Abstract
Hemophilia B is a bleeding disorder caused by mutations in the factor IX gene. The disorder is X-linked recessive with a prevalence of about 1 in 30,000 Caucasian males. Factor IX is naturally synthesized in the liver and secreted into blood. Here we report the construction of recombinant adenoviral vectors containing the canine factor IX cDNA that are capable of transducing hepatocytes in mice at high efficiencies in vivo without partial hepatectomy. The recombinant viral vector was used to treat hemophilia B dogs by direct vector infusion into the portal vasculature of deficient animals. Plasma factor IX concentrations in the treated hemophilia B dogs increased from 0 to 300% of the level present in normal dogs, resulting in complete amelioration of the disease as demonstrated by normal blood coagulation and hemostatic measurements. Although plasma factor IX concentration started to decline after a few days, therapeutic levels of factor IX persisted for 1-2 months in the treated animals. The results validate the principle of in vivo hepatic gene delivery to reconstitute the genetic deficiency in a large animal model and suggest that gene therapy is achievable when long-acting vectors are developed.
Full text
PDF
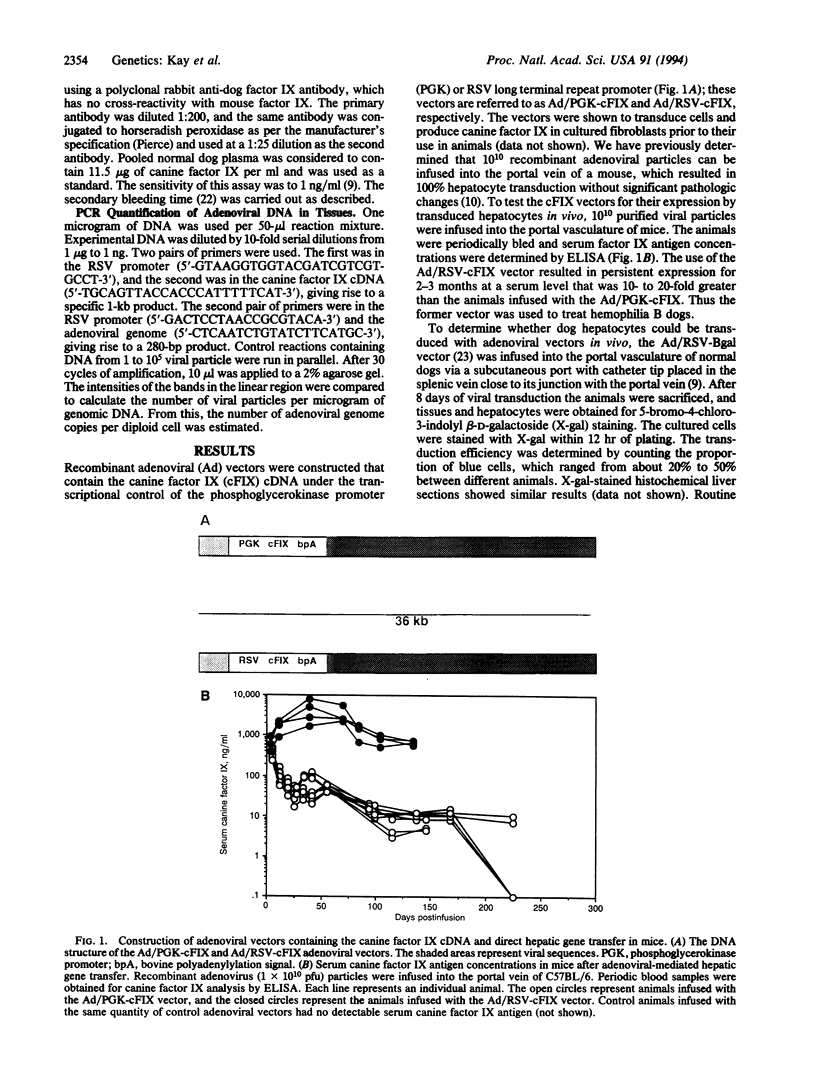
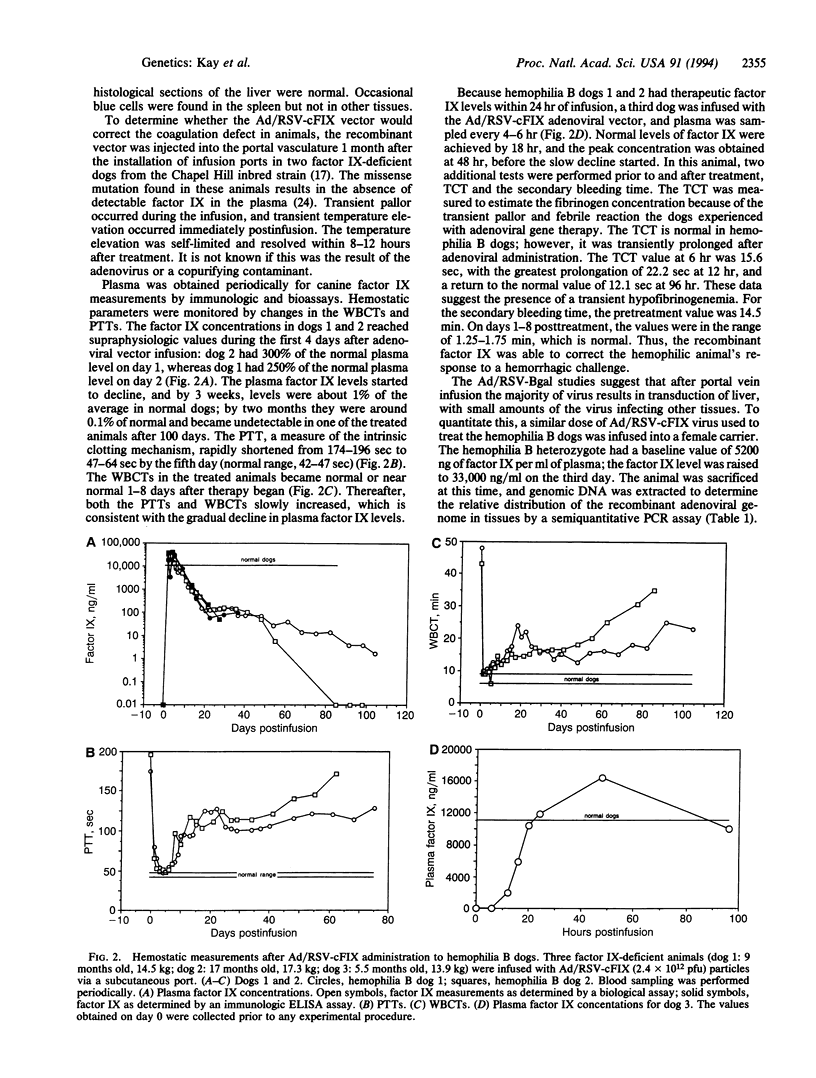


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armentano D., Thompson A. R., Darlington G., Woo S. L. Expression of human factor IX in rabbit hepatocytes by retrovirus-mediated gene transfer: potential for gene therapy of hemophilia B. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6141–6145. doi: 10.1073/pnas.87.16.6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkhous K. M., Davis P. D., Graham J. B., Dodds W. J. Expression and linkage of genes for X-linked hemophilias A and B in the dog. Blood. 1973 Apr;41(4):577–585. [PubMed] [Google Scholar]
- Brinkhous K. M. Gene transfer in the hemophilias: retrospect and prospect. Thromb Res. 1992 Aug 1;67(3):329–338. doi: 10.1016/0049-3848(92)90008-x. [DOI] [PubMed] [Google Scholar]
- Brinkhous K. M., Hedner U., Garris J. B., Diness V., Read M. S. Effect of recombinant factor VIIa on the hemostatic defect in dogs with hemophilia A, hemophilia B, and von Willebrand disease. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1382–1386. doi: 10.1073/pnas.86.4.