated in the mid-1990s with ongoing outcomes assessment (Supplemental Table 1). Locations of cohorts varied in that the AARP study included AARP members who were residents of 6 states (California, Florida, Louisiana, New Jersey, North Carolina, and Pennsylvania) and 2 metropolitan areas (Atlanta, GA, and Detroit, MI), the MEC included residents of Hawaii and the Los Angeles area in California who were from 5 preidentified ethnic groups, and WHI-OS recruited with the use of a national collaboration of 40 clinical centers. Middle-aged and older adults were recruited in all cohorts, with mean ages at baseline in the early 60s. The AARP study and MEC included both sexes, whereas the WHI-OS was restricted to postmenopausal women. The majority of AARP and WHI-OS participants were non-Hispanic white, and the MEC was designed to enroll a multiethnic sample. Each cohort was characterized by a sample size ranging from >63,000 in the WHI-OS to >420,000 in the AARP study.
Investigators from each cohort agreed to the following: 1) to examine 4 dietary quality indices (HEI-2010, AHEI-2010, aMED, and DASH); 2) to follow a uniform process for coding these indices; 3) to adjust for similar covariates in comparable, if not identical, manners, in full multivariate models; and 4) to include harmonized mortality outcomes. Investigators met weekly to discuss all scientific aspects of the project, including decisions related to the selection of dietary indices and definition for statistical analyses, selection of outcomes, exclusion criteria, control of confounders, evaluation of effect modifiers, results, and interpretation of findings. The purpose of these discussions was to standardize the approach as much as possible and to maintain a high level of scientific rigor throughout the project. Issues were discussed until consensus was reached by the entire group. Statistical analyses were conducted locally by each research group by using shared and standardized statistical programs. Each team of investigators sought and received approval for this project in accordance with the policies of the respective institution’s institutional review board and in accordance with publications and presentations policies of each cohort.
Diet assessment methods, dietary index definitions, and covariates in DPMP.
Details with regard to the methods applied in DPMP can be found in the Supplemental Methods. Here, we provide a brief overview. In all 3 cohorts, diet was measured by using a comprehensive self-administered FFQ that assessed dietary intake over the past year in the AARP study and MEC and the past 3 mo in the WHI-OS. The questionnaires were described previously (10–12, 18–23) and can be found at the following websites (24–26). The use of nutrient databases varied between cohorts (11, 12). Conversion of reported food and beverage intake amounts into a uniform system of nutritionally meaningful groups in all 3 cohorts was achieved by merging data with the MPED and calculating components by using MPEDs (17). This is a standardized, guidance-based food grouping method that systematizes calculation of food group and nutrient amounts by disaggregating foods into their ingredients and allocating those ingredients to 1 of 32 food groups and subgroups. MPED units are cup and ounce equivalents vs. servings per day. The MPED units can be converted to metric units by using 1 ounce = 28.3 g and 1 cup = 225 mL. The MPED groups and subgroups were used in the scoring of each dietary index.
Four dietary indices were considered: HEI-2010, AHEI-2010, aMED, and DASH. The HEI was developed to quantify adherence to US federal dietary guidance, with a higher score reflecting better quality and adherence (27). The version used in the DPMP corresponds to the 2010 Dietary Guidelines for Americans (6). The HEI-2010 scores 12 components for a total of 100 points. The AHEI was developed based on foods and nutrients associated with lower chronic disease risk drawn from extensive epidemiologic and clinical studies (28–30) and was recently updated as AHEI-2010 (7). The AHEI-2010 scores 11 components for a total of 110 points. The aMED score used in the DPMP was the one adapted for use in a US population (9) from earlier work on dietary patterns associated with lowered mortality in Mediterranean or southern European populations (31). The aMED scores 9 components for a total of 9 points. The DASH score was designed to capture the diet tested in 2 DASH randomized controlled feeding trials (1, 32), which examined the role of dietary patterns on blood pressure. Several different DASH scores exist, and we used the one most commonly found in the literature with US populations (9). DASH scores 8 components (7 food groups and 1 nutrient)—each worth 5 points—for a total of 40 points. An overview of the scoring criteria is presented in Supplemental Table 2 (10).
To develop a uniform protocol for scoring and coding these indices, the DPMP discussed and agreed upon the standardization process for each FFQ. As with any nutrient or food group variable that is added to a database file, all line items from each FFQ were included. The DPMP group developed a unified approach to consideration of potential covariates, but slight differences between covariate categorizations were present because of the need to respect cohort-specific analysis policies.
Statistical methods.
To evaluate the correlations between the dietary indices, Spearman’s rank correlations were estimated between pairs of indices within each cohort and the ranges across cohorts determined. For each index score, quintiles were computed and a categorical variable representing the 5 quintiles was created, which was represented as design (indicator) variables in the statistical models (10–12). For the analyses related to this article, median index scores were computed within each quintile of dietary intake for each dietary quality index. In addition, index scores were cross-classified in pairs by using the categorical representation of quintiles. We evaluated concordance using 2 definitions: identical rank and identical and/or adjacent rank. In each case, the proportion of the study sample meeting the criterion was estimated. In each cohort, we computed the median intake of the food groups and nutrients in each quintile of each index and by sex where appropriate. For example, the median intake of whole grain was computed for quintile 2 for each of the 4 indices in each of the 3 cohorts, yielding 12 values, of which we show the highest and the lowest value. We present the ranges of these median intake values (i.e., the lowest median intake and the highest intake) per quintile, across all indices of diet quality and cohorts for each food, food group, and nutrient component. SAS statistical software (version 9.2; SAS Institute) and SPSS (IBM SPSS) were used for analyses.
As described previously (10–12), associations of dietary indices with all-cause, CVD, and cancer mortality were assessed by using Cox proportional hazards models in each of the cohorts, with the use of person-years as the time metric. Multivariate HRs and 95% CIs for death were estimated given assignment into quintiles of the dietary index scores by using the lowest quintile as the reference. Separate models were conducted for each outcome and for each dietary quality index.
Results
How do the 3 cohorts included in the DPMP differ in demographic, socioeconomic, and lifestyle characteristics and all-cause, CVD, and cancer mortality risks?
With respect to socioeconomic characteristics, educational levels varied across cohorts (Supplemental Table 3). The WHI-OS had the highest proportion of college graduates among women with 43% (vs. 31% in the AARP study and 24% in the MEC), whereas in men, the AARP study had the highest proportion of college graduates (46% vs. 29% in the MEC). The proportion married was highest among WHI-OS women and AARP men. The proportion of never smokers was highest in MEC women and lowest in MEC men. Mean BMI (in kg/m2) ranged from 26.4 to 27.2. Unadjusted rates of all-cause mortality and CVD mortality differed between WHI-OS women and the women in AARP and MEC, with WHI-OS women having lower rates of all-cause and CVD mortality. In contrast, cancer mortality rates were similar in the 3 samples of women. Mortality rates in men were similar between the 2 cohorts.
What is the magnitude of correlations between the HEI-2010, AHEI-2010, aMED, and DASH scores within and across the cohorts? What proportions of the cohorts are ranked in a similar manner between pairs of dietary quality index scores?
As shown in Table 1, moderate to strong Spearman’s correlation coefficients between pairs of index scores were observed in all cohorts. Correlations between HEI-2010 and DASH scores were highest, ranging from 0.62 to 0.69 in women and 0.69 to 0.72 in men. Correlations between HEI-2010 and aMED scores were consistently the lowest (range: 0.49–0.57).
