Abstract
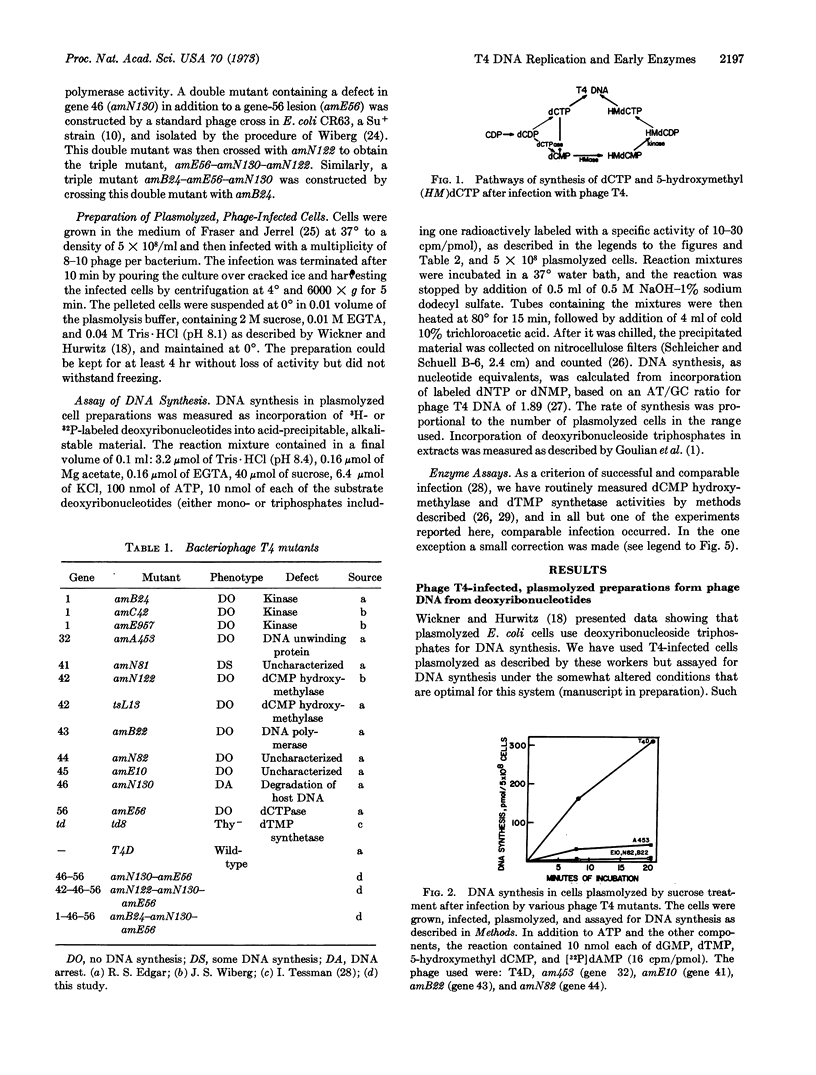
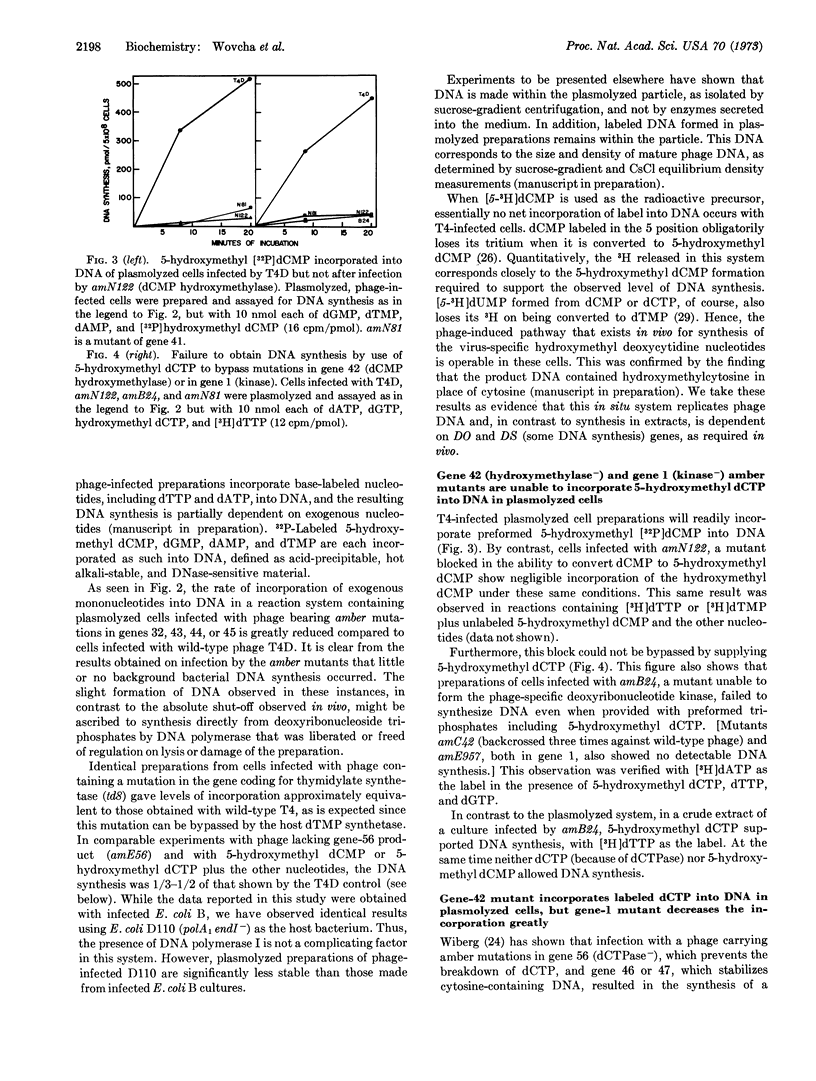
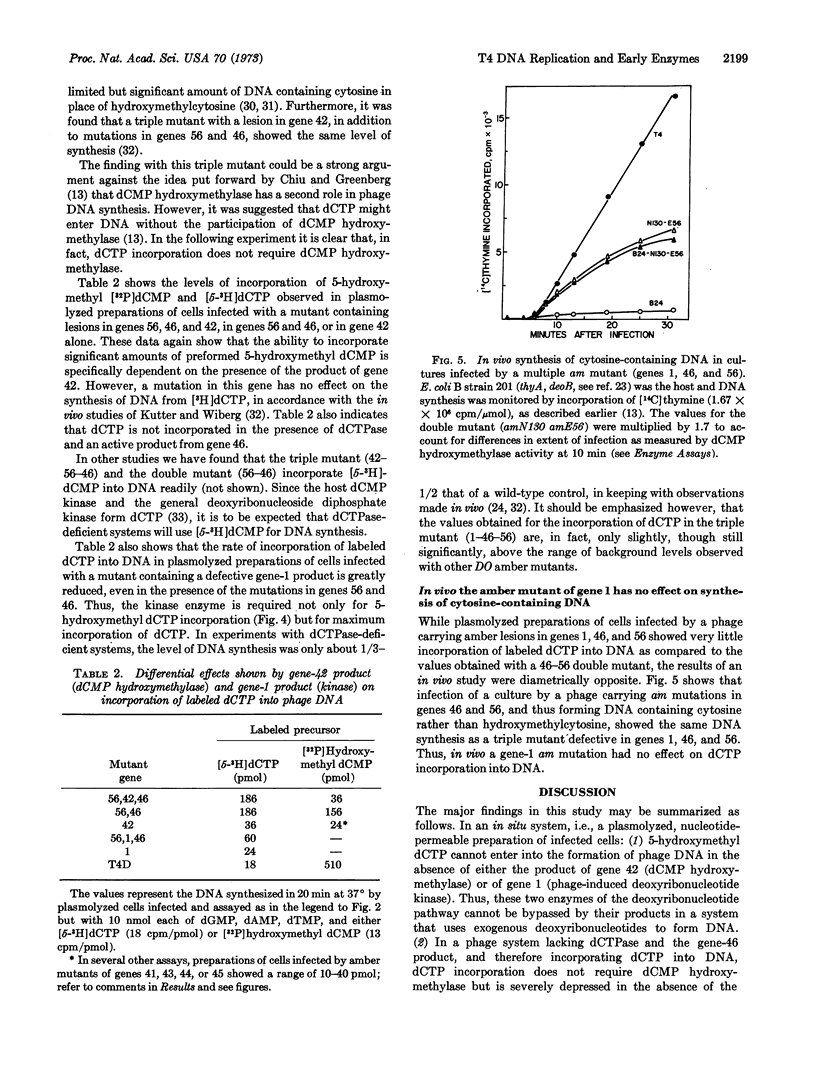
In order to retain in an in situ system the control mechanisms involved in synthesis of bacteriophage T4 DNA, infected cells were made permeable to nucleotides by plasmolysis with concentrated sucrose. Such preparations use exogenous deoxyribonucleotides to synthesize T4 phage DNA. As has been observed with in vivo studies, DNA synthesis was drastically reduced in plasmolyzed preparations from cells infected by amber mutants of genes 1, 32, 41, 42, 43, 44, or 45. Added 5-hydroxymethyl dCTP did not bypass either a mutant of gene 42 (dCMP hydroxymethylase) or of gene 1 (phage-induced deoxyribonucleotide kinase). In a phage system lacking deoxycytidine triphosphatase (gene 56) and the gene-46 product, and therefore incorporating dCTP into DNA, dCTP incorporation did not require dCMP hydroxymethylase, in keeping with in vivo results. With a triple amber mutant of genes 1, 46, and 56 only slight incorporation of dCTP occurred. By contrast, in experiments performed in vivo the synthesis of cytosine-containing DNA was unaffected by an amber mutation in gene 1.
