Abstract
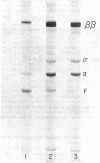
DNA-dependent RNA polymerase was purified from uninfected and ϕ29-infected Bacillus amyloliquefaciens. Differences were observed in the specific activities, template specificities, stability, and sedimentation properties of the two enzymes. A polypeptide of 30,000 molecular weight was found in association with the polymerase of high specific activity from phage-infected cells and was absent from polymerase isolated from uninfected cells. The change in polymerase properties and the appearance of the polypeptide occurred early in phage infection.
Keywords: properties, subunit composition, time of modification
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez G., Salas E., Pérez N., Celis J. E. Phi29 bacteriophage structural proteins. J Gen Virol. 1972 Mar;14(3):243–250. doi: 10.1099/0022-1317-14-3-243. [DOI] [PubMed] [Google Scholar]
- Anderson D. L., Hickman D. D., Reilly B. E. Structure of Bacillus subtilis bacteriophage phi 29 and the length of phi 29 deoxyribonucleic acid. J Bacteriol. 1966 May;91(5):2081–2089. doi: 10.1128/jb.91.5.2081-2089.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babinet C. A new method for the purification of RNA-polymerase. Biochem Biophys Res Commun. 1967 Mar 21;26(6):639–644. doi: 10.1016/s0006-291x(67)80119-0. [DOI] [PubMed] [Google Scholar]
- Bautz E. K., Dunn J. J. DNA-dependent RNA polymerase from phage T4 infected E. coli: an enzyme missing a factor required for transcription of T4 DNA. Biochem Biophys Res Commun. 1969 Jan 27;34(2):230–237. doi: 10.1016/0006-291x(69)90636-6. [DOI] [PubMed] [Google Scholar]
- Bramhall S., Noack N., Wu M., Loewenberg J. R. A simple colorimetric method for determination of protein. Anal Biochem. 1969 Oct 1;31(1):146–148. doi: 10.1016/0003-2697(69)90251-6. [DOI] [PubMed] [Google Scholar]
- Chamberlin M., McGrath J., Waskell L. New RNA polymerase from Escherichia coli infected with bacteriophage T7. Nature. 1970 Oct 17;228(5268):227–231. doi: 10.1038/228227a0. [DOI] [PubMed] [Google Scholar]
- Hagen E. W., Zeece V. M., Anderson D. L. A genetic study of temperature-sensitive mutants of the Bacillus subtilis bacteriophage phi 29. Virology. 1971 Mar;43(3):561–568. doi: 10.1016/0042-6822(71)90281-9. [DOI] [PubMed] [Google Scholar]
- Hagen E. W., Zeece V. M., Anderson D. L. A genetic study of temperature-sensitive mutants of the Bacillus subtilis bacteriophage phi 29. Virology. 1971 Mar;43(3):561–568. doi: 10.1016/0042-6822(71)90281-9. [DOI] [PubMed] [Google Scholar]
- Hermoso J. M., Avila J., Jiménez F., Salas M. RNA polymerase from Bacillus amyloliquefaciens. Biochim Biophys Acta. 1972 Aug 25;277(2):280–283. doi: 10.1016/0005-2787(72)90409-1. [DOI] [PubMed] [Google Scholar]
- Kolenbrander P. E., Hemphill H. E., Whiteley H. R. Nucleic acid synthesis in bacteriophage SPO2c 1 -infected Bacillus subtilis. J Virol. 1972 May;9(5):776–784. doi: 10.1128/jvi.9.5.776-784.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Litman R. M. A deoxyribonucleic acid polymerase from Micrococcus luteus (Micrococcus lysodeikticus) isolated on deoxyribonucleic acid-cellulose. J Biol Chem. 1968 Dec 10;243(23):6222–6233. [PubMed] [Google Scholar]
- Losick R., Sonenshein A. L. Change in the template specificity of RNA polymerase during sporulation of Bacillus subtilis. Nature. 1969 Oct 4;224(5214):35–37. doi: 10.1038/224035a0. [DOI] [PubMed] [Google Scholar]
- Maitra U. Induction of a new RNA polymerase in Escherichia coli infected with bacteriophage T3. Biochem Biophys Res Commun. 1971 Apr 16;43(2):443–450. doi: 10.1016/0006-291x(71)90773-x. [DOI] [PubMed] [Google Scholar]
- Mosharrafa E. T., Schachtele C. F., Reilly B. E., Anderson D. L. Complementary Strands of Bacteriophage phi29 Deoxyribonucleic Acid: Preparative Separation and Transcription Studies. J Virol. 1970 Dec;6(6):855–864. doi: 10.1128/jvi.6.6.855-864.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez E., Ramírez G., Salas M., Viñuela E. Structural proteins of bacteriophage phi 29. Virology. 1971 Sep;45(3):567–576. doi: 10.1016/0042-6822(71)90172-3. [DOI] [PubMed] [Google Scholar]
- Roscoe D. H. Synthesis of DNA in phage-infected Bacillus subtilis. Virology. 1969 Aug;38(4):527–537. doi: 10.1016/0042-6822(69)90173-1. [DOI] [PubMed] [Google Scholar]
- Schachtele C. F., De Sain C. V., Anderson D. L. Transcription during the development of bacteriophage phi29: definition of "early" and "late" phi29 ribonucleic acid. J Virol. 1973 Jan;11(1):9–16. doi: 10.1128/jvi.11.1.9-16.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., Hagen E. W., Anderson D. L. Temperature-shift analysis of bacteriophage phi 29 gene expression in Bacillus amyloliquefaciens. J Virol. 1971 Sep;8(3):352–354. doi: 10.1128/jvi.8.3.352-354.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., Oman R. W., Anderson D. L. Effect of elevated temperature on deoxyribonucleic acid synthesis in bacteriophage phi-29-infected Bacillus amyloliquefaciens. J Virol. 1970 Oct;6(4):430–437. doi: 10.1128/jvi.6.4.430-437.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert W., Qasba P., Walter G., Palm P., Schachner M., Zillig W. Kinetics of the alteration and modification of DNA-dependent RNA-polymerase in T4-infected E. coli cells. Eur J Biochem. 1969 Jun;9(3):319–324. doi: 10.1111/j.1432-1033.1969.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Talavera A., Jimenez F., Salas M., Viñuela E. Temperature-sensitive mutants of bacteriophage phi-29. Virology. 1971 Dec;46(3):586–595. doi: 10.1016/0042-6822(71)90062-6. [DOI] [PubMed] [Google Scholar]
- Travers A. A. Positive control of transcription by a bacteriophage sigma factor. Nature. 1970 Mar 14;225(5237):1009–1012. doi: 10.1038/2251009a0. [DOI] [PubMed] [Google Scholar]
- Walter G., Seifert W., Zillig W. Modified DNA-dependent RNA polymerase from E. coli infected with bacteriophage T4. Biochem Biophys Res Commun. 1968 Feb 15;30(3):240–247. doi: 10.1016/0006-291x(68)90441-5. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Whiteley H. R., McCarthy B. J., Whiteley A. H. Conservation of base sequences in RNA for early development of echinoderms. Dev Biol. 1970 Feb;21(1):216–242. doi: 10.1016/0012-1606(70)90069-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]