TABLE 1.
Cross-cohort ranges of Spearman’s correlation coefficients between dietary quality index scores: the Dietary Patterns Methods Project1
| Women |
Men |
|||||||
| HEI-2010 | AHEI-2010 | aMED | DASH | HEI-2010 | AHEI-2010 | aMED | DASH | |
| HEI-2010 | 1.00 | 0.54–0.65 | 0.48–0.54 | 0.62–0.69 | 1.00 | 0.62–0.68 | 0.53–0.57 | 0.69–0.72 |
| AHEI-2010 | 1.00 | 0.55–0.67 | 0.56–0.66 | 1.00 | 0.60–0.66 | 0.60–0.65 | ||
| aMED | 1.00 | 0.61–0.66 | 1.00 | 0.622 | ||||
| DASH | 1.00 | 1.00 | ||||||
Values for women represent the NIH-AARP Diet and Health Study, Multiethnic Cohort, and Women’s Health Initiative Observational Study, whereas values for men represent the NIH-AARP Diet and Health Study and Multiethnic cohorts. AHEI-2010, Alternative Healthy Eating Index 2010; aMED, alternate Mediterranean Diet; DASH, Dietary Approaches to Stop Hypertension; HEI-2010, Healthy Eating Index 2010.
Spearman’s correlation coefficients were identical across cohorts.
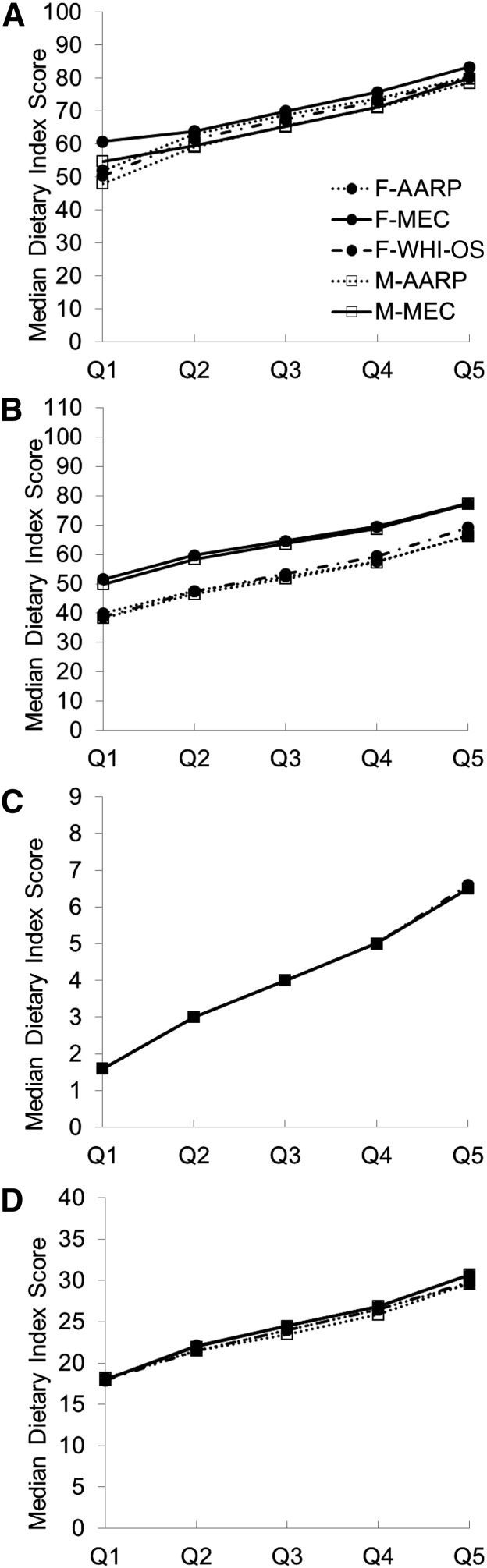
Figure 1 shows the median scores for the quintiles of each dietary quality index for each of the cohorts. Median HEI-2010 scores tracked closely by cohort, in a relatively narrow range, with the range of median values for the highest quintile of 80–83 points in women and 78–80 points in men falling short of the maximum attainable value of 100 points. There was more of a difference between cohorts with respect to median AHEI-2010 scores, with MEC participants having an ∼10 point higher score than all other samples. Again, even in the highest quintile of dietary quality, median scores fell short of the goal (66–77 for the range of AHEI-2010 scores in women and in men compared with the maximum of 110). The near-perfect alignment of aMED median scores across quintiles among all 3 cohorts is a function of creating quintiles on a narrowly defined 9-point scale, where quintile 1 corresponded to the score values 0, 1, and 2; quintile 2 to the value 3; quintile 3 to the value 4; quintile 4 to the value 5; and quintile 5 to the values of 6, 7, 8, and 9. The median DASH scores by quintiles were very consistent across cohorts and, similar to other index scores, were markedly lower than the highest attainable scores (29–31 in women and men in quintile 5 compared with the maximum of 40).
FIGURE 1.
Median HEI-2010 (A), AHEI-2010 (B), aMED (C), and DASH (D) scores by quintile according to respective dietary quality index, cohort, and sex: the Dietary Patterns Methods Project. AARP, NIH-AARP Diet and Health Study; AHEI-2010, Alternative Healthy Eating Index 2010; aMED, alternate Mediterranean Diet; DASH, Dietary Approaches to Stop Hypertension; F, females; HEI-2010, Healthy Eating Index 2010; M, males; MEC, Multiethnic Cohort; Q, quintile; WHI-OS, Women’s Health Initiative Observational Study.
Table 2 shows the proportions of the study samples that are ranked in an identical quintile, comparing 2 indices at a time. Focusing first on the extreme quintiles 1 and 5, cross-classification analyses revealed that ∼8–13% of the sample was consistently ranked into each of these 2 extreme quintiles across all dietary quality index combinations. Considering all quintiles, between 32% and 43% of the samples were ranked identically. However, if one considered classifications into adjacent quintiles in addition to the identical quintiles, these analyses revealed a sizable consistency between classifications across most index pairs, ranging from the lowest consistency for HEI-2010 vs. aMED scores with 69–84% of the study samples to 76–84% of the samples for HEI-2010 vs. DASH scores.
TABLE 2.