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkhous K. M., Sandberg H., Garris J. B., Mattsson C., Palm M., Griggs T., Read M. S. Purified human factor VIII procoagulant protein: comparative hemostatic response after infusions into hemophilic and von Willebrand disease dogs. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8752–8756. doi: 10.1073/pnas.82.24.8752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y., Roman M., Naviaux R. K., Verma I. M. Gene therapy via primary myoblasts: long-term expression of factor IX protein following transplantation in vivo. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10892–10895. doi: 10.1073/pnas.89.22.10892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. P., Brinkhous K. M., Brayer G. D., Reisner H. M., High K. A. Canine hemophilia B resulting from a point mutation with unusual consequences. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10095–10099. doi: 10.1073/pnas.86.24.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard A. J., Hudson D. L., Brownlee G. G., Watt F. M. Towards gene therapy for haemophilia B using primary human keratinocytes. Nat Genet. 1993 Feb;3(2):180–183. doi: 10.1038/ng0293-180. [DOI] [PubMed] [Google Scholar]
- Herz J., Gerard R. D. Adenovirus-mediated transfer of low density lipoprotein receptor gene acutely accelerates cholesterol clearance in normal mice. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2812–2816. doi: 10.1073/pnas.90.7.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIM R. T. A study of the plasma thrombin time. J Lab Clin Med. 1957 Jul;50(1):45–60. [PubMed] [Google Scholar]
- Jaffe H. A., Danel C., Longenecker G., Metzger M., Setoguchi Y., Rosenfeld M. A., Gant T. W., Thorgeirsson S. S., Stratford-Perricaudet L. D., Perricaudet M. Adenovirus-mediated in vivo gene transfer and expression in normal rat liver. Nat Genet. 1992 Aug;1(5):372–378. doi: 10.1038/ng0892-372. [DOI] [PubMed] [Google Scholar]
- Kay M. A., Baley P., Rothenberg S., Leland F., Fleming L., Ponder K. P., Liu T., Finegold M., Darlington G., Pokorny W. Expression of human alpha 1-antitrypsin in dogs after autologous transplantation of retroviral transduced hepatocytes. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):89–93. doi: 10.1073/pnas.89.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. A., Rothenberg S., Landen C. N., Bellinger D. A., Leland F., Toman C., Finegold M., Thompson A. R., Read M. S., Brinkhous K. M. In vivo gene therapy of hemophilia B: sustained partial correction in factor IX-deficient dogs. Science. 1993 Oct 1;262(5130):117–119. doi: 10.1126/science.8211118. [DOI] [PubMed] [Google Scholar]
