Introduction
Due to the frequency of urinary tract infections (UTI) in children with vesicoureteral reflux (VUR), the need exists for a well-designed and appropriately powered study that can determine the effectiveness of antimicrobial prophylaxis on preventing recurrences of UTI and on decreasing the incidence of renal scarring. Since the currently recommended follow-up studies, including repeated urine cultures, renal and genitourinary imaging, antimicrobial therapy and antibiotic prophylaxis as well as compliance with protocols, are costly and cumbersome, these recommendations ought to be based on actual evidence. Because of the relatively low prevalence of renal scarring, the cost of identification of VUR, and the potential problems of long-term antimicrobial prophylaxis and/or surgical correction of VUR, a carefully designed and sufficiently large prospective clinical trial to assess the effectiveness of current evaluation and therapeutic strategies seems warranted. We propose a multi-center, randomized, placebo-controlled, double blind study to determine whether, in the setting of a prompt evaluation of UTI symptoms and early therapy of culture-proven UTI, administration of daily antimicrobial prophylaxis is superior to daily placebo in preventing recurrent UTI and renal scarring in children aged from 2 to 72 months, diagnosed with grades I-IV VUR following an initial episode of UTI.
The Link between Urinary Tract Infection and Vesicoureteral Reflux: Contemporary Issues and Rationale for the Randomized Intervention in Children with Vesicoureteral Reflux (RIVUR) Trial
Urinary tract infection (UTI) remains the most frequently occurring serious bacterial infection during childhood1. Estimates of the cumulative incidence of UTI in children under age 6 years (3–7% in girls and 1–2% in boys) suggest that between 70,000 to 180,000 of the annual US birth cohort will have a UTI by age 62. UTIs have been considered to be the principal cause of permanent renal parenchymal damage and scarring in children, especially those with vesicoureteral reflux (VUR)3. VUR results in urine passing up the ureter in a retrograde fashion. The extent of passage up the ureter is categorized hierarchically, with grades III, IV, and V being defined by progressive dilatation and distention of the renal pelvis3, 4. Since VUR is found in 30% to 40% of children with a UTI, the current standard of care has included performance of an imaging procedure to assess the presence and extent of reflux5, 6. This strategy is dependent upon the hypothesis that reflux, especially of higher grades, increases the risk of recurrent UTIs and renal scarring, with associated sequelae in later life of proteinuria, hypertension, eclampsia and end-stage renal disease (ESRD)2, 5, 7–9. Despite the recommendation that all children with a UTI be evaluated for reflux, there is a failure to do so in contemporary populations. In a recent survey of infants under one year of age who had febrile UTIs, up to 40% were not evaluated for reflux, as recommended by the American Academy of Pediatrics10. This suggests that even at this point in the United States many children with reflux remain undiagnosed after a UTI.
The causal relationship between reflux and pyelonephritic scarring has recently been challenged by long-term studies that demonstrate that renal scarring can occur in children without VUR and that not all children with VUR, including those with higher grades of reflux, have renal scars1, 11. Further, monogenic (and even polygenic) conditions result in reflux and progressive renal damage, often as a component of an identified syndrome; these can be viewed as separate from primary reflux12, 13. Reports from the 1960’s and 1970’s, when reflux was less frequently recognized, revealed that pyelonephritic scarring was the etiology of 50% of the cases of hypertension and 30% of cases of end stage renal disease in children14–17. These rates have been considerably reduced in some contemporary populations as a result of widespread recognition and treatment, with scarring now accounting for 5% of children with hypertension and significant renal impairment in this country18, 19. This has not been the case worldwide, however. Craig has shown that there has been no decrease in the incidence of reflux-related end stage renal disease in Australia and New Zealand over the last several decades20. Approximately 14% of all children enrolled in their end stage renal disease registry had reflux as a cause, and this has remained constant from the 1960’s through the 1990’s. They postulated that the identification of reflux had no impact on the rates of renal failure. It was assumed by the authors, however, that primary care physicians have assiduously evaluated children for reflux in recent decades, but this may not be true, as shown above. Reflux remains a leading cause of chronic renal failure in children and young adults in Italy, accounting for 25% of all cases21. Most of those patients had greater than grade III reflux and 76% were boys. Many of the boys were born with high-grade reflux and renal dysplasia and it is possible that they would have progressed to renal failure despite any type of post-natal management. Again, it is also possible that the relatively high rate of reflux-related end-stage renal disease may be related to failure to diagnose reflux after an initial UTI in many children.
More importantly, studies comparing the effectiveness of combined surgical correction and antimicrobial prophylaxis to antimicrobial prophylaxis alone have not demonstrated differences in rates of renal scarring1, 2, 5, 9, 11, 22. Other concerns about current diagnostic and therapeutic strategies include the cost and potential psychological harm of studies to detect VUR and its follow-up to resolution,5, 11, 22, 23 and the development of antimicrobial resistance with long-term prophylactic antibiotic use24, 25. Doubts have arisen concerning the role of VUR in renal scarring and the efficacy of current therapeutic strategies in comparison to prompt evaluation and treatment of UTI2, 7, 8, 26.