Concordance between pairs of categorized dietary quality indices based on cross-classification: the Dietary Patterns Methods Project1
| Women |
Men |
|||||
| AHEI-2010 | aMED | DASH | AHEI-2010 | aMED | DASH | |
| HEI-2010,% | ||||||
| Identical ranking | ||||||
| Q1 | 9.9–11.6 | 8.3–10.9 | 11.3–12.6 | 10.7–11.9 | 8.9–11.4 | 10.4–11.9 |
| Q5 | 8.5–10.1 | 9.5–11.1 | 10.0–12.2 | 9.8–10.7 | 10.5–12.3 | 11.1–12.7 |
| Q1 − Q5 | 33.5–39.0 | 31.5–40.4 | 37.6–41.1 | 36.8–40.3 | 33.6–42.5 | 40.8–42.4 |
| Identical or adjacent rank | 72.4–78.2 | 69.0–82.0 | 76.5–80.4 | 76.5–80.4 | 71.6–84.0 | 80.7–83.5 |
| AHEI-2010,% | ||||||
| Identical ranking | ||||||
| Q1 | 8.3–10.6 | 10.2–12.4 | 9.5–11.2 | 10.0–10.6 | ||
| Q5 | 10.9–12.1 | 9.4–11.5 | 11.6–11.8 | 9.7–11.4 | ||
| Q1 − Q5 | 33.5–39.4 | 34.5–39.6 | 36.1–41.0 | 35.9–38.7 | ||
| Identical or adjacent rank | 72.0–79.9 | 73.0–78.7 | 74.9–81.3 | 74.7–79.0 | ||
| aMED,% | ||||||
| Identical ranking | ||||||
| Q1 | 9.8–11.1 | 8.4–10.0 | ||||
| Q5 | 11.5–13.7 | 11.6–11.7 | ||||
| Q1 − Q5 | 36.3–39.7 | 36.1–37.0 | ||||
| Identical or adjacent rank | 74.9–77.8 | 76.2–76.4 | ||||
Values are percentages of concordant classifications across cohorts. Note the expected values for single-cell agreement, exact-quintile agreement, and adjacent-quintile agreement are 4%, 20%, and 56%, respectively. Values for women represent the NIH-AARP Diet and Health Study, Multiethnic Cohort, and Women’s Health Initiative Observational Study, whereas values for men represent the NIH-AARP Diet and Health Study and Multiethnic cohorts. AHEI-2010, Alternative Healthy Eating Index 2010; aMED, alternate Mediterranean Diet; DASH, Dietary Approaches to Stop Hypertension; HEI-2010, Healthy Eating Index 2010; Q, quintile.
Is higher dietary quality, as characterized by 4 dietary quality indices, consistently associated with lower all-cause, CVD, and cancer mortality in all cohorts? If so, at what rank (i.e., quintile) of index score does the mortality benefit begin?
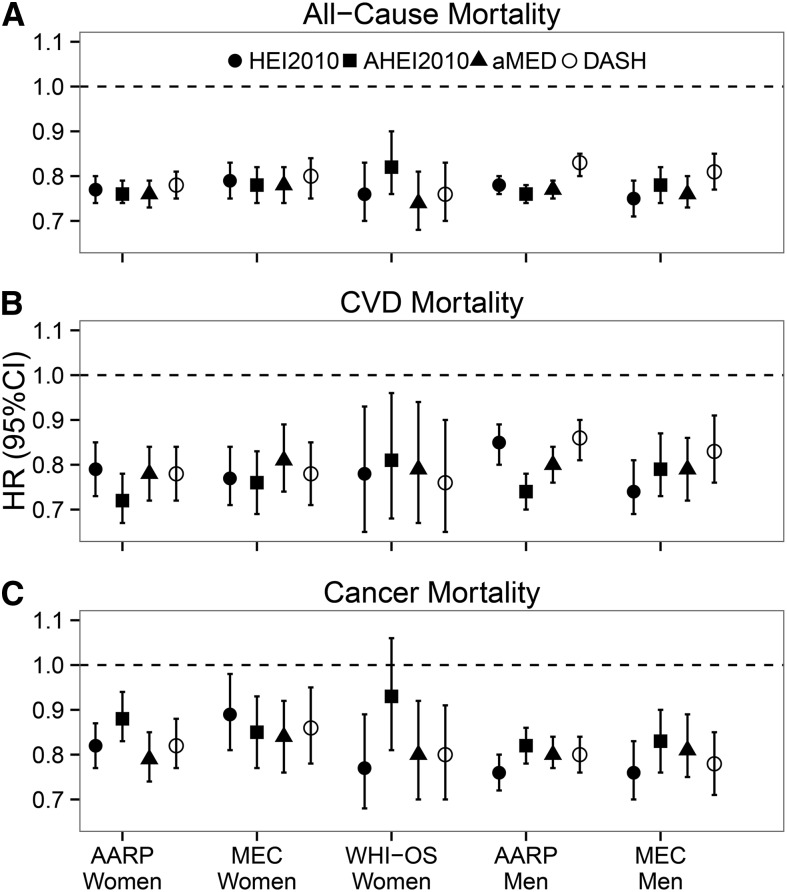
Figure 2 shows HRs and 95% CIs for the associations of high (quintile 5) vs. low (quintile 1) dietary quality from fully adjusted multivariate models, as characterized by 4 indices, with all-cause, CVD, and cancer mortality in the 3 cohorts. Focusing first on the cross-cohort comparisons, we observed remarkable consistency in the magnitude of the associations for each of the indices (10–12). In women, high dietary quality scores were associated with a 18–26% lower risk of all-cause mortality (HRs: 0.74–0.82), a 19–28% lower risk of CVD mortality (HRs: 0.72–0.81), and a 11–23% lower risk of cancer mortality (HRs: 0.77–0.89). The only exception was that the AHEI-2010 was not associated with cancer mortality in the WHI-OS. In men, high diet quality scores were associated with a 17–25% lower risk of all-cause mortality (HRs: 0.75–0.83), a 14–26% lower risk of CVD mortality (HRs: 0.74–0.86), and a 19–24% lower cancer mortality (HRs: 0.76–0.81).
FIGURE 2.
Association of dietary quality indices with all-cause (A), CVD (B), and cancer (C) mortality by cohort and sex (quintile 5 vs. quintile 1): the Dietary Patterns Methods Project. Values are HRs (95% CIs) for quintile 5 vs. quintile 1 from multivariate models adjusted for age, race/ethnicity, education, marital status, physical activity, smoking, energy intake, BMI, diabetes, and alcohol intake (HEI-2010 and DASH only) and hormone replacement therapy (women only). Refer also to data presented in references 10–12. AARP, NIH-AARP Diet and Health Study; AHEI2010, Alternative Healthy Eating Index 2010; aMED, alternate Mediterranean Diet; CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; HEI2010, Healthy Eating Index 2010; MEC, Multiethnic Cohort; WHI-OS, Women’s Health Initiative Observational Study.
To address the question at what rank (i.e., quintile) the mortality benefits became visible, ranges of the HR point estimates across cohorts are shown in Table 3 (i.e., the highest and lowest HRs). Note that this representation does not incorporate statistical significance measures and those details can be obtained in the respective cohort-specific publications (10–12). Here, we focus on the point estimates in order to see trends. In all cohorts and in women and men, reduced risk of all-cause mortality was associated with index scores as low as quintile 2 (i.e., all HRs <1.0, indicating reduced risk). Notably, there was a tight range of the HRs and a consistent, stepwise gain in the strength of the associations at higher diet quality levels (∼3–6% lower mortality risk between index quintiles). Similar trends were seen for CVD and cancer mortality.
TABLE 3.
