Abstract
Neuroinflammation and degeneration of catecholaminergic brainstem nuclei occur early in neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease. Neuroinflammation increases levels of pro-inflammatory cytokines and reactive oxygen species which can alter neuronal calcium (Ca+2) homoeostasis via L-type voltage dependent calcium channels (L-VDCCs) and ryanodine receptors (RyRs). Alterations in Ca+2 channel activity in the SN and LC can lead to disruption of normal pacemaking activity in these areas, contributing to behavioral deficits. Here, we utilized an in vivo model of chronic neuroinflammation: rats were infused intraventricularly with a continuous small dose (0.25 µg/hr) of lipopolysaccharide (LPS) or artificial cerebrospinal fluid (aCSF) for 28 days. Rats were treated with either the L-VDCC antagonist nimodipine or the RyR antagonist dantrolene. LPS-infused rats had significant motor deficits in the accelerating rotarod task as well as abnormal behavioral agitation in the forced swim task and open field. Corresponding with these behavioral deficits, LPS-infused rats also had significant increases in microglia activation and loss of tyrosine hydroxylase (TH) immunoreactivity in the substantia nigra pars compacta (SNpc) and locus coeruleus (LC). Treatment with nimodipine or dantrolene normalized LPS-induced abnormalities in the rotarod and forced swim, restored the number of TH-immunoreactive cells in the LC, and significantly reduced microglia activation in the SNpc. Only nimodipine significantly reduced microglia activation in the LC, and neither drug increased TH immunoreactivity in the SNpc. These findings demonstrate that the Ca+2 dysregulation in the LC and SN brainstem nuclei is differentially altered by chronic neuroinflammation. Overall, targeting Ca+2 dysregulation may be an important target for ameliorating neurodegeneration in the SNpc and LC.
Keywords: LPS, microglia, substantia nigra, locus coeruleus, calcium
Introduction
Degeneration of the locus coeruleus (LC) appears to precede substantia nigra pars compacta (SNpc) and hippocampal damage in Parkinson’s disease (PD) and Alzheimer’s disease (AD), respectively (Simic et al., 2009; Tomlinson et al., 1981; Del Tredici et al., 2002). Chronic neuroinflammation also appears early in the progression of AD and PD, with early stage AD and PD patients showing increased microglia activation in vulnerable brain regions (Yasuno et al., 2012; Iannaccone et al., 2013). The SNpc and LC are vulnerable to neuroinflammation (Bardou et al., 2014; Brothers et al., 2013). Major histocompatibility complex I (MHC-I) is expressed at very low levels in the CNS, although in the presence of neuroinflammation SN and LC neurons specifically upregulate MHC-I, which makes them selectively targeted for degradation (Cebrián et al., 2014). Several aspects of early AD can be reproduced by chronic infusion of lipopolysaccharide (LPS) into the fourth ventricle of young rats (Hauss-Wegrzyniak et al., 1998a). This model may also be relevant for modeling early PD, since following chronic LPS administration in vivo, dopaminergic (DA) cell loss is observed in the SNpc and noradrenergic (NE) neuron loss is observed in the LC (Bardou et al., 2014).
Damage to the LC may account for early behavioral changes in both AD and PD. The LC is important for regulation of sleep, which is disrupted during both AD (Jost and Grossberg, 1996) and PD (Schrempf et al., 2014). Depression, which is also related to LC function (Stone et al., 2011) is highly comorbid with AD (Starkstein and Mizrahi, 2005) and PD (Ravina et al., 2007). Additionally, agitation and damage to the LC are associated with AD (Leverenz et al., 2001). Motor symptoms in both AD and PD are related to loss of SNpc neurons (Horvath et al., 2014; Braak et al., 2004). In rats, moderate LC damage leads to decreased mobility during the forced swim while extensive LC damage leads to increased mobility, following a U-shaped curve (Harro et al., 1999). LC damage can also be correlated with hyperactivity in rodents (Stone et al., 2011), which may be similar to agitation seen in depressed or anxious humans. In rats, the extent of SNpc damage is correlated to motor coordination performance in the rotarod task (Monville et al., 2006). Overall, these data suggest that the forced swim, open field, and rotarod tasks are sensitive to damage in the LC and SNpc and are relevant to physiological changes associated with AD and PD.
Neuroinflammation may lead to neuronal Ca+2 dysregulation and underlie the behavioral impairments and neurodegeneration via excitotoxicity (Mattson et al., 2012). Elevated levels of pro-inflammatory cytokines and oxidative stress can increase the function of L-type voltage-dependent Ca+2 channels (L-VDCCs; Furukawa and Mattson, 1998) and ryanodine receptors (RyRs; Friedrich et al., 2014; Palmi and Meini, 2002). The activity of L-VDCCs and RyRs are also linked to each other (Foster, 2012). Indeed, Ca+2 dysregulation via enhancement of L-VDCC activity in pacemaker neurons in the SNpc and LC may play an important role in the selective vulnerability of these regions in PD (Surmeier and Schumacker, 2013) in addition to their vulnerability to neuroinflammation (Cebrián et al., 2014). Antagonism of L-VDCCs with the dihydropyridine (DHP) isradipine can improve the motor scores of PD patients (Parkinson Study Group, 2013). There are limited data on the role of RyRs in the SNpc and LC neurons, but as in other neurons, RyR activity can increase L-VDCC activity and vice versa (Borde et al., 2000; Chavis et al., 1996). Overall, these data suggest that the anti-inflammatory properties of LVDCC and RyR antagonists such as nimodipine and dantrolene, respectively, could provide protection to the SN and LC during chronic neuroinflammation via control of Ca+2 dysregulation or their direct anti-inflammatory action (Hashioka et al., 2012; Li et al., 2009; Klegeris et al., 2007).
Methods
Subjects and surgical procedures
The subjects were male F-344 (Harlan, Indianapolis, IN, USA) rats, 3 months old, individually housed with ad libitum access to food and water and maintained on a reverse 12/12 light/dark cycle with lights off at 8AM. Artificial cerebrospinal fluid (aCSF, 140 mM NaCl, 3.0 mM KCl, 2.5 mM CaCl2, 1.0 mM MgCl2, 1.2 mM Na2HPO4, pH 7.4; n=30) or lipopolysaccharide (LPS, Sigma, St. Louis, MO, USA, E. coli serotype 055:B5 TCA extraction, 1.0 mg/ml dissolved in aCSF, n=30) was loaded into an osmotic minipump (Alzet model #2004, with a rate of 0.25 µl/hr, Durect Corp., Cupertino, CA, USA)and infused into the brain for 28 days via a cannula surgically implanted into the 4th ventricle as previously described (Brothers et al., 2013a). The day after the osmotic minipump was implanted, rats began to receive daily subcutaneous drug injections at a volume of 1 ml/kg per day with vehicle (polyethylene glycol 300, Thermo Fisher Scientific, Waltham, MA, USA), the RyR antagonist dantrolene sodium salt (5 mg/kg/day, Sigma), or the DHP L-VDCC antagonist nimodipine (5 mg/kg/day, Sigma), resulting in 6 group + drug treatment groups (aCSF + vehicle, n=11; aCSF + dantrolene, n=10; aCSF + nimodipine, n=9; LPS + vehicle, n=10; LPS + dantrolene, n=10; LPS + nimodipine, n=10). Body weights were monitored daily and rats were given saline injections and supplemental food postoperatively to prevent dehydration and weight loss. This research was carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80–23) and The Ohio State University Institutional Animal Care and Use Committee.