Because of the low prevalence of renal scarring, costs of identifying children with VUR, and the potential problems of long-term antimicrobial prophylaxis and/or surgical correction of VUR, the need exists for a carefully designed and adequately powered clinical trial to assess the effectiveness of current evaluation and therapeutic strategies1, 23. In our current trial, we propose a multi-center, randomized, placebo-controlled, double blind study to determine whether, in the setting of prompt evaluation of UTI symptoms and early therapy of culture-proven UTI, daily antimicrobial prophylaxis is superior to daily placebo in preventing recurrent UTI and renal scarring in children aged 2 months to 72 months diagnosed with grades I-IV VUR following an initial episode of UTI12.
A. Background
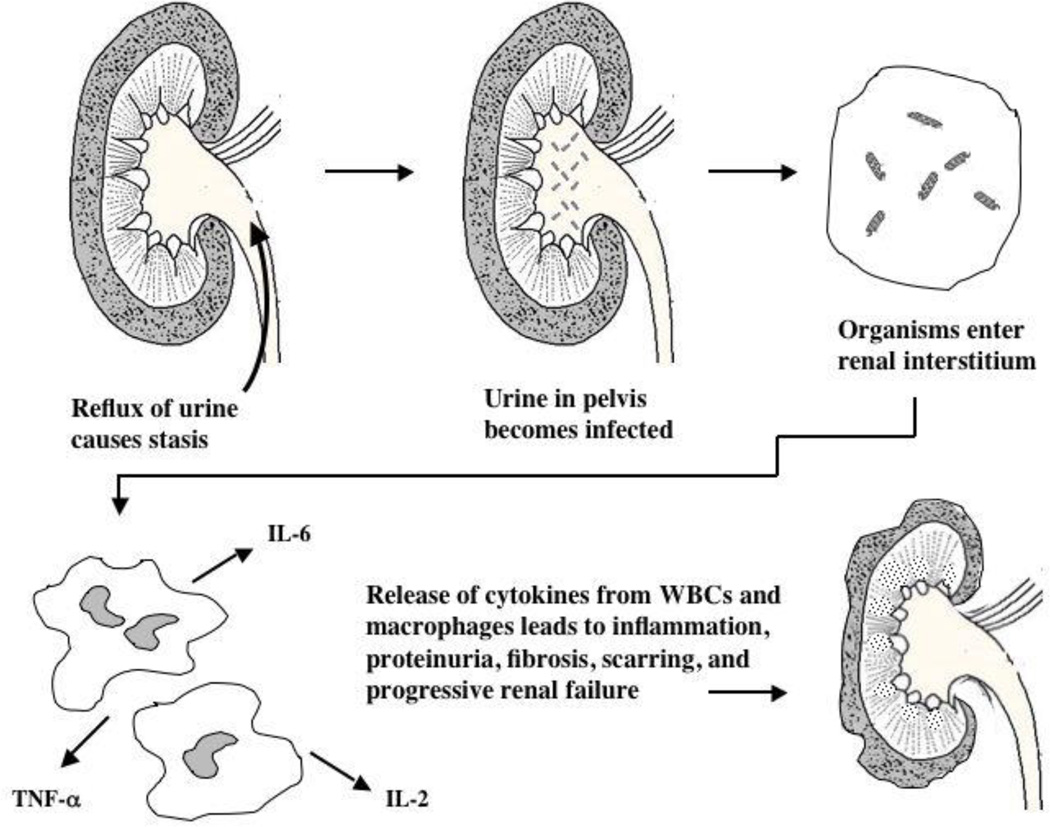
Studies for more than 50 years have suggested a link between recurrent UTI, VUR and renal parenchymal scarring3, 4, 7–9, 25. Renal scarring has been associated with proteinuria, hypertension, failure of renal mass to grow, progression to chronic kidney disease (CKD), and ultimately to ESRD requiring renal replacement therapy4, 24, 27, 28. Under this model of chronic renal failure, either long-term antimicrobial prophylaxis administration or surgical correction of VUR (such as ureteral reimplantation or endoscopic injection of a biocompatible material to diminish the size of the lumen of the ureterovesical orifice) or both have been utilized to prevent the damage related to an inflammatory response after retrograde reflux of infected urine to the ureter to the renal pelvis (Figure 1).
Figure 1.
Theoretical model showing how urinary stasis caused by VUR leads to bacterial growth in the renal parenchyma. Macrophages and leukocytes migrate to the interstitium and secrete pro-inflammatory cytokines, resulting in fibrosis and scarring with a progressive decline in renal function and the development of proteinuria and hypertension. In reality, only a minority of children with primary VUR develop scarring.
Several findings have emerged which strongly question the validity of this hypothesis. First, review of studies that form the basis for our current strategies for preventing UTI-and VUR-related renal scarring reveals an absence of strong supportive evidence, with few randomized controlled trials5, 11, 22, 23, 29. Second, antimicrobial prophylaxis has been shown to be superior to placebo in terms of prevention of recurrent infection in only a limited fashion, and the value of anti-reflux surgery is even less certain1, 2, 5. The finding of renal scarring in children with recurrent of UTIs in the absence of VUR raises issues concerning the model1, 11. Long-term studies have shown that the proportion of children receiving antimicrobial prophylaxis or surgery who develop new scarring is actually quite low, and most children do not progress to chronic kidney disease1, 4, 5, 9.
