Abstract
Novel tumor-targeting drug conjugates, BLT-F2 (1) and BLT-S-F6 (2), bearing a fluorotaxoid as the warhead, a mechanism-based self-immolative disulfide linker, and biotin as the tumor-targeting module, were designed and synthesized as 19F NMR probes. Fluorine atoms and CF3 groups were strategically incorporated into the conjugates to investigate the mechanism of linker cleavage and factors that influence their plasma and metabolic stability by real-time monitoring with 19F NMR. Time-resolved 19F NMR study on probe 1 disclosed a stepwise mechanism for release of a fluorotaxoid, which might not have been detected by other analytical methods. Probe 2 was designed to bear two CF3 groups in the taxoid moiety as “3-FAB” reporters for enhanced sensitivity and a polyethylene glycol oligomer insert to improve solubility. The clean analysis of the linker stability and reactivity of drug conjugates in blood plasma or cell culture media by HPLC and 1H NMR is troublesome, due to the overlap of key signals/peaks with background arising from highly complex ingredients in biological systems. Accordingly, the use of 19F NMR would provide a practical solution to this problem. In fact, our “3-FAB” probe 2 was proven to be highly useful to investigate the stability and reactivity of the self-immolative disulfide linker system in human blood plasma by 19F NMR. It has also been revealed that the use of polysorbate 80 as excipient for the formulation of probe 2 dramatically increases the stability of the disulfide linker system. This finding further indicates that the tumor-targeting drug conjugates with polysorbate 80/EtOH/saline formulation for in vivo studies would have high stability in blood plasma, while the drug release in cancer cells proceeds smoothly.
Keywords: Fluorine Probe, 19F NMR, Fluorotaxoid, Tumor-Targeted Drug Delivery System, Self-immolative Disulfide Linker
1. Introduction
In traditional chemotherapy, highly potent cytotoxic drugs are administered to cancer patients based on the premise that rapidly dividing cancer cells are more likely to be killed than normal cells. However, lack of tumor specificity of these drugs often leads to severe and dose-limiting side effects, and continues to be a serious issue in cancer treatment. Therefore, intensive efforts have been made on the development of tumor-targeted drug delivery systems (TTDDSs) that exploit the unique and intrinsic physiological and biochemical properties of tumors and cancer cells to selectively deliver cytotoxic drugs to cancer cells [1, 2]. A TTDDS consists of a tumor-targeting moiety (TTM) and a cytotoxic drug connected through a “smart” linker system. An ideal “smart” linker must remain stable during circulation in blood, but should readily cleave to release the free drug upon internalization into cancer