- LANGDELL R. D., WAGNER R. H., BRINKHOUS K. M. Effect of antihemophilic factor on one-stage clotting tests; a presumptive test for hemophilia and a simple one-stage antihemophilic factor assy procedure. J Lab Clin Med. 1953 Apr;41(4):637–647. [PubMed] [Google Scholar]
- Li Q., Kay M. A., Finegold M., Stratford-Perricaudet L. D., Woo S. L. Assessment of recombinant adenoviral vectors for hepatic gene therapy. Hum Gene Ther. 1993 Aug;4(4):403–409. doi: 10.1089/hum.1993.4.4-403. [DOI] [PubMed] [Google Scholar]
- McGrory W. J., Bautista D. S., Graham F. L. A simple technique for the rescue of early region I mutations into infectious human adenovirus type 5. Virology. 1988 Apr;163(2):614–617. doi: 10.1016/0042-6822(88)90302-9. [DOI] [PubMed] [Google Scholar]
- Miyanohara A., Johnson P. A., Elam R. L., Dai Y., Witztum J. L., Verma I. M., Friedmann T. Direct gene transfer to the liver with herpes simplex virus type 1 vectors: transient production of physiologically relevant levels of circulating factor IX. New Biol. 1992 Mar;4(3):238–246. [PubMed] [Google Scholar]
- Soriano P., Friedrich G., Lawinger P. Promoter interactions in retrovirus vectors introduced into fibroblasts and embryonic stem cells. J Virol. 1991 May;65(5):2314–2319. doi: 10.1128/jvi.65.5.2314-2319.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spessot R., Inchley K., Hupel T. M., Bacchetti S. Cloning of the herpes simplex virus ICP4 gene in an adenovirus vector: effects on adenovirus gene expression and replication. Virology. 1989 Feb;168(2):378–387. doi: 10.1016/0042-6822(89)90279-1. [DOI] [PubMed] [Google Scholar]
- Stratford-Perricaudet L. D., Makeh I., Perricaudet M., Briand P. Widespread long-term gene transfer to mouse skeletal muscles and heart. J Clin Invest. 1992 Aug;90(2):626–630. doi: 10.1172/JCI115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A. R., Palmer T. D., Lynch C. M., Miller A. D. Gene transfer as an approach to cure patients with hemophilia A or B. Curr Stud Hematol Blood Transfus. 1991;(58):59–62. doi: 10.1159/000419338. [DOI] [PubMed] [Google Scholar]
- Yao S. N., Kurachi K. Expression of human factor IX in mice after injection of genetically modified myoblasts. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3357–3361. doi: 10.1073/pnas.89.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]