Additional issues influence the model. A certain number of children with VUR and recurrent UTIs will have chromosomal, monogenic or polygenic conditions, which may include embryonic malformation of the kidney, renal hypoplasia, dysplasia or obstruction12, 13. Antimicrobial resistance in the patient and the community raises concerns about the safety of antimicrobial prophylaxis in both UTI and acute otitis media studies30–32.
B. Vesicoureteral reflux (VUR)
VUR, retrograde urine flow from the bladder to the ureters, is the most common functional abnormality of the urinary tract in children5, 11. Primary VUR is characterized by short mucosal tunnel length, as opposed to secondary VUR, in which reflux is the result of increased bladder pressure from a neurogenic bladder, outlet obstruction or other vesicular anomalies. The proposed study will address only primary reflux.
Over the past 3 decades, it has become apparent that approximately 30 to 40 percent of children investigated by imaging studies after a UTI show evidence of VUR4, 6, 9. An international grading system of reflux has been established which proceeds from grade I (reflux up a non-dilated ureter) to grade V (massive reflux with marked ureteric dilatation and distention of the pelvis with concavity of the papillae or papillary flattening). These grades are well described and involve increasing degrees of reflux, dilatation and cupping of the papillae3, 4, 6, 9, 27
VUR is also seen in asymptomatic family members of an index case at rates of from 20% to nearly 50% penetrance. Such VUR is found in successive generations without particular influence of consanguinity. Other genetic conditions, to be discussed below, are associated with VUR and recurrent UTIs28.
Another issue for consideration is that primary VUR may be discovered during follow-up investigations for prenatal ultrasound findings or after a UTI8, 26, 31. Because our interest is in patients with primary rather than secondary VUR, the timing of the discovery of reflux is less important. Put differently, all primary VUR exists on an embryologic basis13, 26. The main differences between pre- and post-natal disease are therapeutic approaches, including the choice of antimicrobials, the organisms encountered, and a greater spontaneous remission rate in younger children. Reflux nephropathy is an appreciable cause of progressive renal failure leading to a need renal replacement therapy. Thus, the model of infection, detection of reflux, and treatment is based upon many older studies that are smaller, non-randomized, and may include secondary reflux.
Although numerous studies have examined the importance of anti-reflux surgery, only a few studies are of sufficient duration and size to permit valid statistical analysis5, 11, 22. In general, these studies have examined open vesicoureteric reimplantation and have not evaluated endoscopic ureteral surgery. Some of the earlier studies, which supported the need for treating VUR, either surgically or medically, had significant methodological limitations, and did not particularly address the question for the majority of children who exhibit low-grade reflux. Most of these investigations were uncontrolled, used intravenous pyelograms to assess for scars, and did not all use the International Scale to grade VUR33–35. Yet these studies form the basis of all contemporary therapy. Large uncontrolled studies of children placed on long-term prophylaxis have unquestionably shown, however, that the rate of new scar formation after breakthrough infection is extremely low - less than 3%36, 37. In the later decades of the 20th century, the greater debate was between those who would treat patients prophylactically versus surgically correct the reflux. Therefore, controlled trials such as the International Reflux Study in Children (IRSC) and the Birmingham Cooperative Study were designed to compare the efficacy of prophylaxis to surgery38, 39. The IRSC looked specifically at children with grades III and IV (mostly IV) reflux. Both studies showed that surgery and prophylaxis were equivalent in preventing new renal scars or pyelonephritis in children with reflux. Because none of these studies included a placebo or “observation only” arm, the question has also been raised as to whether surgery or antimicrobial prophylaxis has any effect on renal scarring in children with VUR diagnosed following a UTI.
C. Voiding dysfunction and reflux
Neurologically normal children can demonstrate temporary, but often significant, aspects of lower urinary tract dysfunction during toilet training years. It is assumed that this dysfunction is a result of faulty learning or habituation. These children tend to hold both their urine and bowel movements for an excessive amount of time as they attempt to toilet train40. They do not appropriately relax their perineal sphincters while voiding or defecating. As a result they are constipated and do not empty their bladders completely41. They have what is termed the dysfunctional elimination syndrome (DES). They void at higher than normal voiding pressures, since voiding occurs when the urinary sphincter is not appropriately relaxed42. They also tend to have unstable, “hyper-reflexic” bladders that contract at lower than normal volumes. These children often have recurrent urinary tract infections with or without reflux, along with urinary incontinence and fecal soiling.
Children with DES and vesicoureteral reflux form a special subset. It has been shown that these children have a higher incidence of breakthrough infection while on prophylaxis, more renal scarring, a lower incidence of spontaneous resolution with growth, and a higher failure rate after anti-reflux surgery43–45. DES can be assessed historically by questionnaire or toileting diary40. In particular, families are queried regarding voiding frequency, urinary incontinence, constipation and fecal soiling. Voiding dysfunction can also be confirmed by invasive urodynamic testing42, 46. Many are reluctant to perform these tests on a regular basis in all children with reflux and rely mainly on a clinical history. Based upon a questionnaire, a dysfunctional voiding symptom score (DVSS) has been developed that is valid and reproducible47, 48.