cells or accumulation in the tumor microenvironment [1]. Due to the immense importance of linker dynamics for TTDDS efficacy, significant advancement has been made in the development of effective linker systems, in particular for small-molecule drug conjugates (SMDCs) [3–7] and antibody–drug conjugates (ADCs) [3, 8–12]. Along this line, we have developed novel self-immolative disulfide linkers capable of releasing unmodified drugs via glutathione-triggered linker cleavage and thiolactonization [4, 13–15].
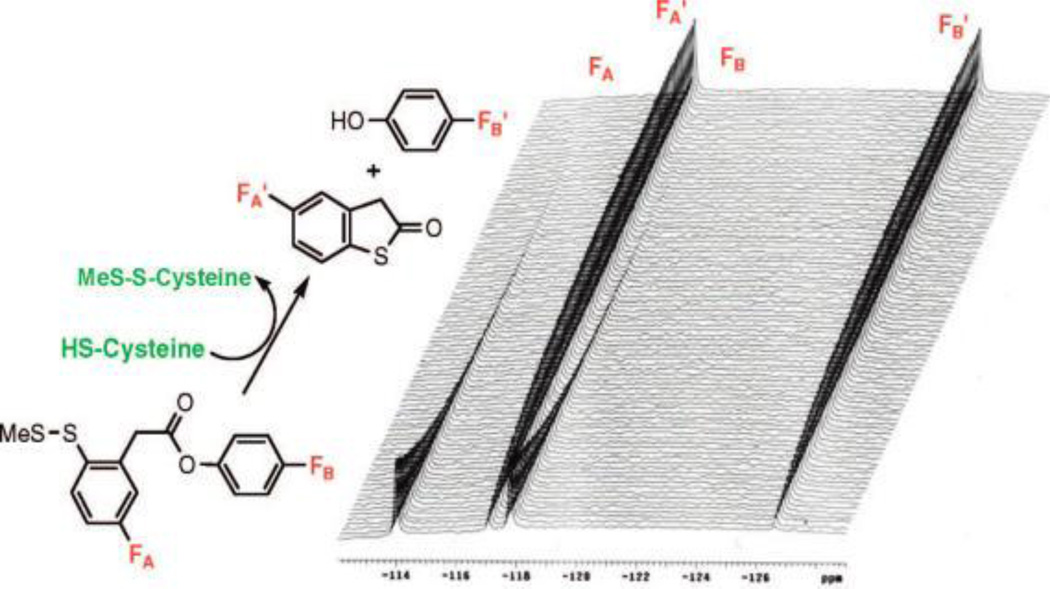
19F NMR spectroscopy allows for direct observation of fluorinated compounds and their metabolites in biological systems without background signal from the tissue or medium due to the absence of fluorine in living systems [16]. Furthermore, 19F has a natural abundance of 100% and a high sensitivity to NMR detection (83% of 1H), allowing for a strong NMR signal with negligible background noise [17]. In the last decade, the “three fluorine atoms for biochemical screening (3-FABS)” has emerged as a useful biochemical tool with heightened signal sensitivity by labeling a substrate with a CF3 moiety and using 19F NMR spectroscopy for the analysis of enzymatic processes [18–20]. Our laboratory has been exploring “fluorine-probes” for structural analysis of paclitaxel and taxoids in the absence and presence of microtubules by 19F NMR in solution and solid state, as well as in combination with computational analysis [21–26]. As Figure 1 shows, we also applied time-resolved 19F NMR spectroscopy to demonstrate a proof of concept for the mechanism-based drug release through thiol-triggered cleavage of a self-immolative disulfide linker and subsequent thiolactonization, using a model system [27].
Figure 1.
A model system for the mechanism-based drug release using cysteine as the trigger for thiolactonization. [Adapted from Reference [27]]
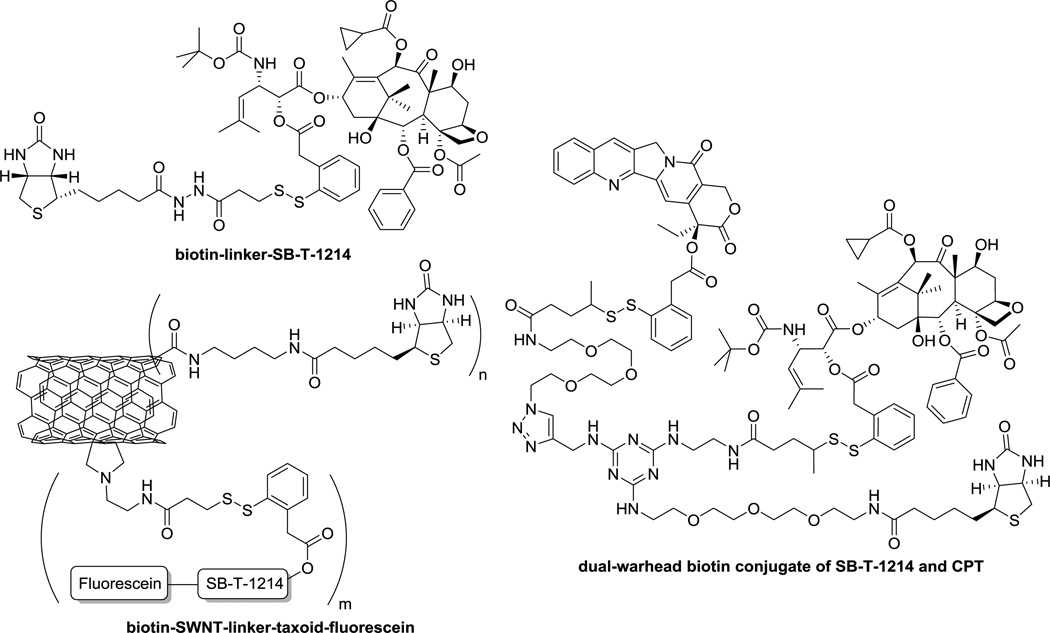
Our laboratory has been successfully developing a series of taxoid-based tumor-targeted drug delivery systems (TTDDSs) using self-immolative disulfide linkers, exemplified in Figure 2 [1, 4, 5, 15]. These vitamin receptor-targeted DDSs are efficiently internalized via a receptor-mediated endocytosis (RME) that traffics the conjugates through highly reduced endosomal and lysosomal compartments, wherein the concentration of endogenous thiols, represented by glutathione (GSH), is >1,000 times higher (2–8 mM) than that in the blood plasma (1–2 µM) [28, 29]. The endogenous thiols trigger the drug release cascade via the cleavage of disulfide linkage and thiolactonization [14]. The internalization of TTDDSs via RME and drug release in cancer cells were validated by means of confocal fluorescence microscopy (CFM) and flow cytometry, using fluorescence-labeled TTDDSs [4, 5, 14, 15].