Behavioral testing
Prior to the onset of behavioral testing, rats were handled daily. The first behavioral test was the accelerating rotarod task, which occurred at the beginning of the third week of LPS infusion and drug treatment. Rotarod testing was completed over two days, and at the end of the second day, rats were subjected to the open field task. On the third and fourth day of behavioral testing, rats completed the forced swim test. For all tasks, rats were first acclimated to the testing room 30 minutes prior to testing.
Accelerating rotarod
The rotarod device consisted of a 6 cm rod raised 40 cm high driven by a SM-S4303R High Torque Full Rotation Servo (Sparkfun, Boulder, CO). Latency to fall off the rod was manually recorded with a stopwatch. On the first day of testing, rats were habituated to the stationary rod for 5 minutes. Rats were then acclimated over 3 trials to a rod rotating at a constant 10 rotations per minute (rpm) for a maximum trial length of 5 minutes, with an intertrial interval of at least 30 minutes. On the second day of testing, rats were tested in 3 trials on a rod that accelerated steadily from 0 to 20 rpm over 5 minutes, with an intertrial interval of at least 30 minutes. Data is expressed as latency to fall off of the rod. All of the results from the accelerating trials were pooled together for statistical analysis.
Open field
Rats were placed in the open field testing box, which was a 50 × 38 × 28 cm chamber. Various behavioral measures including movement, rearing, and distance from the center of the arena were tracked using overhead cameras and Noldus Ethovision 3.1 tracking and analysis system (Noldus, Lessburg, VA, USA).
Forced swim
The forced swim test was composed of two days with 10 minutes of swimming in a small pool with a diameter of 36 cm. Water temperature was adjusted to 25°C ± 1°C and the pool cleaned prior to the introduction of each rat. Rats were observed during the testing, and any rats that showed signs of drowning were immediately rescued. Various behavioral measures including immobility, velocity, and distance traveled were tracked using overhead cameras and Noldus Ethovision 3.1 tracking and analysis system (Noldus, Lessburg, VA, USA).
Tissue preparation and histological procedures
All of the rats were deeply anesthetized using isoflurane prior to sacrifice 28 days after surgery. The rats were rapidly decapitated and the brainstem and cerebellum were isolated and placed in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. The brains were post-fixed for one week in fixative and then stored at 4 °C in phosphate-buffered saline (PBS), pH 7.4. 40 µm sections were generated using a vibratome. The rabbit antibody against tyrosine hydroxylase (TH) was used to label DA and NE neurons (final dilution 1:750; Millipore, Billerica, MA) and the mouse antibody against OX-6 was used to label major histocompatibility complex II (MHC-II) positive microglia. Endogenous peroxidase activity was quenched and nonspecific binding was blocked with 5% normal goat serum (NGS) in PBS. Sections were then incubated overnight at 4 °C in primary antibody diluted in the same blocking solution. The next day, sections were rinsed and then incubated for 1.5 hours at RT in biotinylated anti-rabbit secondary antibody (final dilution 1:200, Vector, Burlingame, CA, USA). Sections were then rinsed and incubated for 1 hour at RT with avidin-biotinylated horseradish peroxidase (Vectastain, ABC kit, Vector). After another rinse, sections were incubated with 0.05% 3, 3’-diaminobenzidine tetrahydrochloride (Vector) as a chromogen. The reaction was stopped by rinsing the sections with PBS. No staining was detected in the absence of primary or secondary antibodies. Sections were mounted on superfrost slides, air-dried, dehydrated with a series of ethanol and xylene rinses and coverslipped with cytoseal (Allan Scientific, Kalamazoo, MI, USA) mounting medium. Images were captured with light microscopy, stitched together, and analyzed with a Nikon 90i system with a DS-5M-L1 digital camera using Elements 3.1 Software (Nikon Instruments, Melville, NY, USA). For the LC cell counts, a uniform sized region of interest was drawn around a portion of the LC and the number of TH immunoreactive cells counted within that region. For SN densitometry, the entire SN was enclosed in a region of interest and a threshold for TH immunoreactivity was identified and applied identically across all of the slices.
Statistical analysis
Statistical analyses were conducted using SigmaPlot 12.5 (Systat, San Jose, CA, USA). Analyses of variance (ANOVA) were performed followed by Fisher's protected least significant difference for post hoc comparisons. Graphs display the mean ± standard error of the mean (SEM). A p < 0.05 was considered statistically significant.
Results
Accelerating rotarod
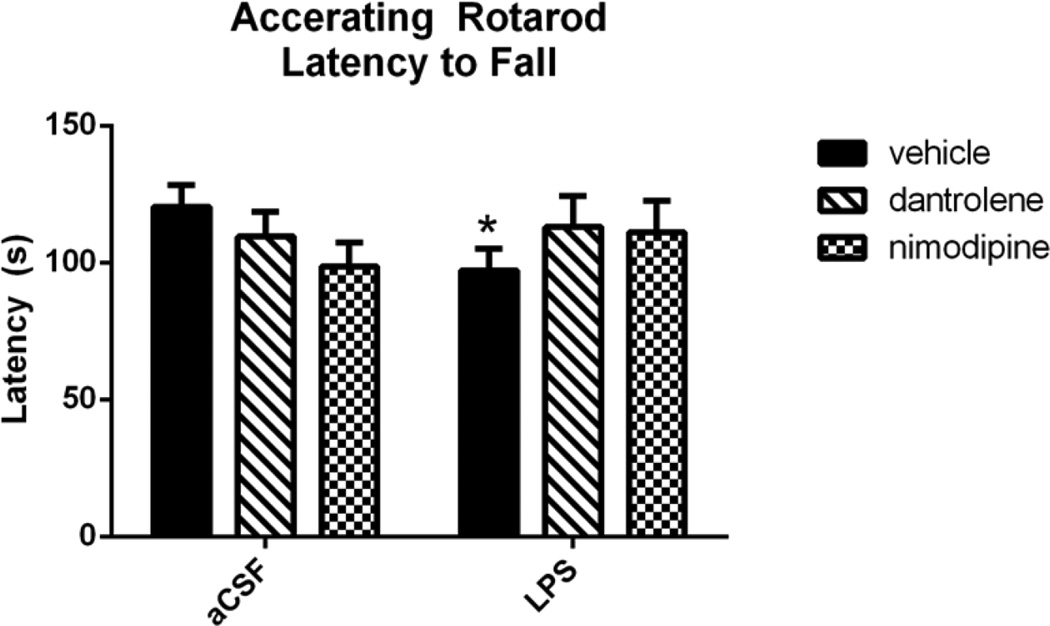
The accelerating rotarod test (Figure 1) was used to investigate the role of L-VDCC and RyR dysregulation in neuroinflammation-induced motor deficits after three weeks of LPS or aCSF infusion and treatment with vehicle, dantrolene, or nimodipine. LPS-infused rats treated with vehicle were significantly impaired in the task, as measured by a shorter latency to fall off of the accelerating rod. A 2-way ANOVA revealed a significant interaction between drug and group (F(2,165=3.070, p=0.049), and post-hoc analyses revealed a significant difference between aCSF and LPS within the vehicle-treated group (p=0.012). There was no difference between rats treated with dantrolene and nimodipine (p>0.3), suggesting a slightly protective effect of these drugs in preserving motor function during chronic neuroinflammation. Outliers that were greater or less than twice the group standard deviation were excluded from analysis.