The etiologic relationship between reflux and DES in the older toilet-trained child is not well established, however. It is not known if reflux in these children results from congenital valvular deficiency or is secondary to abnormal voiding dynamics with a normal ureterovesical junction. In one study, the overall rate of DES in refluxing and non-refluxing populations of toilet-trained children was similar, although the risk of having dysfunctional voiding in the group with both UTI and reflux was greater49. It was shown in a cohort of infants diagnosed with reflux at less than one year of age that they did not have a greater incidence of dysfunctional voiding as they aged as compared to an age-matched population without reflux50.
D. Role of infection and VUR in scarring
At least 1% of boys and 3–5% of girls will experience at least one UTI during childhood: of these 30–50% are likely to have a recurrence8. Permanent renal scarring after pyelonephritis is detected 5–20% of the time when children are evaluated with intravenous urography and up to 40% of the time when evaluated by a DMSA renal scan1, 5. The finding of scarring increases with each episode of pyelonephritis25. The conventional view is that the incidence of scarring falls after the 5th–7th birthday, even with new infections, but in a large study by Benador et al. the frequency of scarring was the same in children aged 1–5 years as it was in children over 5 years51. Rushton et al., in a now classic study, emphasized that new renal scars form less frequently in kidneys with VUR than those without52. In a meta-analysis of randomized, controlled trials of antimicrobials and anti-reflux surgery for VUR, Wheeler et al. concluded that, "it is uncertain whether the identification and treatment of children with VUR confers clinically important benefit"11, 22. It also appears that scarring may be identical in refluxing and non-refluxing units3, 11, 22, challenging routine initiation of antibacterial prophylaxis following detection of VUR in all patients53
Another major problem with the published literature is the aggregation of cases with secondary VUR due to genetic causes with primary VUR. A number of malformation syndromes, some with a recognizable inheritance pattern, can be associated with VUR, infection and scarring12, 13. Among these are VATER-VACTERL (vertebral, anal, cardiac, tracheoesophageal, renal, limb) syndrome and other syndromes with a variety of renal malformations, including renal agenesis, dysgenesis, horseshoe kidney, a duplex pelvis, hydronephrosis, and cloacal anomalies12, 13. Some of these disorders are chromosomal and others involve mutations in developmental genes. (Table 1, 2) In a series of 317 children with anorectal malformations, 138 had associated renal anomalies and 27 had VUR. Mutations of developmental genes, including PAX2, EYE1, and WT-1, can result in syndromes with reflux, scarring, and reflux nephropathy12.
Table 1.
Representative syndromes that may display VUR, scarring, recurrent infection and progressive renal disease11, 22
|
Table 2.
|
E. The characterization of scarring
Renal scarring is the consequence of focal areas of inflammation with massive cytokine release from tissue macrophages and lymphocytes5. Fibrosis is another post-infection finding. Other features are renal cortical thinning, and evidence of microalbuminuria1, 22. In all series a small number of children go on to have significant hypertension and ESRD.
F. Background to therapy
The use of antimicrobials to reduce recurrent UTI dates back to the 1940's and 50's. The now classical studies of Jean Smellie and her colleagues form the most compelling reason to consider antimicrobial prophylaxis6–9, 22, 25, 27. Goals of antimicrobial therapy and prophylaxis include to: (a) prevent or reduce the number of recurrent UTIs; and (b) 49/66 reduce the incidence of scarring. Again, this set of assumptions has come under criticism because of many of the facts listed previously (Table 3).
Table 3.
|
F. Background to imaging studies
A number of imaging techniques have been utilized to evaluate children with UTIs. Radiographic VCUG remains the gold standard for identification and evaluation of VUR. Renal ultrasound identifies hydronephrosis but is insensitive to identifying renal scarring. A number of procedures and tests have been used to try to localize the site of UTI to the upper (acute pyelonephritis, or APN) or lower (cystitis) urinary tract. An acute phase response consisting of elevated peripheral white blood cell (WBC) count, erythrocyte sedimentation rate, C-reactive protein and procalcitonin were used in several studies to indicate infection of the upper urinary tract. However, as noted in a review article by Rushton and in editorials by Andrich and Majd, Conway, and Hellerstein, children who have a first UTI accompanied by fever and toxicity cannot be diagnosed reliably as having APN based on clinical signs and symptoms or laboratory parameters alone52, 54.
Currently, DMSA scintigraphy is the imaging agent of choice for the detection and evaluation of APN and renal cortical scarring in children. Using strict histopathologic criteria in the refluxing infected piglet model, DMSA renal scans have been found to be highly sensitive and specific for the detection and localization of APN52, 55. The DMSA scan also has shown higher sensitivity and specificity than intravenous pyelography (IVP) in documenting renal scars in several clinical studies, and has shown good correlation with histopathology in animal data56–60. Consequently, DMSA renal scintigraphy provides a unique opportunity to study the progression of renal damage and functional loss from the initial insult of APN to the subsequent development of irreversible renal scarring52. Accordingly, DMSA renal scanning is currently the “gold standard” for identifying renal parenchymal changes, including scarring52, 54, 61, 62. As such, DMSA renal scans will be used in the NIH RIVUR study as the outcome measurement for the detection and semi-quantification of both pre-existent and newly acquired renal parenchymal damage secondary to UTIs in children with grades I–IV VUR.