Cross-cohort ranges of HRs by type of mortality outcome and quintile of dietary quality index scores: the Dietary Patterns Methods Project1
| Women |
Men |
|||||||
| Q2 | Q3 | Q4 | Q5 | Q2 | Q3 | Q4 | Q5 | |
| All-cause mortality | ||||||||
| HEI-2010 | 0.89–0.91 | 0.82–0.90 | 0.80–0.84 | 0.76–0.89 | 0.89–0.91 | 0.85–0.86 | 0.82–0.83 | 0.75–0.78 |
| AHEI-2010 | 0.91–0.94 | 0.85–0.90 | 0.79–0.85 | 0.76–0.82 | 0.82–0.91 | 0.88–0.90 | 0.83–0.88 | 0.76–0.78 |
| aMED | 0.87–0.94 | 0.83–0.89 | 0.80–0.84 | 0.74–0.78 | 0.922 | 0.86–0.88 | 0.832 | 0.76–0.77 |
| DASH | 0.91–0.93 | 0.86–0.89 | 0.82–0.86 | 0.76–0.80 | 0.952 | 0.90–0.91 | 0.86–0.87 | 0.81–0.83 |
| CVD mortality | ||||||||
| HEI-2010 | 0.90–0.91 | 0.87–0.92 | 0.79–0.82 | 0.77–0.79 | 0.87–0.95 | 0.83–0.90 | 0.84–0.89 | 0.74–0.85 |
| AHEI-2010 | 0.90–0.91 | 0.81–0.87 | 0.77–0.87 | 0.72–0.81 | 0.882 | 0.892 | 0.82–0.92 | 0.74–0.79 |
| aMED | 0.91–0.92 | 0.82–0.89 | 0.80–0.87 | 0.78–0.81 | 0.922 | 0.87–0.90 | 0.84–0.85 | 0.79–0.80 |
| DASH | 0.88–0.91 | 0.84–0.86 | 0.78–0.80 | 0.76–0.78 | 0.952 | 0.912 | 0.86–0.88 | 0.83–0.86 |
| Cancer mortality | ||||||||
| HEI-2010 | 0.892 | 0.76–0.92 | 0.85–0.86 | 0.77–0.89 | 0.87–0.90 | 0.85–0.87 | 0.79–0.84 | 0.762 |
| AHEI-2010 | 0.932 | 0.88–0.90 | 0.87–0.92 | 0.85–0.88 | 0.90–0.94 | 0.89–0.90 | 0.89–0.91 | 0.82–0.83 |
| aMED | 0.87–0.92 | 0.85–0.91 | 0.832 | 0.79–0.84 | 0.922 | 0.91–0.92 | 0.87–0.88 | 0.80–0.81 |
| DASH | 0.822 | 0.85–0.90 | 0.84–0.90 | 0.80–0.86 | 0.942 | 0.89–0.91 | 0.87–0.88 | 0.78–0.80 |
Values are HRs adjusted for age, race/ethnicity, education, marital status, physical activity, smoking, energy intake, BMI, diabetes, and alcohol intake (HEI-2010 and DASH only) and hormone replacement therapy (women only). For complete data refer to references 10–12. Values for women represent the NIH-AARP Diet and Health Study, Multiethnic Cohort, and Women’s Health Initiative Observational Study, whereas values for men represent the NIH-AARP Diet and Health Study and Multiethnic cohorts. AHEI-2010, Alternative Healthy Eating Index 2010; aMED, alternate Mediterranean Diet; DASH, Dietary Approaches to Stop Hypertension; HEI-2010, Healthy Eating Index 2010; Q, quintile.
HRs were identical across cohorts.
Some variation in statistical significance across quintiles 2–4 was observed, which seemed to be more evident in women than in men and varied with outcome, diet index, and cohort. Full details can be obtained in the cohort-specific studies (10–12). In brief, all dietary quality levels (i.e., quintiles 2–5 on all index scores) were inversely associated with reduced risk of all-cause mortality in women, except for HEI-2010 and AHEI-2010 scores in quintile 2 in the WHI-OS. With respect to CVD mortality in women, all diet indices were significantly associated with reduced CVD mortality in the AARP study and MEC starting at quintile 2. In contrast, WHI-OS findings did not reach significance for any index in quintile 2, only for aMED in quintile 3, for aMED and AHEI-2010 in quintile 4, and for all indices in quintile 5. For cancer mortality in women, generally consistent and significant associations were seen for quintile 5 scores in all cohorts and for all indices (with the exception that AHEI-2010 in the WHI-OS was not significantly associated with cancer mortality) but not at lower diet quality levels.
In contrast, a more consistent pattern emerged with diet index score quality levels in quintiles 2–5 for men. All indices were consistently and significantly associated with reduced all-cause mortality in both cohorts, starting at quintile 2. With respect to CVD mortality, quintiles 4 and 5 of all indices were significantly associated with the outcome in both cohorts. In addition, quintiles 2 and 3 of HEI-2010 and quintile 3 of AHEI-2010 and aMED were significantly associated with CVD mortality in both cohorts. Quintile 2 of AHEI-2010, aMED, and DASH was associated with CVD mortality only in the AARP study. With respect to cancer mortality, quintiles 3–5 of all indices were significantly associated with cancer mortality in both cohorts, as was quintile 2 for both HEI-2010 and AHEI-2010. Quintile 2 of aMED was only associated with cancer mortality in MEC and quintile 2 of DASH in the AARP study.
How does diet quality, as characterized by the 4 indices, relate to absolute intake amounts of food groups, foods, beverages, and nutrients across the cohorts? What conclusions can be drawn relative to mortality benefits?
Table 4 shows ranges of median intakes of foods, food groups, and nutrients expressed in MPED equivalents/1000 kcal for women and men. We show here only those index components that are common to at least 2 of the dietary indices, grouping first those that are included in the HEI-2010 and then all other index components. The values represent the lowest and the highest median intakes by index quintiles across all cohorts and the 4 dietary indices. We also show recommended intake amounts according to the HEI-2010 standard in the last column of the table for comparison.
TABLE 4.