These studies provide evidence that dCMP hydroxymethylase, in addition to its known catalytic function, has a second, more direct, role in phage T4 DNA synthesis, apparently in recognition of hydroxymethyl dCTP. The role of the phage-induced deoxyribonucleotide kinase in T4 DNA synthesis in the plasmolyzed system remains unresolved.
Keywords: 5-hydroxymethyl dCMP kinase, DNA replication, permeable cells
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Frey L. T4 bacteriophage gene 32: a structural protein in the replication and recombination of DNA. Nature. 1970 Sep 26;227(5265):1313–1318. doi: 10.1038/2271313a0. [DOI] [PubMed] [Google Scholar]
- Barry J., Alberts B. In vitro complementation as an assay for new proteins required for bacteriophage T4 DNA replication: purification of the complex specified by T4 genes 44 and 62. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2717–2721. doi: 10.1073/pnas.69.9.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C. S., Greenberg G. R. Evidence for a possible direct role of dCMP hydroxymethylase in T4 phage DNA synthesis. Cold Spring Harb Symp Quant Biol. 1968;33:351–359. doi: 10.1101/sqb.1968.033.01.041. [DOI] [PubMed] [Google Scholar]
- De Waard A., Paul A. V., Lehman I. R. The structural gene for deoxyribonucleic acid polymerase in bacteriophages T4 and T5. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1241–1248. doi: 10.1073/pnas.54.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake J. W., Allen E. F., Forsberg S. A., Preparata R. M., Greening E. O. Genetic control of mutation rates in bacteriophageT4. Nature. 1969 Mar 22;221(5186):1128–1132. [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- Fareed G. C., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. II. The structural gene for polynucleotide ligase in bacteriophage T4. Proc Natl Acad Sci U S A. 1967 Aug;58(2):665–672. doi: 10.1073/pnas.58.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBERG G. R., SOMERVILLE R. L. Deoxyuridylate kinase activity and deoxyuridinetriphosphatase in Escherichia coli. Proc Natl Acad Sci U S A. 1962 Feb;48:247–257. doi: 10.1073/pnas.48.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulian M., Lucas Z. J., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXV. Purification and properties of deoxyribonucleic acid polymerase induced by infection with phage T4. J Biol Chem. 1968 Feb 10;243(3):627–638. [PubMed] [Google Scholar]
- Gross J. D. DNA replication in bacteria. Curr Top Microbiol Immunol. 1972;57:39–74. doi: 10.1007/978-3-642-65297-4_2. [DOI] [PubMed] [Google Scholar]
- Hiraga S., Sugino Y. Nucleoside monophosphokinases of Escherichia coli infected and uninfected with an RNA phage. Biochim Biophys Acta. 1966 Feb 21;114(2):416–418. doi: 10.1016/0005-2787(66)90324-8. [DOI] [PubMed] [Google Scholar]
- Huberman J. A., Kornberg A., Alberts B. M. Stimulation of T4 bacteriophage DNA polymerase by the protein product of T4 gene 32. J Mol Biol. 1971 Nov 28;62(1):39–52. doi: 10.1016/0022-2836(71)90129-x. [DOI] [PubMed] [Google Scholar]
- Kornberg A. Active center of DNA polymerase. Science. 1969 Mar 28;163(3874):1410–1418. doi: 10.1126/science.163.3874.1410. [DOI] [PubMed] [Google Scholar]