H. Potential harm of current management of VUR
Concerns exist regarding the potential harm of current diagnostic tests and therapeutic approaches. Tests for defining reflux - its nature and extent - include voiding cystourethrography and radionuclide cystography. Major concerns are as follows:
Imaging – problems include cost and the long-term effect of repeated exposure to ionizing radiation.
- Antimicrobials - the daily administration of an antimicrobial is problematic for several reasons:
- Resistance leads to the use of other agents and classes of antimicrobials, which may be costlier, are not fully tested in younger children, may be excreted in sites other than the renal parenchyma, and have limited antibacterial spectra28.
Length of follow-up - this is a complex issue with myriad unanswered questions2. After infancy, boys experience far fewer recurrences of UTI. In general, new scarring does not occur after age 5–7 years, but as noted, can occur in the absence of VUR. Does the risk of antimicrobial resistance outweigh the possibility of infection and the even smaller risk of scarring?1, 5.
Psychological - the process of inserting a urinary catheter into the urethra of a young child, followed by putting the child under an X-ray machine on a hard table, is clearly a source of psychological stress and the cause of tears, nightmares, and retained memories. If these tests are of only marginal value, or no value, then perhaps they should not be performed1, 11, 22, 63.
I. Need for a well-designed study
To date, a small number of studies in children and numerous larger studies in adults have indicated that antibacterial prophylaxis can reduce the number of recurrent UTIs5, 11, 22. While this could indicate that randomized, prospective, placebo-controlled trials (in children with VUR diagnosed following UTI) are not indicated, this is not the case. As succinctly stated by J. Craig in his commentary on clinical trials in children, "We do not simply need more studies. We need the right studies done right."20. In May 2003, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) sponsored a strategic planning meeting to assess the need and feasibility of conducting clinical trials in children diagnosed with VUR. The need for definitive research in three areas was identified: 1) Resolution of conflicting data related to use of antimicrobial prophylaxis in a placebo-controlled trial that includes assessments of dysfunctional voiding, pyelonephritis and renal scarring; 2) Comparison of an endoscopic injection with a bulking agent with open repair; 3) Comparison of a preemptive surgical intervention with use of antimicrobial prophylaxis. The RIVUR trial is an outgrowth of this NIDDK planning meeting. In order to accomplish the first charge, children with a resolved first febrile or symptomatic UTI who have grades I-IV VUR will be randomized to receive placebo or an antimicrobial prophylaxis with a primary endpoint of recurrent UTI and a secondary endpoint of renal scarring. We intend to study children with primary VUR recruited following their first occurrence of a UTI. We anticipate randomizing 600 children to antimicrobial prophylaxis or placebo treatment arms. Children with urinary tract obstruction, genetic syndromes, chromosomal syndromes, and complex anomalies that influence bladder function and urinary flow will be excluded, in part because many of these children have secondary or obstruction-related VUR12, 13. Each child will be followed for at least 2 years, and both infection rates and scarring will be monitored. Such a study should have the statistical power to answer whether antimicrobial prophylaxis protects against these outcomes.
I. Summary
Because of the frequency of UTIs in children, off-label use of antimicrobial prophylaxis is often the usual treatment of children with VUR, and such use is increasingly being called into question, hence a definitive study to determine the value of antimicrobial prophylaxis with regards to the recurrence of UTI and the incidence of renal scarring1, 20 is essential. The currently recommended follow-up procedures (repeated urine cultures, renal and genitourinary imaging, antimicrobial therapy and prophylaxis, as well as other factors including cleanliness, adequate bladder and bowel emptying, and compliance with protocols) are expensive (in terms of time, attention to detail, and cost) and cumbersome. Such recommendations should be evidence-based53.
