Abstract
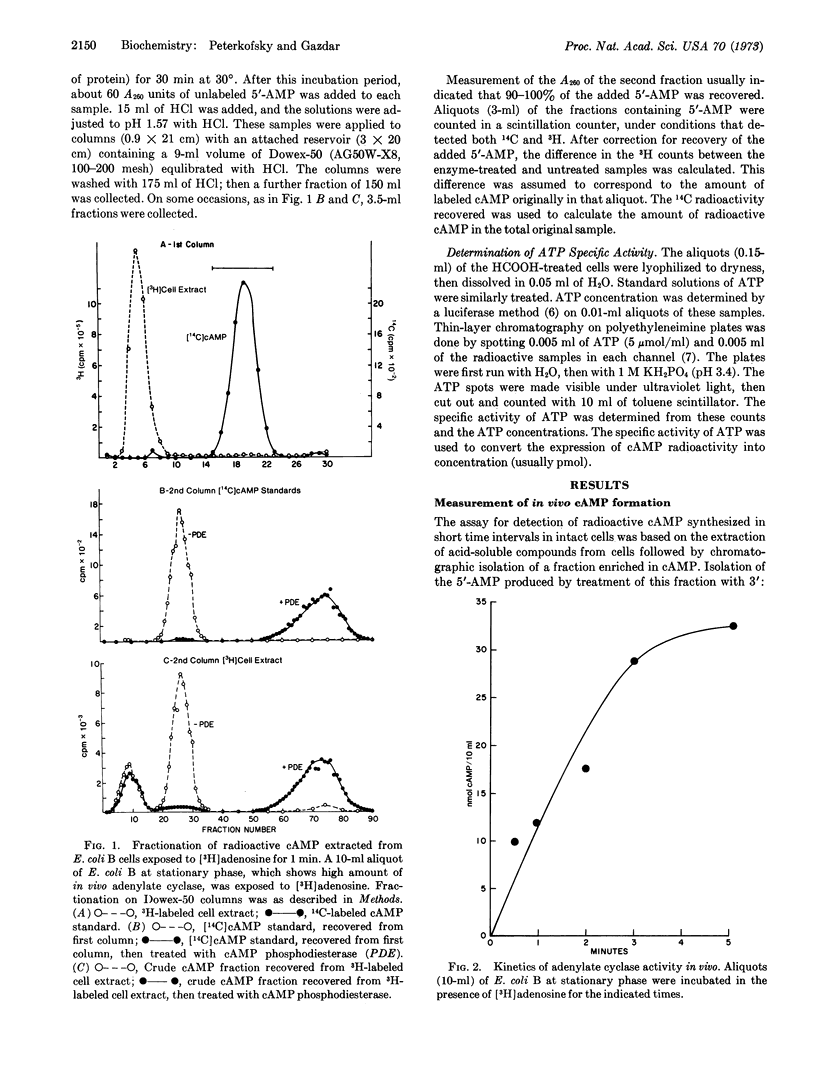
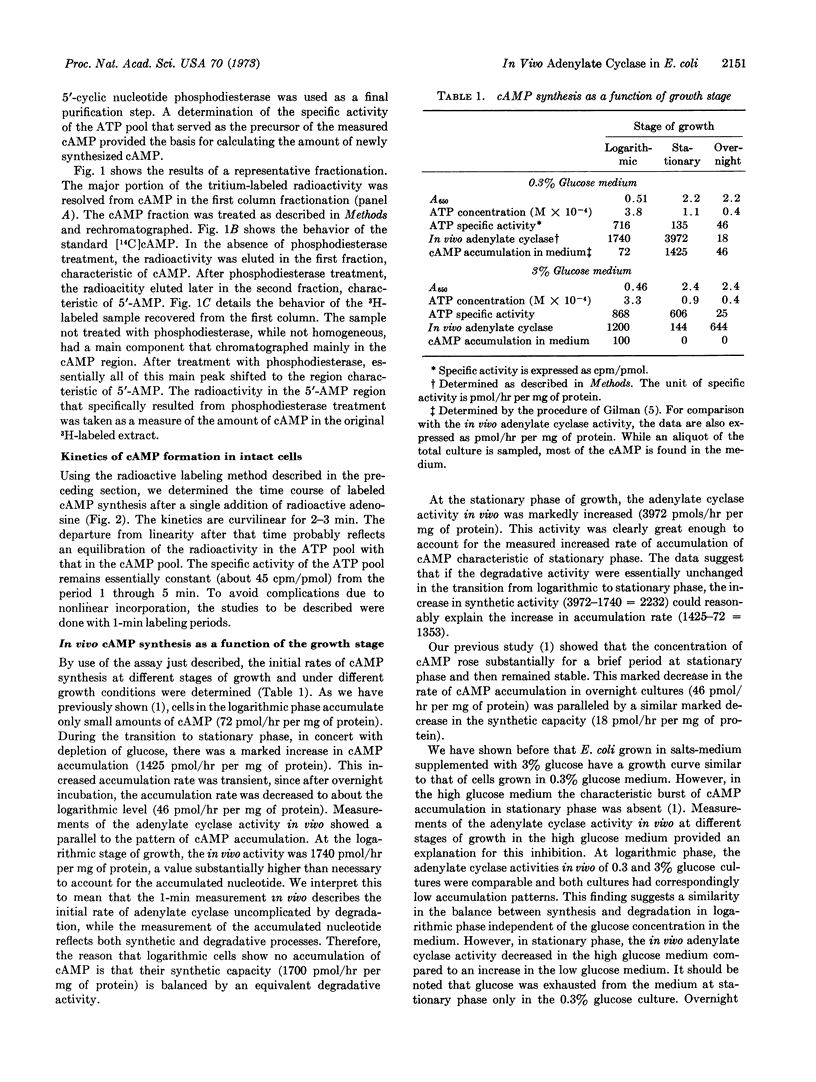
A method for labeling adenosine 3′:5′-cyclic monophosphate in vivo from precursor adenosine followed by quantitative analysis of the labeled nucleotide is described. When the labeling period is short and the specific activity of the ATP pool is determined, the rate of incorporation of radioactivity corresponds to a determination of adenylate cyclase activity in vivo. In E. coli B the specific activity of adenylate cyclase in vivo varies under different growth conditions. Under all conditions tested, the adenylate cyclase activity in vivo is great enough to account for the pattern of accumulation of cyclic AMP. This is in contrast to previous adenylate cyclase assays in vitro, where the measured enzyme activities were insufficient to account for the amounts of cyclic AMP accumulated.
Keywords: adenylate cyclase, glucose utilization
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cashel M., Lazzarini R. A., Kalbacher B. An improved method for thin-layer chromatography of nucleotide mixtures containing 32P-labelled orthophosphate. J Chromatogr. 1969 Mar 11;40(1):103–109. doi: 10.1016/s0021-9673(01)96624-5. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klofat W., Picciolo G., Chappelle E. W., Freese E. Production of adenosine triphosphate in normal cells and sporulation mutants of Bacillus subtilis. J Biol Chem. 1969 Jun 25;244(12):3270–3276. [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- Kuo J. F., De Renzo E. C. A comparison of the effects of lipolytic and antilipolytic agents on adenosine 3',5'-monophosphate levels in adipose cells as determined by prior labeling with adenine-8-14C. J Biol Chem. 1969 May 10;244(9):2252–2260. [PubMed] [Google Scholar]
- MAKMAN R. S., SUTHERLAND E. W. ADENOSINE 3',5'-PHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1965 Mar;240:1309–1314. [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Glucose and the metabolism of adenosine 3':5'-cyclic monophosphate in Escherichia coli. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2794–2798. doi: 10.1073/pnas.68.11.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]