Figure 1.
Rats infused with LPS and treated with vehicle had a significantly faster latency to fall off of the accelerating rotarod compared to aCSF rats treated with vehicle. LPS rats treated with dantrolene or nimodipine did not perform significantly worse than their aCSF treatment-matched controls. Data expressed as mean ± SEM. * indicates a significant difference from aCSF treatmentmatched control. Significance set to p<0.05.
Open field
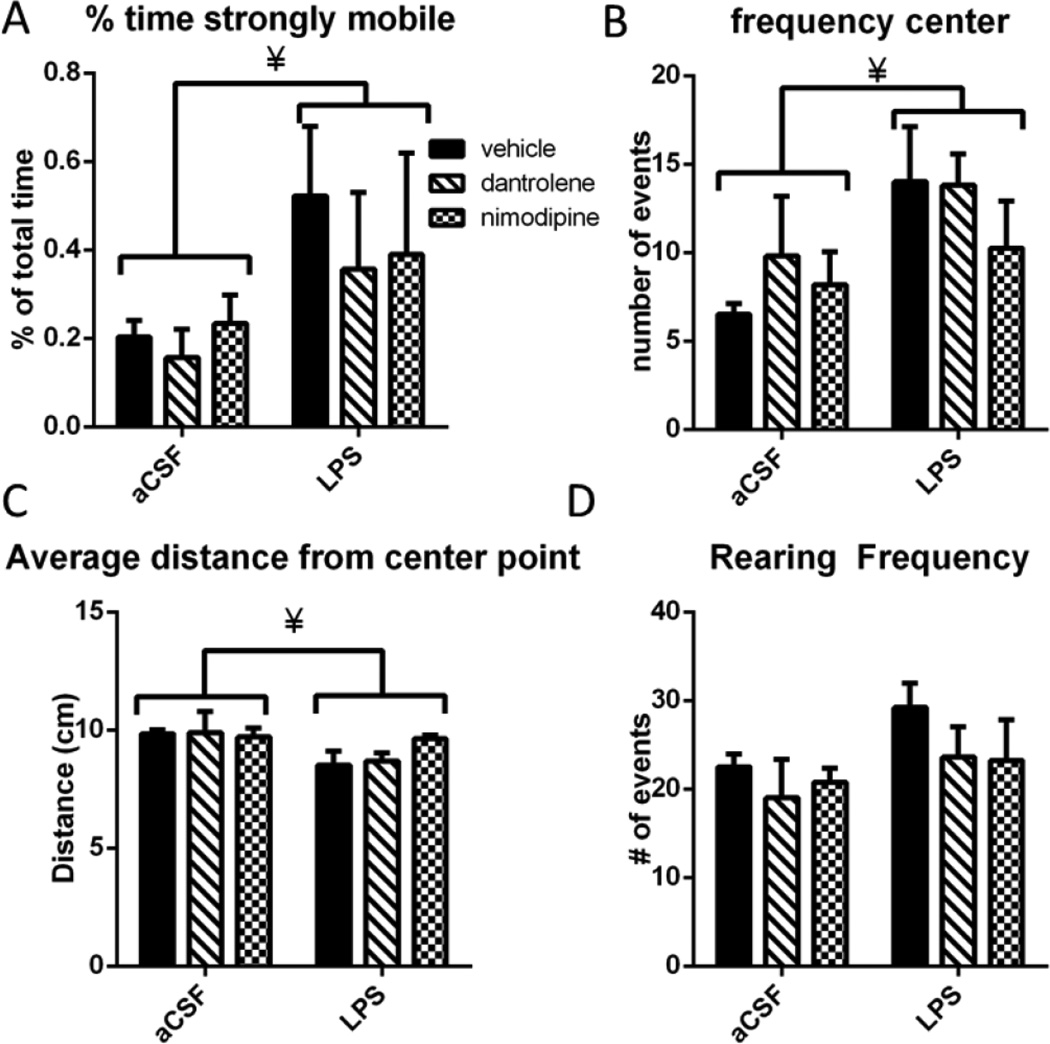
Rats were exposed to a novel open field environment to examine gross motor behavior as well as anxiety-like behaviors (Figure 2). LPS-infused rats were significantly more hyperactive, spending significantly more time strongly mobile (F(1,24)=4.639, p=0.042; Figure 2A). Strong mobility is determined automatically by the tracking software, and indicates that more than 60% of the pixels that make up the rat changed between each 1 second sampling period. LPS-infused rats entered the center of the arena more frequently (F(1,24=5.584, p=0.027; Figure 2B), which may be indicative of a reduced anxiety-like behavioral phenotype or may be due to their increased mobility. We measured the rats’ average distance from the center of the arena in order to further examine this potential anxiety-like behavior (Figure 2C). On average, the LPS-infused rats were significantly closer to the center point at any given time compared to the aCSF-treated rats (F(1,24)=4.602, p=0.042). There was also a trend toward a main effect of group (p=0.083) on rearing frequency during the open field (Figure 2D). Overall, these data indicate that LPS-infused rats are significantly more active than aCSF-treated rats and show reduced anxiety in an open field environment.
Figure 2.
A. Rats infused with LPS spent significantly more time strongly mobile. B. LPS infused rats visited the center of the open field arena significantly more frequently. C. LPS infused rats on average were significantly closer to the center of the arena at any given time. D. LPS infused rats reared more frequently (trend toward statistical significance. Data expressed as mean ± SEM. ¥ indicates significant main effect of LPS vs. aCSF. Significance set at p<0.05.