Acknowledgments
This research was supported by grant number DK74059 from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Department of Health and Human Service
References
- 1.Beetz R. May we go on with antibacterial prophylaxis for urinary tract infections? Pediatr Nephrol. 2006;21:5–13. doi: 10.1007/s00467-005-2083-6. [DOI] [PubMed] [Google Scholar]
- 2.Elder JS, Peters CA, Arant BS, Jr, et al. Pediatric Vesicoureteral Reflux Guidelines Panel summary report on the management of primary vesicoureteral reflux in children. J Urol. 1997;157:1846–1851. [PubMed] [Google Scholar]
- 3.Olbing H. Comparison of the surgical and nonsurgical treatment of primary vesicoureteral-renal reflux--an international reflux study. Kinderarztl Prax. 1986;54:493–499. [PubMed] [Google Scholar]
- 4.Weiss R, Duckett J, Spitzer A. Results of a randomized clinical trial of medical versus surgical management of infants and children with grades III and IV primary vesicoureteral reflux (United States). The International Reflux Study in Children. J Urol. 1992;148:1667–1673. doi: 10.1016/s0022-5347(17)36998-7. [DOI] [PubMed] [Google Scholar]
- 5.Fanos V, Cataldi L. Antibiotics or surgery for vesicoureteric reflux in children. Lancet. 2004;364:1720–1722. doi: 10.1016/S0140-6736(04)17359-5. [DOI] [PubMed] [Google Scholar]
- 6.Weiss R, Tamminen-Mobius T, Koskimies O, et al. Characteristics at entry of children with severe primary vesicoureteral reflux recruited for a multicenter, international therapeutic trial comparing medical and surgical management. The International Reflux Study in Children. J Urol. 1992;148:1644–1649. doi: 10.1016/s0022-5347(17)36993-8. [DOI] [PubMed] [Google Scholar]
- 7.Smellie J, Edwards D, Hunter D, et al. Vesicoureteric reflux and renal scarring. Kidney Int (Suppl) 1975;4:65–72. [PubMed] [Google Scholar]
- 8.Smellie JM, Poulton A, Prescod NP. Retrospective study of children with renal scarring associated with reflux and urinary infection. Bmj. 1994;308:1193–1196. doi: 10.1136/bmj.308.6938.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smellie JM, Prescod NP, Shaw PJ, et al. Childhood reflux and urinary infection: a follow-up of 10–41 years in 226 adults. Pediatr Nephrol. 1998;12:727–736. doi: 10.1007/s004670050535. [DOI] [PubMed] [Google Scholar]
- 10.Cohen AL, Rivara FP, Davis R, et al. Compliance with guidelines for the medical care of first urinary tract infections in infants: a population-based study. Pediatrics. 2005;115:1474–1478. doi: 10.1542/peds.2004-1559. [DOI] [PubMed] [Google Scholar]
- 11.Wheeler D, Vimalachandra D, Hodson EM, et al. Antibiotics and surgery for vesicoureteric reflux: a meta-analysis of randomised controlled trials. Arch Dis Child. 2003;88:688–694. doi: 10.1136/adc.88.8.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berger S, Goppl M, Zachariou Z. Syndromology of anorectal malformations revisited: from patterns of associated malformations to the recognition of syndromes. World J Pediatr. 2005;1:8–14. [Google Scholar]
- 13.Hahn H, Ku SE, Kim KS, et al. Implication of genetic variations in congenital obstructive nephropathy. Pediatr Nephrol. 2005;20:1541–1544. doi: 10.1007/s00467-005-1999-1. [DOI] [PubMed] [Google Scholar]
- 14.Bakshandeh K, Lynne C, Carrion H. Vesicoureteral reflux and end stage renal disease. J Urol. 1976;116:557–558. doi: 10.1016/s0022-5347(17)58909-0. [DOI] [PubMed] [Google Scholar]
- 15.Kincaid-Smith P, Bastos MG, Becker GJ. Reflux nephropathy in the adult. In: Hodson CJ, Heptinstall RH, Winberg J, Basel S, editors. Reflux Nephropathy Update. Karger; 1984. pp. 94–101. [Google Scholar]
- 16.Londe S. Causes of hypertension in the young. Pediatr Clin North Am. 1978;25:55–65. doi: 10.1016/s0031-3955(16)33532-5. [DOI] [PubMed] [Google Scholar]
- 17.Still JL, Cottom D. Severe hypertension in childhood. Arch Dis Child. 1967;42:34–39. doi: 10.1136/adc.42.221.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vallee JP, Vallee MP, Greenfield SP, et al. Contemporary incidence of morbidity related to vesicoureteral reflux. Urology. 1999;53:812–815. doi: 10.1016/s0090-4295(98)00587-1. [DOI] [PubMed] [Google Scholar]
- 19.Warady BA, Hebert D, Sullivan EK, et al. Renal transplantation, chronic dialysis, and chronic renal insufficiency in children and adolescents. The 1995 Annual Report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Nephrol. 1997;11:49–64. doi: 10.1007/s004670050232. [DOI] [PubMed] [Google Scholar]
- 20.Craig JC, Irwig LM, Knight JF, et al. Does treatment of vesicoureteric reflux in childhood prevent end-stage renal disease attributable to reflux nephropathy? Pediatrics. 2000;105:1236–1241. doi: 10.1542/peds.105.6.1236. [DOI] [PubMed] [Google Scholar]