Cross-cohort and cross-index ranges of dietary intake amounts by quintile of dietary quality index scores: the Dietary Patterns Methods Project1
| Women |
Men |
||||||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | Q1 | Q2 | Q3 | Q4 | Q5 | HEI-2010 standard | |
| HEI-2010 dietary index components | |||||||||||
| Whole grain, ounce2 equivalents/1000 kcal | 0.3–0.5 | 0.4–0.6 | 0.5–0.7 | 0.5–1.0 | 0.6–1.4 | 0.2–0.3 | 0.3–0.4 | 0.5–0.6 | 0.5–0.8 | 0.6–1.3 | ≥1.5 |
| Total vegetables, cup3 equivalents/1000 kcal | 0.7–1.0 | 0.9–1.1 | 1.0–1.2 | 1.1–1.3 | 1.2–1.6 | 0.7–0.8 | 0.8–0.9 | 0.9–1.0 | 1.0–1.1 | 1.2–1.3 | ≥1.1 |
| Total fruit, cup equivalents/1000 kcal | 0.4–0.9 | 0.8–1.1 | 1.0–1.2 | 1.1–1.4 | 1.3–1.7 | 0.3–0.6 | 0.5–0.8 | 0.7–0.9 | 0.8–1.2 | 1.0–1.5 | ≥0.8 |
| Seafood and plant proteins, ounce equivalents/1000 kcal | 0.4–0.6 | 0.5–0.7 | 0.6–0.8 | 0.6–0.9 | 0.8–1.1 | 0.4–0.6 | 0.5–0.7 | 0.6–0.8 | 0.7–0.9 | 0.8–1.1 | ≥0.8 |
| Low-fat dairy foods, cup equivalents/1000 kcal | 0.4–0.9 | 0.5–1.0 | 0.5–1.0 | 0.5–1.1 | 0.5–1.3 | 0.3–0.6 | 0.4–0.6 | 0.4–0.6 | 0.5–0.7 | 0.4–0.8 | ≥1.3 |
| (PUFAs + MUFAs)/SFAs, ratio of FAs | 1.6–2.0 | 1.7–2.0 | 1.8–2.0 | 1.8–2.1 | 1.9–2.3 | 1.7–2.0 | 1.9–2.0 | 2.04 | 2.0–2.1 | 2.1–2.3 | ≥2.5 |
| Refined grains, ounce equivalents/1000 kcal | 2.2–3.3 | 2.2–3.0 | 2.2–2.7 | 2.1–2.6 | 1.8–2.3 | 2.2–3.2 | 2.2–3.1 | 2.2–2.9 | 2.2–2.7 | 2.1–2.6 | ≤1.8 |
| Empty calories, % of kcal | 25.0–35.9 | 22.9–28.4 | 21.5–24.7 | 19.5–23.0 | 16.8–20.9 | 25.9–38.5 | 24.8–31.0 | 23.1–26.9 | 20.9–25.0 | 17.7–22.3 | ≤19 |
| Sodium, mg/1000 kcal | 1.5–1.7 | 1.5–1.7 | 1.5–1.7 | 1.5–1.7 | 1.4–1.7 | 1.4–1.6 | 1.5–1.6 | 1.5–1.6 | 1.5–1.6 | 1.4–1.6 | ≤1.1 |
| Other dietary index components | |||||||||||
| Vegetables (no potatoes), cup equivalents/1000 kcal | 0.5–0.8 | 0.7–0.9 | 0.8–1.0 | 0.9–1.2 | 1.0–1.4 | 0.4–0.6 | 0.6–0.7 | 0.7–0.8 | 0.8–0.9 | 0.9–1.1 | — |
| Nuts and legumes, ounce equivalents/1000 kcal | 0.1–0.2 | 0.1–0.3 | 0.2–0.3 | 0.2–0.3 | 0.2–0.4 | 0.1–0.2 | 0.2–0.3 | 0.2–0.3 | 0.2–0.3 | 0.3–0.4 | — |
| MUFAs:SFAs, ratio of FAs | 1.1–1.2 | 1.1–1.2 | 1.1–1.2 | 1.1–1.3 | 1.1–1.3 | 1.1–1.2 | 1.24 | 1.24 | 1.2–1.3 | 1.2–1.3 | — |
| Alcohol, g/1000 kcal | 0.0–0.7 | 0.0–0.8 | 0.0–0.8 | 0.0–1.0 | 0.0–2.2 | 0.8–2.6 | 0.8–2.5 | 0.9–2.3 | 1.0–2.9 | 0.8–5.1 | — |
| Red and processed meat, ounce equivalents/1000 kcal | 0.4–1.2 | 0.3–0.9 | 0.3–0.8 | 0.2–0.7 | 0.2–0.5 | 0.5–1.3 | 0.5–1.1 | 0.4–1.0 | 0.4–0.9 | 0.3–0.7 | — |
| SSBs and juices, cup equivalents/1000 kcal | 0.3–0.8 | 0.3–0.6 | 0.3–0.5 | 0.2–0.5 | 0.1–0.5 | 0.3–0.6 | 0.3–0.5 | 0.3–0.4 | 0.2–0.5 | 0.1–0.5 | — |
| SSBs, cup equivalents/1000 kcal | 0.0–0.4 | 0.0–0.2 | 0.0–0.1 | 0.0–0.1 | 0.0–0.1 | 0.0–0.5 | 0.0–0.2 | 0.0–0.2 | 0.0–0.1 | 0.0–0.1 | — |
Q1 to Q5 represent quintiles 1 to 5 of the 4 dietary indices. Values shown are the lowest and highest median values across cohorts and indices (within quintiles). Values for women represent the NIH-AARP Diet and Health Study, Multiethnic Cohort, and Women’s Health Initiative Observational Study, whereas values for men represent the NIH-AARP Diet and Health Study and Multiethnic cohorts. HEI-2010, Healthy Eating Index 2010; Q, quintile; SSB, sugar-sweetened beverage.
1 ounce = 28.3 g.
1 cup = 225 mL.
Rounded values are identical across dietary indices and cohorts.
First, focusing within quintiles, for diet quality described by quintiles 1–4 for both women and men, the range of median intake values of whole grains, vegetables, fruit, nuts, legumes, seafood and plant protein, and low-fat dairy was quite narrow, corresponding to 0.2–0.6 ounce or cup equivalents/1000 kcal (1 ounce = 28.3 g; 1 cup = 225 mL). Ranges for these food groups were substantially wider for quintile 5, which was to be expected given that the quintile is unbounded at the upper end. The same can be said of the ratio of FAs and sodium. This trend of narrow ranges applied to red and processed meat and sugar-sweetened beverages and juices, albeit for quintiles 2–5 because reverse coding led to quintile 1 being the widest. In contrast, the range of median intake values was substantially wider for alcohol, especially among men. Second, comparing across quintiles, note that a relatively small intake difference distinguished each quintile from the next. For instance, in women, a per-1000 kcal difference of 0.2–0.8 ounce equivalents of whole grains; 0.3–0.5 and 0.5–0.6 cup equivalents of vegetables and fruit, respectively; 0.3 cup equivalents of low-fat dairy; and 0.1–0.4 ounce equivalents of red and processed meats would correspond to a difference in dietary quality ranking from quintile 2 to quintile 5. Similar findings were observed in men.
Discussion
In the 3 DPMP cohorts of women and the 2 cohorts of men, we observed consistent inverse associations of each of the 4 measures of diet quality, as characterized by HEI-2010, AHEI-2010, aMED, and DASH scores, with all-cause, CVD, and cancer mortality, with one exception in the WHI-OS. In women, high diet quality was associated with an 18–26% lower risk of all-cause mortality, a 19–28% lower risk of CVD mortality, and an 11–23% lower risk of cancer mortality. In men, high diet quality was associated with a 17–25% lower risk of all-cause mortality, a 14–26% lower risk of CVD mortality, and a 19–24% lower cancer mortality. Although a number of previous cohort studies showed highly consistent associations between dietary indices and all-cause mortality, including with the HEI (33), AHEI (34–36), a Mediterranean-style diet (13, 31, 37–43), and DASH (36, 44), evidence has been inconclusive with respect to cause-specific mortality (33–36, 40, 43). The DPMP findings confirm previous findings on diet quality and all-cause mortality and offer new evidence for significant and strong associations between diet quality and CVD and cancer mortality. In 3 prospective cohorts, the HEI-2010, AHEI-2010, aMED, and DASH scores showed significant associations with CVD and cancer mortality outcomes and were consistent, with the exception of AHEI-2010, which lacked significance in association with cancer mortality in WHI-OS women.
Some commonalities and differences in the criteria underlying the 4 dietary quality indices are worth pointing out, given that each was developed with a different set of recommendations in mind and some as refinements of a previously existing dietary index (6–9). Consideration of the food groups whole grains, vegetables, fruit, and plant-based protein is common to all 4 indices (6–9). In addition, PUFAs and MUFAs are components of HEI-2010, AHEI-2010, and aMED and reduced sodium is a component of HEI-2010, AHEI-2010, and DASH. Other components that varied between indices, some of which were unique to a single index, included intakes of alcohol (AHEI-2010 and aMED), low-fat dairy (HEI-2010 and DASH), and refined grains (HEI-2010). The HEI-2010 and AHEI-2010 distinguish between whole fruit and fruit juice, DASH considers sugar-sweetened beverages, and the AHEI-2010 includes trans fats. In addition, there are some differences in the scoring systems underlying each component and consideration of energy intake, with aMED and DASH using the population’s median or quintiles of intake, HEI-2010 using standards defined on a density basis (or per 1000 kcal), and AHEI-2010 using cutoffs based on absolute intakes (6–9).