Figure 2.
Examples of biotin-based SMDCs: BLT (top left), dual-warhead conjugate of SB-T-1214 and camptothecin (CPT) (right), and SWNT-based drug conjugate of SB-T-1214 (bottom left).
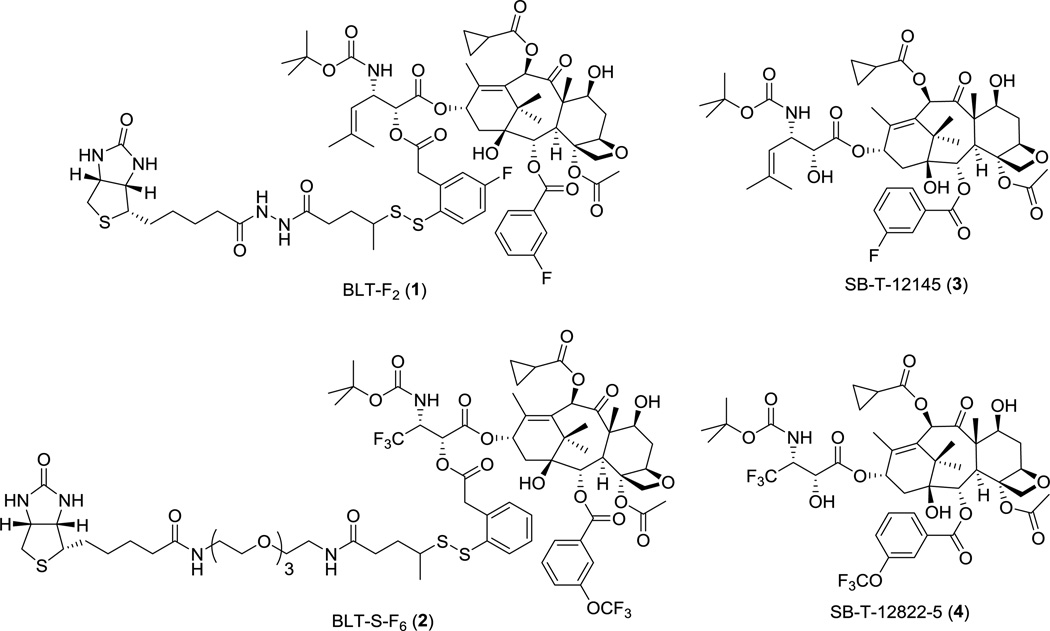
We describe here the design and synthesis of two novel 19F NMR probes, BLT-F2 (1) and BLT-S-F6 (2), to investigate the factors that affect the rate of disulfide cleavage and drug release in biologically relevant media such as human blood plasma, which would otherwise be difficult to assess accurately by conventional HPLC or 1H NMR analysis, due to complex background peaks/signals. These 19F NMR probes, 1 and 2, are TTDDSs, bearing fluorotaxoids, SB-T-12145 (3) and SB-T-12822-5 (4), respectively, as “warheads”, a self-immolative disulfide linker unit, and biotin as the tumor-targeting module (Figure 3). Fluorine atoms are strategically incorporated into each conjugate to monitor the dynamics of disulfide linker cleavage and drug release by 19F NMR.
Figure 3.
BLT-F2 (1), BLT-S-F6 (2), SB-T-12145 (3) and SB-T-12822-5 (4)
2. Results and Discussion
2.1. Design and Synthesis of Fluorine-Probe 1
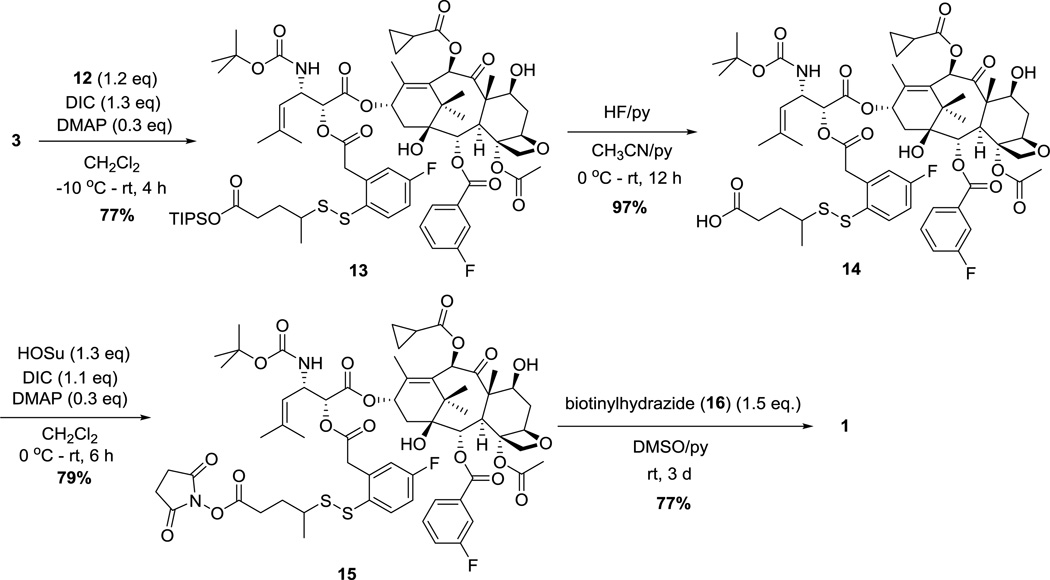
Among possible cytotoxic agents for use in SMDCs, our laboratory has been developing highly potent next-generation taxoids, which exhibit 2–3 orders of magnitude greater potency than paclitaxel and docetaxel against multidrug-resistant and paclitaxel-resistant cancer cell lines [30–35]. For fluorine-probe 1, we designed and synthesized fluorotaxoid SB-T-12145 (3) as the warhead with site-specific incorporation of fluorine at the meta-position of the C2-benzoate moiety as a close mimic of a highly efficacious next-generation taxoid, SB-T-1214 [35–38]. Another fluorine was introduced to the 4 position of the self-immolative disulfanylphenylacetate moiety in the linker unit, i.e., para to the disulfide moiety.