Forced swim
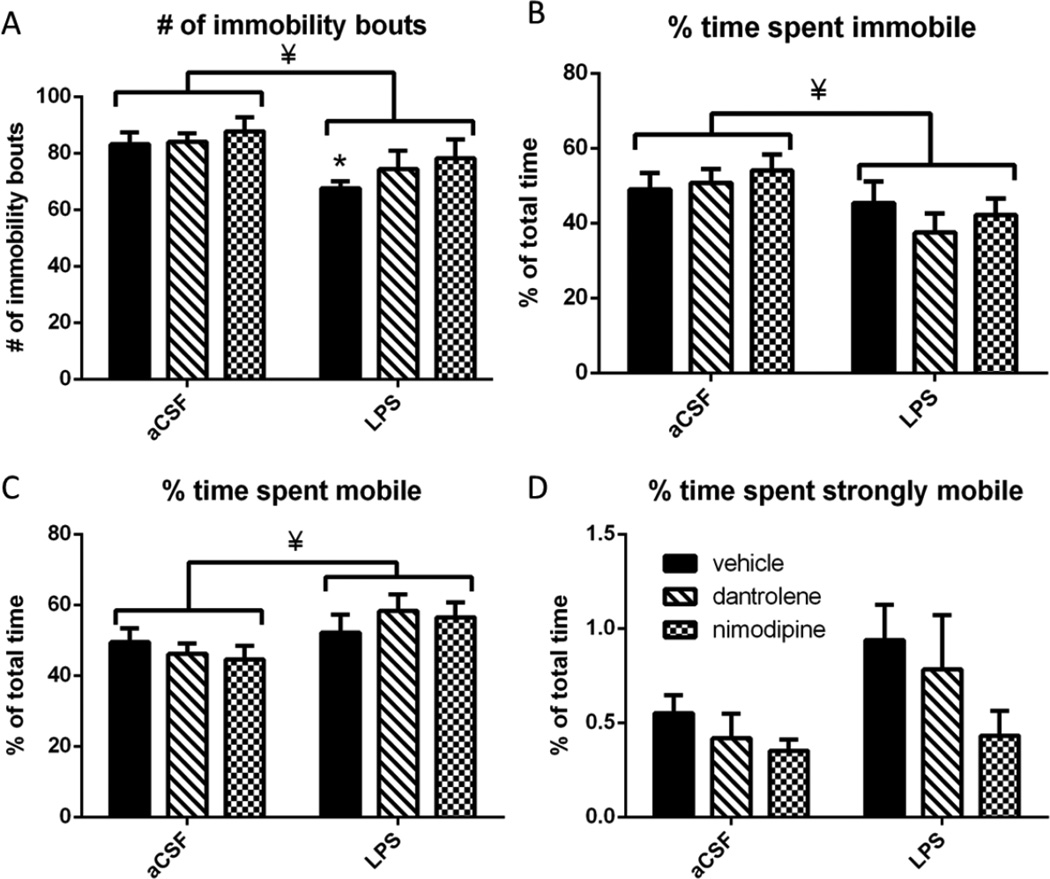
Rats were tested in the forced swim task in order to measure behavioral despair or depressivelike behavior. (Figure 3). LPS-infused rats had significantly fewer immobility bouts (F(1,54)=8.229, p=0.006), with a post-hoc test revealing that LPS-infused vehicle rats had significantly (p<0.05) fewer bouts of immobility than their aCSF controls. Dantrolene or nimodipine treatment did not significantly (p>0.05) alter the number of immobility bouts, suggesting a partial preservation of the normal behavior observed in the aCSF rats. We also examined the percentage of total time that rats spent immobile, mobile, or strongly immobile. Rats are immobile when fewer than 20% of the pixels that make up the rat changed within each 10 second sampling window, mobile when 20% to 60% change within each 10 second sampling window, and strongly mobile when more than 60% change within each 10 second sampling window. LPS-infused rats spent a significantly greater portion of time mobile than aCSF-treated rats (F(1,54)=6.769, p=0.012) and a significantly smaller portion of time immobile compared to aCSF-treated rats (F(1,54)=6.282, p=0.015). There was no statistically significant (p>0.05) change in the amount of time spent strongly mobile. Overall, these data demonstrate that LPS-infused rats are hyperactive in the forced swim task, spending more time mobile than their aCSF-treated controls. LPS-infused rats treated with either dantrolene or nimodipine had similar number of immobility bouts compared to their aCSF controls, but these drugs did not significantly reduce the overall hyperactive phenotype induced by chronic neuroinflammation.
Figure 3.
A. LPS-infused rats had significantly fewer immobility bouts in the forced swim task. Post-hoc analysis revealed that vehicle treated LPS rats were significantly different from vehicle treated aCSF rats. LPS rats treated with dantrolene or nimodipine were no different than their treatmentmatched aCSF controls. B. LPS infused rats spent significantly less time immobile compared to aCSF infused rats. C. LPS infused rats spent significantly more time mobile compared to aCSF infused rats. D. LPS infused rats trended toward spending significantly more time strongly mobile than aCSF infused rats. Data expressed as mean ± SEM. * indicates significant difference from treatment-matched aCSF controls. ¥ indicates significant main effect of LPS vs. aCSF. Significance set at p<0.05.
SNpc DA neurons
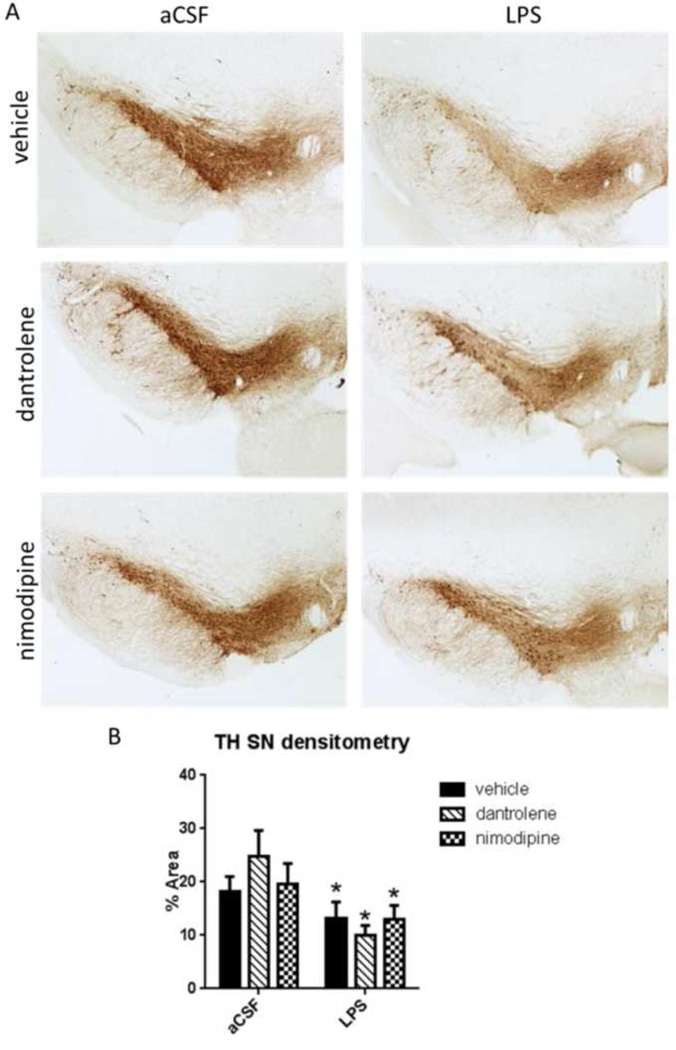
Deficits in accelerating rotarod are associated with damage to the SNpc (Monville et al., 2006). In order to assess SNpc damage due to chronic LPS infusion we performed immunohistochemistry of TH-positive neurons in brainstem sections containing SN (Figure 4). TH is the rate-limiting enzyme in the synthesis reaction for DA (Daubner et al., 2011), making it a relevant marker for DA neurons. LPS-infused rats had significantly less TH immunoreactivity in their SNpc cells as compared to aCSF-treated rats (F(1,181)=6.572, p<0.001). There was no effect of drug treatment on the amount of TH immunoreactivity in SNpc cells.