From a statistical perspective, the consistency of categorical classifications of dietary index scores between pairs of indices was noteworthy, with ∼33%–42% of participants being ranked in an identical quintile across all index pairs and an additional 40% being classified in the adjacent quintile. This suggests that all 4 dietary quality indices not only differentiate persons who eat a higher quality diet from those who do not but that a large proportion of persons would be ranked similarly across indices. Considering both this finding and the high level of consistency in the diet index–mortality associations, we infer that the common components of diet quality captured by these 4 dietary indices may have a substantial effect on mortality.
This project also provides a partial answer to the question as to whether there is one overarching approach to healthful eating associated with reduced mortality or whether there are multiple ways that a healthy, high-quality diet can be achieved. Our findings suggest that all 4 indices are capturing the essential and common components (i.e., foods and nutrients) of a healthy diet, although there are likely multiple ways to prepare and consume foods that would include the aforementioned common and essential components. However, the nature of FFQ data and the aggregation of items into food groups do not allow us to reach conclusions that would distinguish between food and meal preparation methods.
The present DPMP analyses evaluated each of the 4 dietary quality indices as a whole. To date, only the findings from the AARP cohort speak to the question of whether any one component of a given dietary quality index may explain its association with mortality. As shown by Reedy et al. (10), AARP study data suggest that each diet quality measure as a whole was more strongly associated with mortality than any one component alone. Replicating this approach in the MEC and WHI-OS could be worthwhile. Moreover, given the consistency of findings across cohorts and indices to date, it may also be informative to quantify how much of present associations between high dietary quality and mortality are due to the common components underlying the indices.
Given the multitude of available dietary indices in the literature, of which we selected only 4, it is reasonable to ask whether in the DPMP one dietary index was consistently associated with stronger mortality benefits than another. In totality, the DPMP findings did not offer a clear indication that any one index performed better than another. The differences in HRs between the various index-specific associations were very small and likely not clinically meaningful. However, given that we did not conduct an exhaustive study of all available dietary indices and quality measures and that available measures may not capture all healthful ways of eating, we cannot exclude the possibility that there are other diet quality measures that might better capture the nature of a healthy diet. To date, the body of evidence from the DPMP project suggests that, especially if focusing on mortality, all 4 indices are performing similarly and that all can reasonably be used as a basis for dietary recommendations.
The DPMP also offers some insights into how diet intake amounts in the cohort populations compare with dietary recommendations. The 2010 Dietary Guidelines for Americans includes 2 templates that “translate and integrate dietary recommendations into an overall healthy way to eat,” the USDA Food Patterns and the DASH Eating Plan (4). Both of these templates have corresponding indices. Of these, the HEI-2010 was chosen for comparison to median diet intake amounts across cohorts because it is easily applicable given its density-based nature (and we show the criteria in the last column of Table 4), whereas the DASH standards are specific to the highest (or lowest) quintile of intake within a given cohort (6, 9). Persons who consume a dietary pattern that achieved a high dietary quality index score (quintile 5) met or exceeded the recommendations for seafood and plant proteins and total vegetables and fruit but did not meet recommendations for whole grains, FA ratio, and sodium. Intakes of dairy, refined grains, and energy from solid fats and added sugars were somewhat close to the recommendations in this high-diet-quality group but included a range of values that did not meet recommendations for dairy or that exceeded the standard for refined grains, solid fats, and added sugars. Across all cohorts we noted that most persons who consume a high-quality diet still had some room for improvement (i.e., their dietary index scores were not at ideal levels). An interesting exercise would be to see how much stronger the reductions in mortality might be in groups achieving optimal dietary quality index scores.
Focusing on the ranges of median intakes of foods, food groups, and nutrients across quintiles of dietary quality index scores revealed that the ranges of values were quite narrow for a large number of foods, specifically whole grains, vegetables, fruit, nuts, legumes, seafood and plant protein, and low-fat dairy. In other words, a relatively small intake difference distinguished each quintile from the next. Thus, our findings suggest that even quite small improvements in dietary intake quality could have a meaningful influence on mortality risk in the total population.
There are a number of limitations to this study. Analyses were based on a single FFQ administered at baseline and hence do not account for potential changes in dietary intakes over time. Although FFQs are not designed to provide estimates of absolute intake (because they draw from only a defined list of foods and beverages), they do provide a means to rank individuals (45). Furthermore, measurement error inherent in a self-reported FFQ may have led to an attenuation or distortion of the associations found (46, 47). One could speculate whether the associations we found with mortality might be stronger with measurement error correction methods (48, 49), which we hope to explore in the future. Even though we adjusted for a large number of covariates, residual confounding cannot be excluded in observational studies. Finally, there is some opportunity for survival bias in that even though recruitment criteria for all cohorts included persons as young as 45 or 50 y of age, the mean age at baseline was quite consistent across cohorts, in the early 60s. Future studies should consider including assessments of diet over time; the use of instruments such as 24-h dietary recalls or food records, which are designed to estimate absolute intake; and measurement error correction methods.
Few studies to date have evaluated and compared multiple index-based measures of dietary quality in the same cohort in relation to mortality (30, 35, 36, 39, 42, 50), and to our knowledge this has never been done in a standardized and parallel approach across multiple cohorts. This was possible because the DPMP group was committed to conducting research that would be of direct utility to the development of federal dietary guidance on the topic of dietary patterns and hence formed a consensus-based collaboration to standardize the approaches across cohorts and maintain the highest level of scientific rigor. The DPMP research group agreed to follow jointly developed and clearly defined protocols for statistical analyses in each cohort. This approach, in conjunction with attributes of the cohorts (i.e., the large sample sizes, long follow-up durations, and the highly developed, validated FFQs available in all cohorts), is a unique strength of this study.
In the future, the DPMP is positioned to address additional research questions in a consistent and standardized manner. For instance, the AARP study conducted additional by-component analyses of the indices (10), the MEC explored the associations in 5 ethnic groups (12), and the WHI-OS explored associations in different levels of overweight and obesity (11). Replicating these cohort-specific analyses across all DPMP cohorts (and in other cohorts who wish to join the effort) would add to the generalizability of findings, which currently extend to a US population of middle-aged and older adults. Furthermore, the DPMP’s approach to studying healthy dietary patterns focused on 4 dietary quality measures, operationalized as indices and their scores. Although each score represents the sum of scoring multiple dietary components (i.e., food groups, foods and beverages, and nutrients), the index scores used to date in DPMP statistical analyses are single variables and have been used in a unidimensional manner. It is important to recognize that this approach aggregates many different combinations of amounts of specific food groups and nutrients into each index score. To more fully evaluate the dietary intake patterns underlying each of the dietary quality index scores, multidimensional approaches would be needed.
In conclusion, in 3 distinct US cohorts, analyses indicate marked reductions in all-cause, CVD, and cancer mortality associated with higher dietary quality as characterized by high scores on 4 dietary indices. Despite the known limitations of observational cohort data used in the DPMP (51), note that the findings for the DASH and the aMED indices align with results from previously published randomized controlled trials (1–3, 51). We therefore agree with the conclusions of Maki et al. (51) that the type of congruence of observational research as conducted by the DPMP group and previous randomized controlled trial findings can be considered as the basis for some of the strongest recommendations in an evidence-based review such as the one conducted for the Dietary Guidelines for Americans. Finding significant, consistent, and strong associations of high dietary quality with all-cause, CVD, and cancer mortality in all 3 prospective cohorts (with one exception)—which satisfies the Bradford-Hill causality criteria of strength, consistency, temporality, coherence, and biological gradient—is an important contribution to the field (52).