- 21.Ardissino G, Avolio L, Dacco V, et al. Long-term outcome of vesicoureteral reflux associated chronic renal failure in children. Data from the ItalKid Project. J Urol. 2004;172:305–310. doi: 10.1097/01.ju.0000129067.30725.16. [DOI] [PubMed] [Google Scholar]
- 22.Wheeler DM, Vimalachandra D, Hodson EM, et al. Interventions for primary vesicoureteric reflux. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD001532.pub2. CD001532. [DOI] [PubMed] [Google Scholar]
- 23.Craig J. Quality research meets urinary tract infection. J Pediatr. 1999;135:664–666. doi: 10.1016/s0022-3476(99)70081-8. [DOI] [PubMed] [Google Scholar]
- 24.Duckett JW, Walker RD, Weiss R. Surgical results: International Reflux Study in Children--United States branch. J Urol. 1992;148:1674–1675. doi: 10.1016/s0022-5347(17)36999-9. [DOI] [PubMed] [Google Scholar]
- 25.Olbing H, Smellie JM, Jodal U, et al. New renal scars in children with severe VUR: a 10-year study of randomized treatment. Pediatr Nephrol. 2003;18:1128–1131. doi: 10.1007/s00467-003-1256-4. [DOI] [PubMed] [Google Scholar]
- 26.Silva J, Oliviera E, Diniz J, et al. Clinical course of prenatally detected primary vesicoureteral reflux. Pediatr Nephrol. 2006;21:86–91. doi: 10.1007/s00467-005-2058-7. [DOI] [PubMed] [Google Scholar]
- 27.Jodal U, Koskimies O, Hanson E, et al. Infection pattern in children with vesicoureteral reflux randomly allocated to operation or long-term antibacterial prophylaxis. The International Reflux Study in Children. J Urol. 1992;148:1650–1652. doi: 10.1016/s0022-5347(17)36994-x. [DOI] [PubMed] [Google Scholar]
- 28.Wong S-N. Urinary tract infection and vesicoureteral reflux. Hong Kong: Medcom Ltd.; 2005. [Google Scholar]
- 29.Garin EH, Olavarria F, Garcia Nieto V, et al. Clinical significance of primary vesicoureteral reflux and urinary antibiotic prophylaxis after acute pyelonephritis: a multicenter, randomized, controlled study. Pediatrics. 2006;117:626–632. doi: 10.1542/peds.2005-1362. [DOI] [PubMed] [Google Scholar]
- 30.Bitsori M, Maraki S, Raissaki M, et al. Community-acquired enterococcal urinary tract infections. Pediatr Nephrol. 2005;20:1583–1586. doi: 10.1007/s00467-005-1976-8. [DOI] [PubMed] [Google Scholar]
- 31.McGillivray D, Mok E, Mulrooney E, et al. A head-to-head comparison:"cleanvoid" bag versus catheter urinalysis in the diagnosis of urinary tract infection in young children. J Pediatr. 2005;147:451–456. doi: 10.1016/j.jpeds.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Wald ER. To bag or not to bag. J Pediatr. 2005;147:418–420. doi: 10.1016/j.jpeds.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 33.Govan DE, Fair WR, Friedland GW, et al. Urinary tract infections in children. Part III--Treatment of ureterovesical reflux. West J Med. 1974;121:382–389. [PMC free article] [PubMed] [Google Scholar]
- 34.Lenaghan D, Whitaker JG, Jensen F, et al. The natural history of reflux and long-term effects of reflux on the kidney. J Urol. 1976;115:728–730. doi: 10.1016/s0022-5347(17)59352-0. [DOI] [PubMed] [Google Scholar]
- 35.O'Donnell B, Moloney MA, Lynch V. Vesico-ureteric reflux in infants and children: results of "supervision", chemotherapy and surgery. Br J Urol. 1969;41:6–13. doi: 10.1111/j.1464-410x.1969.tb09901.x. [DOI] [PubMed] [Google Scholar]
- 36.Greenfield SP, Ng M, Wan J. Experience with vesicoureteral reflux in children: clinical characteristics. J Urol. 1997;158:574–577. [PubMed] [Google Scholar]
- 37.Greenfield SP, Wan J. Experience with vesicoureteral reflux in children: clinical characteristics. In: Gearhart JP, Rink RC, Mouriquand PDE, Philadelphia WB, editors. Pediatric Urology. Saunders Co.; 2001. pp. 382–410. [Google Scholar]
- 38.Medical versus surgical treatment of primary vesicoureteral reflux: a prospective international reflux study in children. J Urol. 1981;125:277–283. doi: 10.1016/s0022-5347(17)55009-0. [DOI] [PubMed] [Google Scholar]
- 39.Prospective trial of operative versus non-operative treatment of severe vesicoureteric reflux in children: five years' observation. Birmingham Reflux Study Group. Br Med J (Clin Res Ed) 1987;295:237–241. doi: 10.1136/bmj.295.6592.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wan J, Kaplinsky R, Greenfield S. Toilet habits of children evaluated for urinary tract infection. J Urol. 1995;154:797–799. doi: 10.1097/00005392-199508000-00126. [DOI] [PubMed] [Google Scholar]
- 41.Koff SA. Relationship between dysfunctional voiding and reflux. J Urol. 1992;148:1703–1705. doi: 10.1016/s0022-5347(17)37007-6. [DOI] [PubMed] [Google Scholar]
- 42.Sillen U. Bladder dysfunction in children with vesico-ureteric reflux. Acta Paediatr Suppl. 1999;88:40–47. doi: 10.1111/j.1651-2227.1999.tb01318.x. [DOI] [PubMed] [Google Scholar]
- 43.Koff SA, Wagner TT, Jayanthi VR. The relationship among dysfunctional elimination syndromes, primary vesicoureteral reflux and urinary tract infections in children. J Urol. 1998;160:1019–1022. doi: 10.1097/00005392-199809020-00014. [DOI] [PubMed] [Google Scholar]