Figure 4.
A. Immunohistochemistry against TH in the SN labels DA neurons in the pars compacta subregion. B. Densitometric measurement showed a significant decrease in TH immunoreactivity due to LPS infusion with no effect of drug treatment. Data expressed as mean ± SEM. ¥ indicates significant main effect of LPS vs. aCSF. Significance set at p<0.05.
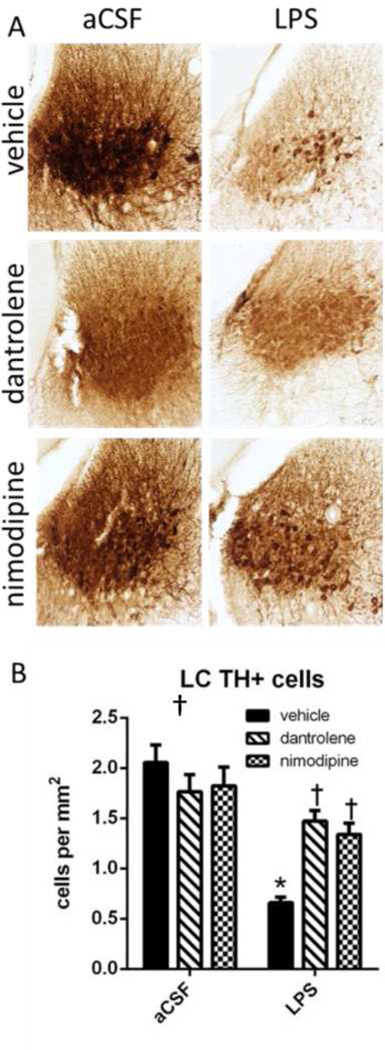
LC NE neurons
Hyperactivity in the forced swim task and open field are associated with decreased LC activity or increased LC lesion sizes (Stone et al., 2011; Harro et al., 1999). In order to assess LC damage due to chronic LPS infusion we performed immunohistochemistry of NE neurons in the LC by staining for TH (Error! Reference source not found.). TH is the first step in the production of NE (Daubner et al., 2011), making it a relevant target for labeling NE neurons in the LC. Chronic LPS infusion led to a significant decrease in the number of TH-immunoreactive neurons in the LC (F(1,303)=40.050, p<0.001). An ANOVA also revealed that there was a significant drug × group interaction (F(1,303)=9.367, p<0.001). Post-hoc analyses revealed that LPS + vehicle rats had significantly fewer TH-immunoreactive cells compared to aCSF + vehicle rats (p<0.001) and treatment with dantrolene or nimodipine, in the presence of LPS, restored the number of TH-immunoreactive cells to control levels in LPS-infused (p<0.001).
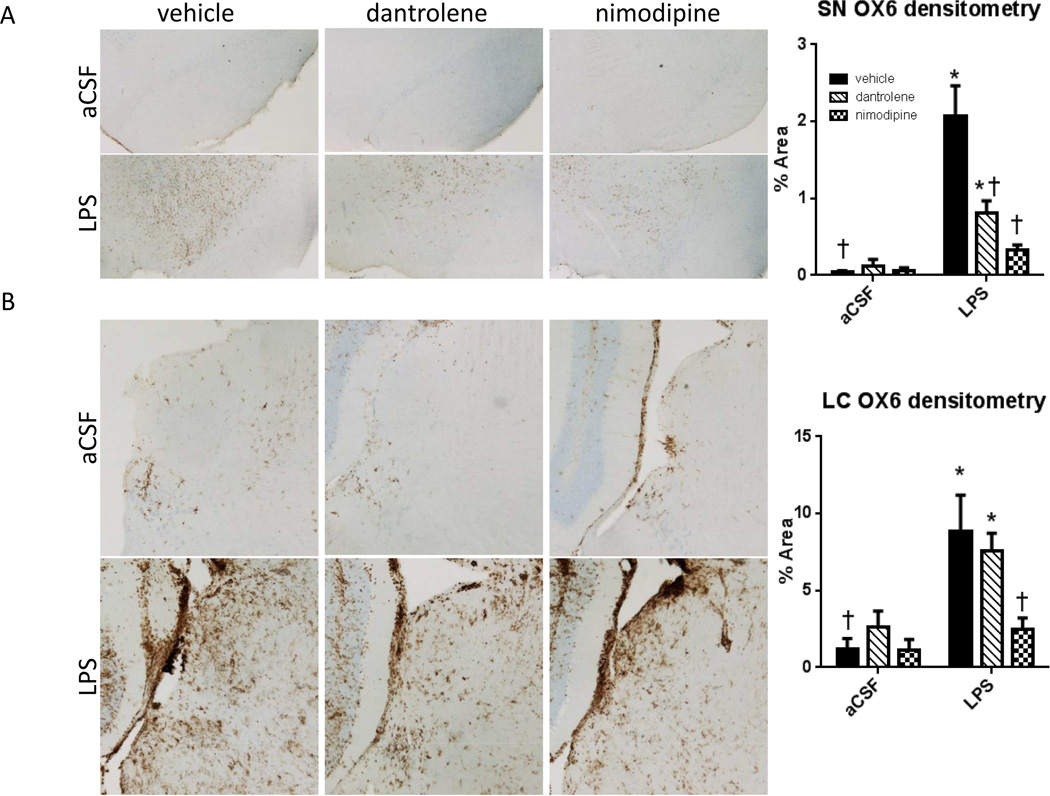
Activated microglia in the SN and LC
Microglia activation in the SN and LC were examined by MHC-II immunohistochemistry (Figure 6). LPS-infused rats had significantly more MHC-II immunoreactivity in their SNpc as measured by densitometry (F(1,145)=28.047, p<0.001). There was a significant main effect of drug (F(2,145)=7.673, p<0.001) and an interaction between drug and group (F(2, 145)=8.4, p<0.001). Post-hoc analyses revealed that LPS-infused rats treated with vehicle or dantrolene had significantly more MHC-II staining as compared to their treatment-matched aCSF controls (p<0.05). LPS-infused rats treated with either dantrolene or nimodipine had significantly less MHC-II immunoreactivity in the SNpc compared to LPS-infused rats treated with vehicle (p<0.001). Similar to the SNpc, the LC of LPS-infused rats had significantly more MHC-II immunoreactivity (F(1,85)=14.74, p<0.001). There was a significant main effect of drug (F(2,85)=3.414, p=0.037) on LC MHC-II staining. Post-hoc analyses revealed that LPS-infused rats treated with either vehicle or dantrolene had significantly (p<0.05) more MHC-II staining in their LCs as compared to their treatment-matched aCSF controls. LPS-infused rats treated with nimodipine had significantly less MHC-II immunoreacitivty in the LC compared to LPS-infused rats treated with vehicle (p=0.002), but there was no significant anti-inflammatory effect of dantrolene (p=0.551).