Supplementary Material
Acknowledgments
The short list of WHI investigators is as follows—Program Office: Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller (National Heart, Lung, and Blood Institute, Bethesda, MD); Clinical Coordinating Center: Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg (Fred Hutchinson Cancer Research Center, Seattle, WA); investigators and academic centers: JoAnn E Manson (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA), Barbara V Howard (MedStar Health Research Institute/Howard University, Washington, DC), Marcia L Stefanick (Stanford Prevention Research Center, Stanford, CA), Rebecca Jackson (Ohio State University, Columbus, OH), Cynthia A Thomson (University of Arizona, Tucson/Phoenix, AZ), Jean Wactawski-Wende (State University of New York, Buffalo, NY), Marian Limacher (University of Florida, Gainesville/Jacksonville, FL), Robert Wallace (University of Iowa, Iowa City/Davenport, IA), Lewis Kuller (University of Pittsburgh, Pittsburgh, PA), Sally Shumaker (Wake Forest University School of Medicine, Winston-Salem, NC); Women’s Health Initiative Memory Study: Sally Shumaker (Wake Forest University School of Medicine, Winston-Salem, NC). Additional information: A full list of all the investigators who have contributed to WHI science is available from: https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Short%20List.pdf.
We thank Xiaonan Ma, University of South Carolina Arnold School of Public Health, for assistance with manuscript preparation. We also thank Lisa Kahle at Information Management Services, Inc., for programming expertise and acknowledge the analytical assistance of Yurii Shvetsov, Reynolette Ettienne, and Anne Tome. We acknowledge the MEC participants and lead investigators: Larry Kolonel, Lynne Wilkens, Loic Le Marchand, and Brian Henderson. We also thank the WHI investigators, staff, and the trial participants for their outstanding dedication and commitment. ADL, SMK-S, MLN, CJB, and JR designed the research; ADL and JR wrote the manuscript; and ADL, SMK-S, AFS, SMG, BEH, MLN, CJB, TES, and JR reviewed the manuscript drafts, provided critical input to content and wording, and have final responsibility for the content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AARP study, NIH-AARP Diet and Health Study; AHEI-2010, Alternative Healthy Eating Index 2010; aMED, alternate Mediterranean Diet; CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; DPMP, Dietary Patterns Methods Project; HEI-2010, Healthy Eating Index 2010; MEC, Multiethnic Cohort; MPED, MyPyramid Equivalents Database; WHI-OS, Women’s Health Initiative Observational Study.
References
- 1.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997;336:1117–24. [DOI] [PubMed] [Google Scholar]
- 2.de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation 1999;99:779–85. [DOI] [PubMed] [Google Scholar]
- 3.Estruch R, Ros E, Salas-Salvadó, Covas M, Corella D, Arós F, Gómez-Gracia E., Ruiz-Gutiérrez V, Fiol M, Lapetra J. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90. [DOI] [PubMed] [Google Scholar]
- 4.Office of Disease Prevention and Health Promotion, US Department of Health and Human Services. Dietary guidelines for Americans 2010 [cited 2014 Aug 26]. Available from: http://www.dietaryguidelines.gov.
- 5.USDA. 2015 Dietary Guidelines Advisory Committee. Federal presentations [cited 2014 Aug 26]. Available from: http://www.health.gov/dietaryguidelines/2015-binder/2015/historyCurrentUse.aspx.
- 6.Guenther PM, Casavale KO, Reedy J, Kirkpatrick SI, Hiza HA, Kuczynski KJ, Kahle LL, Krebs-Smith SM. Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet. 2013;113:569–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fung TT, McCullough ML, Newby PK, Manson JE, Meigs JB, Rifai N, Willett WC, Hu FB. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 2005;82:163–73. [DOI] [PubMed] [Google Scholar]
- 9.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 2008;168:713–20. [DOI] [PubMed] [Google Scholar]
- 10.Reedy J, Krebs-Smith SM, Miller PE, Liese AD, Kahle LL, Park Y, Subar AF. Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J Nutr 2014;144:881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George SM, Ballard-Barbash R, Manson JE, Reedy J, Shikany JM, Subar AF, Tinker LF, Vitolins MZ, Neuhouser ML. Comparing indices of diet quality with chronic disease mortality in postmenopausal women in the Women's Health Initiative Observational Study: evidence to inform national dietary guidance. Am J Epidemiol 2014;180:616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harmon BE, Boushey CJ, Shvetsov YB, Ettienne R, Reedy J, Wilkens LR, Le Marchand L, Henderson BE, Kolonel LN. Association of key diet quality indexes with mortality in the Multiethnic Cohort: The Dietary Patterns Methods Project. Am J Clin Nutr. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trichopoulou A, Orfanos P, Norat T, Bueno-de-Mesquita B, Ocké MC, Peeters PH, van der Schouw YT, Boeing H, Hoffmann K, Boffetta P. Modified Mediterranean diet and survival: EPIC-Elderly Prospective Cohort Study. BMJ 2005;330:991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bamia C, Trichopoulos D, Ferrari P, Overvad K, Bjerregaard L, Tjønneland A, Halkjaer J, Clavel-Chapelon F, Kesse E, Boutron-Ruault MC. Dietary patterns and survival of older Europeans: the EPIC-elderly study (European Prospective Investigation into Cancer and Nutrition). Public Health Nutr 2007;10:590–8. [DOI] [PubMed] [Google Scholar]
- 15.Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr 2010;92:1189–96. [DOI] [PubMed] [Google Scholar]
- 16.National Cancer Institute. Dietary Patterns Methods Project [cited 2014 Aug 26]. Available from: http://appliedresearch.cancer.gov/dietary_patterns/.
- 17.USDA, Agricultural Research Service. MyPyramid Equivalents Database for USDA Survey Food Codes. Version 1.0 [cited 2014 Aug 26]. Available from: http://www.ars.usda.gov/News/docs.htm?docid=8503.
- 18.Thompson FE, Kipnis V, Midthune D, Freedman LS, Carroll RJ, Subar AF, Brown CC, Butcher MS, Mouw T, Leitzmann M, et al. Performance of a food-frequency questionnaire in the US NIH-AARP (National Institutes of Health-American Association of Retired Persons) Diet and Health Study. Public Health Nutr 2008;11:183–95. [DOI] [PubMed] [Google Scholar]
- 19.Stram DO, Hankin JH, Wilkens LR, Pike MC, Monroe KR, Park S, Henderson BE, Nomura AMY, Earle ME, Nagamine FS. Calibration of the dietary questionnaire for a multiethnic cohort in Hawaii and Los Angeles. Am J Epidemiol 2000;151:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Block G, Hartman A, Dresser C, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol 1986;124:453–69. [DOI] [PubMed] [Google Scholar]
- 21.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Gurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol 1999;9:178–87. [DOI] [PubMed] [Google Scholar]
- 22.Schatzkin A, Subar AF, Thompson FE, Harlan LC, Tangrea J, Hollenbeck AR, Hurwitz PE, Coyle L, Schussler N, Michaud DS. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health−American Association of Retired Persons Diet and Health Study. Am J Epidemiol 2001;154:1119–25. [DOI] [PubMed] [Google Scholar]
- 23.Thompson FE, Subar AF, Brown CC, Smith AF, Sharbaugh CO, Jobe JB, Mittl B, Gibson JT, Ziegler RG. Cognitive research enhances accuracy of food frequency questionnaire reports: results of an experimental validation study. J Am Diet Assoc 2002;102:212–25. [DOI] [PubMed] [Google Scholar]
- 24.NIH and AARP: Diet and Health Study. Questionnaire [cited 2014 Nov 20]. Available from: http://dietandhealth.cancer.gov/docs/diet_questionnaire_baseline.pdf.