- Kutter E. M., Wiberg J. S. Biological effects of substituting cytosine for 5-hydroxymethylcytosine in the deoxyribonucleic acid of bacteriophage T4. J Virol. 1969 Oct;4(4):439–453. doi: 10.1128/jvi.4.4.439-453.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutter E. M., Wiberg J. S. Degradation of cytosin-containing bacterial and bacteriophage DNA after infection of Escherichia coli B with bacteriophage T4D wild type and with mutants defective in genes 46, 47 and 56. J Mol Biol. 1968 Dec;38(3):395–411. doi: 10.1016/0022-2836(68)90394-x. [DOI] [PubMed] [Google Scholar]
- Lomax M. I., Greenberg G. R. A new assay of thymidylate synthetase activity based on the release of tritium from deoxyuridylate-5-3-H. J Biol Chem. 1967 Jan 10;242(1):109–113. [PubMed] [Google Scholar]
- Lomax M. S., Greenberg G. R. Characteristics of the deo operon: role in thymine utilization and sensitivity to deoxyribonucleosides. J Bacteriol. 1968 Aug;96(2):501–514. doi: 10.1128/jb.96.2.501-514.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOMURA M., OKAMOTO K., ASANO K. RNA metabolism in Escherichia coli infected with bacteriophage T4. Inhibition of host ribosomal and soluble RNA synthesis by phage and effect of chloromycetin. J Mol Biol. 1962 May;4:376–387. doi: 10.1016/s0022-2836(62)80018-7. [DOI] [PubMed] [Google Scholar]
- Price A. R., Warner H. R. Bacteriophage T4-induced deoxycytidine triphosphate-deoxyuridine triphosphate nucleotidohydrolase: its properties and its role during phage infection of Escherichia coli. Virology. 1969 Dec;39(4):882–892. doi: 10.1016/0042-6822(69)90024-5. [DOI] [PubMed] [Google Scholar]
- Richardson C. C. Enzymes in DNA metabolism. Annu Rev Biochem. 1969;38:795–840. doi: 10.1146/annurev.bi.38.070169.004051. [DOI] [PubMed] [Google Scholar]
- SHAPIRO D. M., EIGNER J., GREENBERG G. R. INABILITY OF THYMINE-DEPENDENT MUTANTS OF BACTERIOPHAGE T4 TO INDUCE THYMIDYLATE SYNTHETASE. Proc Natl Acad Sci U S A. 1965 Apr;53:874–881. doi: 10.1073/pnas.53.4.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speyer J. F. Mutagenic DNA polymerase. Biochem Biophys Res Commun. 1965 Oct 8;21(1):6–8. doi: 10.1016/0006-291x(65)90417-1. [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Okazaki T., Okazaki R. Mechanism of DNA chain growth, II. Accumulation of newly synthesized short chains in E. coli infected with ligase-defective T4 phages. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1356–1362. doi: 10.1073/pnas.60.4.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WYATT G. R., COHEN S. S. The bases of the nucleic acids of some bacterial and animal viruses: the occurrence of 5-hydroxymethylcytosine. Biochem J. 1953 Dec;55(5):774–782. doi: 10.1042/bj0550774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. R., Barnes J. E. Deoxyribonucleic acid synthesis in Escherichia coli infected with some deoxyribonucleic acid polymerase-less mutants of bacteriophage T4. Virology. 1966 Jan;28(1):100–107. doi: 10.1016/0042-6822(66)90310-2. [DOI] [PubMed] [Google Scholar]
- Wiberg J. S. Mutants of bacteriophage T4 unable to cause breakdown of host DNA. Proc Natl Acad Sci U S A. 1966 Mar;55(3):614–621. doi: 10.1073/pnas.55.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B., Hurwitz J. DNA replication in Escherichia coli made permeable by treatment with high sucrose. Biochem Biophys Res Commun. 1972 Apr 14;47(1):202–211. doi: 10.1016/s0006-291x(72)80029-9. [DOI] [PubMed] [Google Scholar]
- Yeh Y. C., Greenberg G. R. Tetrahydrofolate-dependent labilization of the hydrogen atom on carbon 5 of 5'-deoxycytidylate, a step in the deoxycytidylate hydroxymethylase reaction. J Biol Chem. 1967 Mar 25;242(6):1307–1313. [PubMed] [Google Scholar]