Figure 6.
A. Densitometry against OX6 in the SN shows an increase in MHC-II immunoreactivity in LPSinfused rats treated with vehicle, which is significantly reduced in dantrolene and nimodipine treated LPS-infused rats, with a full recovery observed with nimodipine. B. Densitometry against OX6 in the LC shows an increase in MHC-II immunoreactivity in LPS-infused rats treated with vehicle or dantrolene, which is significantly reduced in nimodipine treated LPS-infused rats, indicating a full recovery. Data expressed as mean ± SEM. * indicates a significant difference from treatment-matched aCSF controls, †indicates a significant difference from LPS + vehicle rats. Significance set as p<0.05.
Discussion
Chronic neuroinflammation disrupts motor behavior and reduces SNpc TH; partial rescue with calcium channel blockers
Neuroinflammation in the SN is associated with motor deficits that occur during normal aging (Beach et al., 2007), PD (Tansey and Goldberg, 2010; McGeer et al., 1988), and AD (McGeer et al., 1988). SN damage is the primary pathogenic feature of PD; due to the association between neuroinflammation and PD, several animal models utilizing LPS as an inflammagen have been characterized (Liu and Bing, 2011). All of these studies observed microglia activation, cytokine release, and DA cell degeneration; behavioral correlates for these studies are usually limited to ipsilateral deficits since the inflammagen is injected unilaterally (Liu and Bing, 2011). Our chronic LPS infusion model allows for examination of bilateral chronic neuroinflammation in the SNpc, more similar to that which is observed in PD. Chronic infusion of LPS significantly reduced the density of TH staining in the SNpc and induced a motor deficit. In contrast, LPS-infused rats treated with nimodipine and dantrolene did not show significant motor deficits. Although these results suggest a neuroprotective effect, we did not observe an increase in TH density in the SNpc of LPS-infused rats treated following treatment with either nimodipine or dantrolene. The absence of an apparent increase in TH density in the SNpc might be related to the detection limits presented by immunohistochemistry. However, we did observe a significant anti-inflammatory effect of both drugs, which may account for the behavioral recovery. Isradipine, a DHP L-VDC antagonist in the same class as nimodipine, has been shown to slightly reduce motor symptoms in humans, but its neuroprotective action on DA cells in the SNpc in humans has not been directly investigated (Parkinson Study Group, 2013). Similar to L-3–4-dihydroxyphenylalanine (L-DOPA), nimodipine may be enhancing the function of remaining SNpc neurons independent of preventing TH loss. Since RyR activity can increase L-VDCC activity and vice versa (Borde et al., 2000; Chavis et al., 1996), dantrolene may act via a similar mechanism.
Chronic neuroinflammation induces hyperactivity: the role of LC neurons
LPS infusion significantly reduced the number of TH-expressing cells in the LC, which is a phenomenon also observed early in AD (Simic et al., 2009) and influences disease severity (Tomlinson et al., 1981). Here, LPS-infused rats displayed reduced “anxiety-like” behaviors in the open field task. However, this apparent decrease in anxiety-like behavior may be due to an overall increase in hyperactivity in LPS-infused rats that was similar to that seen following extensive neurotoxin-induced lesions of the LC (Murrough et al., 2000). The observed increase in hyperactivity may be a manifestation of anxiety-like behavior, or agitation. Indeed, agitation and damage to the LC are related in AD patients (Leverenz et al., 2001). The forced swim task can be utilized as a measure of agitation: a normal rodent will alternate between two behavioral strategies, “agitation” during which rodents actively search for an escape, and “immobility” during which they wait for an escape to present itself in order to save energy (Thierry et al., 1984; Steru et al., 1985). Increased mobility, as observed in LPS-infused rats may not be an adaptive strategy as it leads to increased energy expenditure. Increased mobility during the forced swim task and open field task is associated with damage to the LC (Lin et al., 2011); conversely, increased LC activity is related to increased depressive-like behavior (Stone et al., 2011). Overall, the behavioral data presented here demonstrate that chronic LPS infusion induces an agitation-like phenotype that is maladaptive.
Previous studies measuring anxiety and depression during neuroinflammation utilized peripheral LPS infusion and observed increases in these anxiety-like and depressive-like behaviors (Custódio et al., 2013; Salazar et al., 2012; Swiergiel and Dunn, 2007). However, these acute inflammatory models may be modeling peripheral sickness behavior, which shares many characteristics of anxiety and depression (Dantzer et al., 2008). Our model of chronic LPS infusion does not increase levels of peripheral (serum) cytokines (Bardou et al., 2013) nor produces fever (Hauss-Wegrzyniak et al., 1998b), suggesting that our model does not induce sickness behavior, which may account for the lack of depressive- and anxiety-like behaviors.
Blockade of L-VDCCs or RyRs with nimodipine or dantrolene, respectively, reduced LPS-induced hyperactivity. Similarly, dantrolene and nimodipine also reduced loss of TH immunoreactive cells associated with LPS infusion. In the LC, nimodipine also showed an anti-inflammatory effect similar to that which was observed in the SN, but there was no anti-inflammatory effect of dantrolene. L-VDCC and RyR blockade may exert beneficial effects in the LC by normalizing LPS-induced disruption in LC pacemaking activity (Borsody and Weiss, 2004). LC pacemaking activity is L-VDCC-dependent (Williams et al., 1984) and may play an important role in the selective vulnerability of both the LC and SN during PD (Surmeier and Schumacker, 2013). As described earlier, pro-inflammatory cytokines increase the function of L-VDCCs (Furukawa and Mattson, 1998) and RyRs (Friedrich et al., 2014; Palmi and Meini, 2002) in the hippocampus. Taken together, these data suggest that neuroinflammation may disrupt normal L-VDCC and RyR activity in the LC.
Conclusions
Our results herein demonstrate, for the first time, that chronic neuroinflammation is sufficient to disrupt SNpc- and LC- dependent behaviors. Blockade of L-VDCCs or RyRs was able to improve both LC- and SNpc- dependent behaviors following LPS infusion, but this blockade only increased TH expression in the LC, suggesting a regional selectivity for neuroprotection with these drugs during chronic neuroinflammation. These data suggest that selectively vulnerable regions have differential underlying etiologies despite the common feature of calcium dysregulation. The differential effect of dantrolene and nimodipine treatment in these two different brain areas may be due to several factors. The SN may be more resistant to treatment due to the presence of DA, which itself can exacerbate pathology via a variety of mechanisms (Muñoz et al., 2012), or due to SN iron enrichment (Zhang et al., 2014). Overall, the differential effects of dantrolene and nimodipine in the SN and LC highlight the need for targeted pharmacological therapies in treatment of neurodegenerative diseases such as AD and PD.
Figure 5.