- 25.University of Hawaii Cancer Center; University of Southern California. The Multiethnic Cohort Study: participant questionnaires [cited 2014 Nov 20]. Available from: http://www.crch.org/multiethniccohort/mec_questionnaires.htm.
- 26.Fred Hutchinson Cancer Research Center. Women's Health Initiative: forms [cited 2014 Nov 20]. Available from: https://www.whi.org/studydoc/WHI%20Forms/Forms/WHI.aspx.
- 27.Kennedy ET, Ohls J, Carlson S, Fleming K. The Healthy Eating Index: design and applications. J Am Diet Assoc 1995;95:1103–8. [DOI] [PubMed] [Google Scholar]
- 28.McCullough ML, Feskanich D, Stampfer MJ, Rosner BA, Hu FB, Hunter DJ, Variyam JN, Colditz GA, Willett WC. Adherence to the Dietary Guidelines for Americans and risk of major chronic disease in women. Am J Clin Nutr 2000;72:1214–22. [DOI] [PubMed] [Google Scholar]
- 29.McCullough ML, Feskanich D, Rimm EB, Giovannucci EL, Ascherio A, Variyam JN, Spiegelman D, Stampfer MJ, Willett WC. Adherence to the Dietary Guidelines for Americans and risk of major chronic disease in men. Am J Clin Nutr 2000;72:1223–31. [DOI] [PubMed] [Google Scholar]
- 30.McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, Spiegelman D, Hunter DJ, Colditz GA, Willett WC. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr 2002;76:1261–71. [DOI] [PubMed] [Google Scholar]
- 31.Trichopoulou A, Kouris-Blazos A, Wahlqvist ML, Gnardellis C, Lagiou P, Polychronopoulos E, Vassilakou T, Lipworth L, Trichopoulos D. Diet and overall survival in elderly people. BMJ 1995;311:1457–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, Simons-Morton DG. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 33.Rathod AD, Bharadwaj AS, Badheka AO, Kizilbash M, Afonso L. Healthy Eating Index and mortality in a nationally representative elderly cohort. Arch Intern Med 2012;172:275–7. [DOI] [PubMed] [Google Scholar]
- 34.Akbaraly TN, Ferrie JE, Berr C, Brunner EJ, Head J, Marmot MG, Singh-Manoux A, Ritchie K, Shipley MJ, Kivimaki M. Alternative Healthy Eating Index and mortality over 18 y of follow-up: results from the Whitehall II cohort. Am J Clin Nutr 2011;94:247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mursu J, Steffen LM, Meyer KA, Duprez D, Jacobs DR. Diet quality indexes and mortality in postmenopausal women: the Iowa Women's Health Study. Am J Clin Nutr 2013;98:444–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu D, Zhang X, Xiang YB, Yang G, Li H, Gao YT, Zheng W, Shu XO. Adherence to dietary guidelines and mortality: a report from prospective cohort studies of 134,000 Chinese adults in urban Shanghai. Am J Clin Nutr 2014;100:693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osler M, Schroll M. Diet and mortality in a cohort of elderly people in a north European community. Int J Epidemiol 1997;26:155–9. [DOI] [PubMed] [Google Scholar]
- 38.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med 2003;348:2599–608. [DOI] [PubMed] [Google Scholar]
- 39.Knoops KTB. Comparison of three different dietary scores in relation to 10-year mortality in elderly European subjects: the HALE project. Eur J Clin Nutr 2006;60:746–55. [DOI] [PubMed] [Google Scholar]
- 40.Mitrou PN, Kipnis V, Thiébaut ACM, Reedy J, Subar AF, Wirfält E, Flood A, Mouw T, Hollenbeck AR, Leitzmann MF. Mediterranean dietary pattern and prediction of all-cause mortality in a US population: results from the NIH-AARP Diet and Health Study. Arch Intern Med 2007;167:2461–8. [DOI] [PubMed] [Google Scholar]
- 41.Lagiou P, Trichopoulos D, Sandin S, Lagiou A, Mucci L, Wolk A, Elisabete E, Adami HO. Mediterranean dietary pattern and mortality among young women: a cohort study in Sweden. Br J Nutr 2006;96:384–92. [DOI] [PubMed] [Google Scholar]
- 42.McNaughton SA, Bates CJ, Mishra GD. Diet quality is associated with all-cause mortality in adults aged 65 years and older. J Nutr 2012;142:320–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buckland G, Agudo A, Travier N, Huerta JM, Cirera L, Tormo MJ, Navarro C, Chirlaque MD, Moreno-Iribas C, Ardanaz E, et al. Adherence to the Mediterranean diet reduces mortality in the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain). Br J Nutr 2011;106:1581–91. [DOI] [PubMed] [Google Scholar]
- 44.Parikh A, Lipsitz SR, Natarajan S. Association between a DASH-like diet and mortality in adults with hypertension: findings from a population-based follow-up study. Am J Hypertens 2009;22:409–16. [DOI] [PubMed] [Google Scholar]
- 45.Willett WC. Nutritional epidemiology. 3rd ed. New York: Oxford University Press; 2013.
- 46.Kipnis V, Subar AF, Midthune D, Freedman LS, Ballard-Barbash R, Troiano RP, Bingham S, Schoeller DA, Schatzkin A, Carroll RJ. Structure of dietary measurement error: results of the OPEN biomarker study. Am J Epidemiol 2003;158:14–21. [DOI] [PubMed] [Google Scholar]
- 47.Subar AF, Kipnis V, Troiano RP, Midthune D, Schoeller DA, Bingham S, Sharbaugh CO, Trabulsi J, Runswick S, Ballard-Barbash R, et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am J Epidemiol 2003;158:1–13. [DOI] [PubMed] [Google Scholar]
- 48.Freedman LS, Schatzkin A, Midthune D, Kipnis V. Dealing with dietary measurement error in nutritional cohort studies. J Natl Cancer Inst 2011;103:1086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang S, Midthune D, Guenther PM, Krebs-Smith SM, Kipnis V, Dodd KW, Buckman DW, Tooze JA, Freedman L, Carroll RJ. A new multivariate measurement error model with zero-inflated dietary data, and its application to dietary assessment. Ann Appl Stat 2011;5:1456–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCullough ML, Willett WC. Evaluating adherence to recommended diets in adults: the Alternate Healthy Eating Index. Public Health Nutr 2006;9:152–7. [DOI] [PubMed] [Google Scholar]
- 51.Maki KC, Slavin JL, Rains TM, Kris-Etherton PM. Limitations of observational evidence: implications for evidence-based dietary recommendations. Adv Nutr. 2014;5:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hill AB. The environment and disease: association or causation? Proc R Soc Med 1965;58:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.