- 44.Naseer SR, Steinhardt GF. New renal scars in children with urinary tract infections, vesicoureteral reflux and voiding dysfunction: a prospective evaluation. J Urol. 1997;158:566–568. [PubMed] [Google Scholar]
- 45.Soygur T, Arikan N, Yesilli C, et al. Relationship among pediatric voiding dysfunction and vesicoureteral reflux and renal scars. Urology. 1999;54:905–908. doi: 10.1016/s0090-4295(99)00291-5. [DOI] [PubMed] [Google Scholar]
- 46.Willemsen J, Nijman RJ. Vesicoureteral reflux and videourodynamic studies: results of a prospective study. Urology. 2000;55:939–943. doi: 10.1016/s0090-4295(00)00549-5. [DOI] [PubMed] [Google Scholar]
- 47.Akbal C, Genc Y, Burgu B, et al. Dysfunctional voiding and incontinence scoring system: quantitative evaluation of incontinence symptoms in pediatric population. J Urol. 2005;173:969–973. doi: 10.1097/01.ju.0000152183.91888.f6. [DOI] [PubMed] [Google Scholar]
- 48.Farhat W, Bagli DJ, Capolicchio G, et al. The dysfunctional voiding scoring system: quantitative standardization of dysfunctional voiding symptoms in children. J Urol. 2000;164:1011–1015. doi: 10.1097/00005392-200009020-00023. [DOI] [PubMed] [Google Scholar]
- 49.Chen JJ, Mao W, Homayoon K, et al. A multivariate analysis of dysfunctional elimination syndrome, and its relationships with gender, urinary tract infection and vesicoureteral reflux in children. J Urol. 2004;171:1907–1910. doi: 10.1097/01.ju.0000120288.82950.a2. [DOI] [PubMed] [Google Scholar]
- 50.Shaikh N, Hoberman A, Wise B, et al. Dysfunctional elimination syndrome: is it related to urinary tract infection or vesicoureteral reflux diagnosed early in life? Pediatrics. 2003;112:1134–1137. doi: 10.1542/peds.112.5.1134. [DOI] [PubMed] [Google Scholar]
- 51.Benador D, Benador N, Slosman D, et al. Are younger children at highest risk of renal sequelae after pyelonephritis? Lancet. 1997;349:17–19. doi: 10.1016/s0140-6736(96)06126-0. [DOI] [PubMed] [Google Scholar]
- 52.Rushton HG, Majd M, Jantausch B, et al. Renal scarring following reflux and nonreflux pyelonephritis in children: evaluation with 99mtechnetium-dimercaptosuccinic acid scintigraphy. J Urol. 1992;147:1327–1332. doi: 10.1016/s0022-5347(17)37555-9. [DOI] [PubMed] [Google Scholar]
- 53.Hunter D. First, gather the data. N Engl J Med. 2006;354:329–331. doi: 10.1056/NEJMp058235. [DOI] [PubMed] [Google Scholar]
- 54.Hellerstein S. Evolving concepts in the evaluation of the child with a urinary tract infection. J Pediatr. 1994;124:589–592. doi: 10.1016/s0022-3476(05)83138-5. [DOI] [PubMed] [Google Scholar]
- 55.Parkhouse HF, Godley ML, Cooper J, et al. Renal imaging with 99Tcm-labelled DMSA in the detection of acute pyelonephritis: an experimental study in the pig. Nucl Med Commun. 1989;10:63–70. doi: 10.1097/00006231-198901000-00010. [DOI] [PubMed] [Google Scholar]
- 56.Farnsworth RH, Rossleigh MA, Leighton DM, et al. The detection of reflux nephropathy in infants by 99mtechnetium dimercaptosuccinic acid studies. J Urol. 1991;145:542–546. doi: 10.1016/s0022-5347(17)38391-x. [DOI] [PubMed] [Google Scholar]
- 57.Goldraich NP, Ramos OL, Goldraich IH. Urography versus DMSA scan in children with vesicoureteric reflux. Pediatr Nephrol. 1989;3:1–5. doi: 10.1007/BF00859614. [DOI] [PubMed] [Google Scholar]
- 58.Merrick MV, Uttley WS, Wild SR. The detection of pyelonephritic scarring in children by radioisotope imaging. Br J Radiol. 1980;53:544–556. doi: 10.1259/0007-1285-53-630-544. [DOI] [PubMed] [Google Scholar]
- 59.Monsour M, Azmy AF, MacKenzie JR. Renal scarring secondary to vesicoureteric reflux. Critical assessment and new grading. Br J Urol. 1987;60:320–324. doi: 10.1111/j.1464-410x.1987.tb04976.x. [DOI] [PubMed] [Google Scholar]
- 60.Risdon RA, Godley ML, Parkhouse HF, et al. Renal pathology and the 99mTc-DMSA image during the evolution of the early pyelonephritic scar: an experimental study. J Urol. 1994;151:767–773. doi: 10.1016/s0022-5347(17)35084-x. [DOI] [PubMed] [Google Scholar]
- 61.Andrich MP, Majd M. Diagnostic imaging in the evaluation of the first urinary tract infection in infants and young children. Pediatrics. 1992;90:436–441. [PubMed] [Google Scholar]
- 62.Conway JJ, Cohn RA. Evolving role of nuclear medicine for the diagnosis and management of urinary tract infection. J Pediatr. 1994;124:87–90. doi: 10.1016/s0022-3476(94)70258-6. [DOI] [PubMed] [Google Scholar]
- 63.Moorthy I, Easty M, McHugh K, et al. The presence of vesicoureteric reflux does not identify a population at risk for renal scarring following a first urinary tract infection. Arch Dis Child. 2005;90:733–736. doi: 10.1136/adc.2004.057604. [DOI] [PMC free article] [PubMed] [Google Scholar]