A. Immunohistochemistry against TH in the LC labels NE neurons. B. Cell counting revealed a significant reduction in the number of TH+ cells in the LC of LPS-infused vehicle rats compared to aCSF-infused vehicle rats. Treatment with dantrolene or nimodipine significantly increased the number of TH+ cells in rats infused with LPS compared to LPS rats treated with vehicle. Data expressed as mean ± SEM. * indicates a significant difference from treatment-matched aCSF controls, †indicates a significant difference from LPS + vehicle rats. Significance set as p<0.05.
Acknowledgements
Supported by U.S. Public Health Service, RO1 AG030331, RO1 AG037320, The Ohio State University Women and Philanthropy Program to GLW, and Howard Hughes Medical Institute Med-into-Grad fellowship to SCH.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bardou I, Brothers HM, Kaercher RM, Hopp SC, Wenk GL. Differential effects of duration and age on the consequences of neuroinflammation in the hippocampus. Neurobiol Aging. 2013;34(10):2293–2301. doi: 10.1016/j.neurobiolaging.2013.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardou I, Kaercher RM, Brothers HM, Hopp SC, Royer S, Wenk GL. Age and duration of inflammatory environment differentially affect the neuroimmune response and catecholaminergic neurons in the midbrain and brainstem. Neurobiol Aging. 2014;35(5):1065–1073. doi: 10.1016/j.neurobiolaging.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Sue LI, Walker DG, Lue LF, Connor DJ, Caviness JN, Sabbagh MN, Adler CH. Marked microglial reaction in normal aging human substantia nigra: correlation with extraneuronal neuromelanin pigment deposits. Acta Neuropathol. 2007;114(4):419–424. doi: 10.1007/s00401-007-0250-5. [DOI] [PubMed] [Google Scholar]
- Borde M, Bonansco C, Fernández de Sevilla D, Le Ray D, Buño W. Voltage-clamp analysis of the potentiation of the slow Ca2+-activated K+ current in hippocampal pyramidal neurons. Hippocampus. 2000;10(2):198–206. doi: 10.1002/(SICI)1098-1063(2000)10:2<198::AID-HIPO9>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Borsody MK, Weiss JM. The effects of endogenous interleukin-1 bioactivity on locus coeruleus neurons in response to bacterial and viral substances. Brain Res. 2004;1007(1–2):39–56. doi: 10.1016/j.brainres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318(1):121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Brothers HM, Bardou I, Hopp SC, Marchalant Y, Kaercher RM, Turner SM, Mitchem MR, Kigerl K, Wenk GL. Time-Dependent Compensatory Responses to Chronic Neuroinflammation in Hippocampus and Brainstem: The Potential Role of Glutamate Neurotransmission. J Alzheimers Dis Parkinsonism. 2013;3:110. doi: 10.4172/2161-0460.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebrián C, Zucca FA, Mauri P, Steinbeck JA, Studer L, Scherzer CR, Kanter E, Budhu S, Mandelbaum J, Vonsattel JP, Zecca L, Loike JD, Sulzer D. MHC-I expression renders catecholaminergic neurons susceptible to T-cell-mediated degeneration. Nat Commun. 2014;5:3633. doi: 10.1038/ncomms4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavis P, Fagni L, Lansman JB, Bockaert J. Functional coupling between ryanodine receptors and L-type calcium channels in neurons. Nature. 1996;382(6593):719–722. doi: 10.1038/382719a0. [DOI] [PubMed] [Google Scholar]
- Custódio CS, Mello BSF, Cordeiro RC, de Araújo FYR, Chaves JH, Vasconcelos SMM, Macêdo DS. Time course of the effects of lipopolysaccharide on prepulse inhibition and brain nitrite content in mice. Eur J Pharmacol. 2013;713(1–3):31–38. doi: 10.1016/j.ejphar.2013.04.040. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubner SC, Le T, Wang S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys. 2011;508(1):1–12. doi: 10.1016/j.abb.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Tredici K, Rüb U, De Vos RAI, Bohl JRE, Braak H. Where does parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol. 2002;61(5):413–426. doi: 10.1093/jnen/61.5.413. [DOI] [PubMed] [Google Scholar]
- Foster TC. Dissecting the age-related decline on spatial learning and memory tasks in rodent models: N-methyl-D-aspartate receptors and voltage-dependent Ca2+ channels in senescent synaptic plasticity. Prog Neurobiol. 2012;96(3):283–303. doi: 10.1016/j.pneurobio.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich O, Yi B, Edwards JN, Reischl B, Wirth-Hücking A, Buttgereit A, Lang R, Weber C, Polyak F, Liu I, von Wegner F, Cully TR, Lee A, Most P, Völkers M. Interleukin-1α Reversibly Inhibits Skeletal Muscle Ryanodine Receptor: A Novel Mechanism for Critical Illness Myopathy? Am J Respir Cell Mol Biol. 2014;50(6):1096–1106. doi: 10.1165/rcmb.2013-0059OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K, Mattson MP. The transcription factor NF-kappaB mediates increases in calcium currents and decreases in NMDA- and AMPA/kainate-induced currents induced by tumor necrosis factor-alpha in hippocampal neurons. J Neurochem. 1998;70(5):1876–1886. doi: 10.1046/j.1471-4159.1998.70051876.x. [DOI] [PubMed] [Google Scholar]
- Harro J, Pähkla R, Modiri A-R, Harro M, Kask A, Oreland L. Dose-dependent effects of noradrenergic denervation by DSP-4 treatment on forced swimming and beta-adrenoceptor binding in the rat. J Neural Transm. 1999;106(7–8):619–629. doi: 10.1007/s007020050184. [DOI] [PubMed] [Google Scholar]
- Hashioka S, Klegeris A, McGeer PL. Inhibition of human astrocyte and microglia neurotoxicity by calcium channel blockers. Neuropharmacology. 2012;63(4):685–691. doi: 10.1016/j.neuropharm.2012.05.033. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Dobrzanski P, Stoehr JD, Wenk GL. Chronic neuroinflammation in rats reproduces components of the neurobiology of Alzheimer’s disease. Brain Res. 1998a;780(2):294–303. doi: 10.1016/s0006-8993(97)01215-8. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Lukovic L, Bigaud M, Stoeckel ME. Brain inflammatory response induced by intracerebroventricular infusion of lipopolysaccharide: an immunohistochemical study. Brain Res. 1998b;794(2):211–224. doi: 10.1016/s0006-8993(98)00227-3. [DOI] [PubMed] [Google Scholar]
- Horvath J, Burkhard PR, Herrmann FR, Bouras C, Kövari E. Neuropathology of parkinsonism in patients with pure Alzheimer’s disease. J Alzheimers Dis. 2014;39(1):115–120. doi: 10.3233/JAD-131289. [DOI] [PubMed] [Google Scholar]
- Iannaccone S, Cerami C, Alessio M, Garibotto V, Panzacchi A, Olivieri S, Gelsomino G, Moresco RM, Perani D. In vivo microglia activation in very early dementia with Lewy bodies, comparison with Parkinson’s disease. Parkinsonism Relat Disord. 2013;19(1):47–52. doi: 10.1016/j.parkreldis.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Jost BC, Grossberg GT. The evolution of psychiatric symptoms in Alzheimer’s disease: a natural history study. J Am Geriatr Soc. 1996;44(9):1078–1081. doi: 10.1111/j.1532-5415.1996.tb02942.x. [DOI] [PubMed] [Google Scholar]
- Klegeris A, Choi HB, McLarnon JG, McGeer PL. Functional ryanodine receptors are expressed by human microglia and THP-1 cells: Their possible involvement in modulation of neurotoxicity. J Neurosci Res. 2007;85(10):2207–2215. doi: 10.1002/jnr.21361. [DOI] [PubMed] [Google Scholar]
- Leverenz JB, Miller MA, Dobie DJ, Peskind ER, Raskind MA. Increased alpha 2-adrenergic receptor binding in locus coeruleus projection areas in dementia with Lewy bodies. Neurobiol Aging. 2001;22(4):555–561. doi: 10.1016/s0197-4580(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Li Y, Hu X, Liu Y, Bao Y, An L. Nimodipine protects dopaminergic neurons against inflammation-mediated degeneration through inhibition of microglial activation. Neuropharmacology. 2009;56(3):580–589. doi: 10.1016/j.neuropharm.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Lin Y, Sarfraz Y, Jensen A, Dunn AJ, Stone EA. Participation of brainstem monoaminergic nuclei in behavioral depression. Pharmacol Biochem Behav. 2011;100(2):330–339. doi: 10.1016/j.pbb.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Bing G. Lipopolysaccharide animal models for Parkinson’s disease. Parkinson’s Dis. 2011;2011:327089. doi: 10.4061/2011/327089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Parkinson’s disease: don't mess with calcium. J Clin Invest. 2012;122(4):1195–1198. doi: 10.1172/JCI62835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer's disease brains. Neurology. 1988;38(8):1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- Monville C, Torres EM, Dunnett SB. Comparison of incremental and accelerating protocols of the rotarod test for the assessment of motor deficits in the 6-OHDA model. J Neurosci Methods. 2006;158(2):219–223. doi: 10.1016/j.jneumeth.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Muñoz P, Huenchuguala S, Paris I, Segura-Aguilar J. Dopamine oxidation and autophagy. Parkinson’s Dis. 2012;20:920–953. doi: 10.1155/2012/920953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough J, Boss-Williams K, Emery M, Bonsall R, Weiss J. Depletion of brain norepinephrine does not reduce spontaneous ambulatory activity of rats in the home cage. Brain Res. 2000;883:125–130. doi: 10.1016/s0006-8993(00)02850-x. [DOI] [PubMed] [Google Scholar]
- Palmi M, Meini A. Role of the nitric oxide/cyclic GMP/Ca2+ signaling pathway in the pyrogenic effect of interleukin-1beta. Mol Neurobiol. 2002;25(2):133–147. doi: 10.1385/MN:25:2:133. [DOI] [PubMed] [Google Scholar]
- Parkinson Study Group. Phase II safety, tolerability, and dose selection study of isradipine as a potential disease-modifying intervention in early Parkinson’s disease (STEADY-PD) Mov Dis. 2013;28(13):1823–1831. doi: 10.1002/mds.25639. [DOI] [PubMed] [Google Scholar]
- Ravina B, Camicioli R, Como PG, Marsh L, Jankovic J, Weintraub D, Elm J. The impact of depressive symptoms in early Parkinson disease. Neurology. 2007;69(4):342–347. doi: 10.1212/01.wnl.0000268695.63392.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar A, Gonzalez-Rivera BL, Redus L, Parrott JM, O’Connor JC. Indoleamine 2,3-dioxygenase mediates anhedonia and anxiety-like behaviors caused by peripheral lipopolysaccharide immune challenge. Horm Behav. 2012;62(3):202–209. doi: 10.1016/j.yhbeh.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrempf W, Brandt MD, Storch A, Reichmann H. Sleep disorders in Parkinson’s disease. J Parkinsons Dis. 2014;4(2):211–221. doi: 10.3233/JPD-130301. [DOI] [PubMed] [Google Scholar]
- Simic G, Stanic G, Mladinov M, Jovanov-Milosevic N, Kostovic I, Hof PR. Does Alzheimer’s disease begin in the brainstem? Neuropathol Appl Neurobiol. 2009;35(6):532–554. doi: 10.1111/j.1365-2990.2009.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkstein SE, Mizrahi R. Depression in Alzheimer’s disease. Expert Rev Neurother. 2006;6(6):887–895. doi: 10.1586/14737175.6.6.887. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology. 1985;85(3):367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Stone EA, Lin Y, Sarfraz Y, Quartermain D. The role of the central noradrenergic system in behavioral inhibition. Brain Res Rev. 2011;67(1–2):193–208. doi: 10.1016/j.brainresrev.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Schumacker PT. Calcium, bioenergetics, and neuronal vulnerability in Parkinson’s disease. J Biol Chem. 2013;288(15):10736–10741. doi: 10.1074/jbc.R112.410530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiergiel AH, Dunn AJ. Effects of interleukin-1beta and lipopolysaccharide on behavior of mice in the elevated plus-maze and open field tests. Pharmacol Biochem Behav. 2007;86(4):651–659. doi: 10.1016/j.pbb.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey MG, Goldberg MS. Neuroinflammation in Parkinson’s disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis. 2010;37(3):510–518. doi: 10.1016/j.nbd.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry B, Steru L, Chermat R, Simon P. Searching-waiting strategy: a candidate for an evolutionary model of depression? Behav Neural Biol. 1984;41(2):180–189. doi: 10.1016/s0163-1047(84)90555-7. [DOI] [PubMed] [Google Scholar]
- Tomlinson BE, Irving D, Blessed G. Cell loss in the locus coeruleus in senile dementia of Alzheimer type. J Neurol Sci. 1981;49(3):419–428. doi: 10.1016/0022-510x(81)90031-9. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7217992. [DOI] [PubMed] [Google Scholar]
- Williams JT, North RA, Shefner SA, Nishi S, Egan TM. Membrane properties of rat locus coeruleus neurones. Neuroscience. 1984;13(1):137–156. doi: 10.1016/0306-4522(84)90265-3. [DOI] [PubMed] [Google Scholar]
- Yasuno F, Kosaka J, Ota M, Higuchi M, Ito H, Fujimura Y, Nozaki S, Takahashi S, Mizukami K, Asada T, Suhara T. Increased binding of peripheral benzodiazepine receptor in mild cognitive impairment-dementia converters measured by positron emission tomography with [11C]DAA1106. Psychiatry Res. 2012;203(1):67–74. doi: 10.1016/j.pscychresns.2011.08.013. [DOI] [PubMed] [Google Scholar]
- Zhang W, Yan Z, Gao J, Sun L, Huang X, Liu Z, Yu S, Cao C-J, Zuo L, Chen Z-J, Hu Y, Wang F, Hong J, Wang X. Role and mechanism of microglial activation in iron-induced selective and progressive dopaminergic neurodegeneration. Mol Neurobiol. 2014;49(3):1153–1165. doi: 10.1007/s12035-013-8586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]