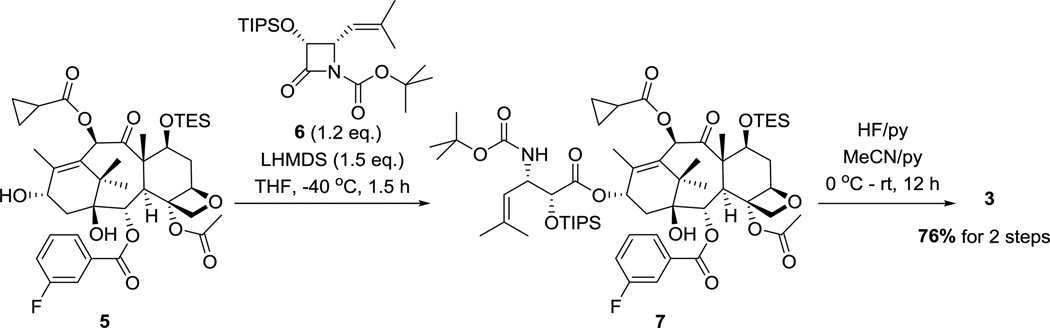
The synthesis of fluorotaxoid 3 is shown in Scheme 1. 7-TES-2-(3-fluorobenzoyl)-10-cyclopropanecarbonylbaccatin 5 and (3R,4S)-4-isobutenyl-3-TIPSO-β-lactam 6 were prepared according to literature methods [35, 39] and subjected to the Ojima-Holton coupling under the standard conditions to afford 7. Deprotection of the silyl groups of 7 with HF-pyridine gave SB-T-12145 (3) in 76% yield for 2 steps (Scheme 1).
Scheme 1.
Synthesis of SB-T-12145 (3).
The synthesis of the fluorine-labeled disulfide linker 12 is illustrated in Scheme 2. First, the reaction of 5-fluoro-3H–benzo[b]thiophene (8) [40] with n-BuLi and tributyl borate afforded 2,4,6-trisubstituted-1,3,5-trioxa-2,4,6-triborane, which was treated with 30% hydrogen peroxide to give fluorobenzothiolactone 9. 4-Fluoro-2-sulfhydrylphenylacetic acid (10), which was obtained by the basic hydrolysis of 9, was subjected to a thiol-disulfide exchange with 2-pyridyldisulfide 11 [5, 41] to afford the coupling-ready disulfide linker 12.
Scheme 2.
Synthesis of 19F-labeled linker intermediate (12).
Synthesis of conjugate 1 is illustrated in Scheme 3. Esterification of the hydroxyl group at C2 of 3 with 12 in the presence of DIC and DMAP afforded taxoid-linker conjugate 13. Deprotection of the TIPS ester with HF-pyridine, followed by condensation with N-hydroxysuccinimide gave activated ester 15 in 77% yield for 2 steps. Coupling of 15 with biotinylhydrazine (16) [42] in the presence of pyridine afforded doubly fluorine-labeled probe 1, in 56% overall yield from 3.
Scheme 3.
Synthesis of BLT-F2 probe (1).
2.2. 19F NMR Analysis of the Linker Cleavage and Drug Release Using Probe 1
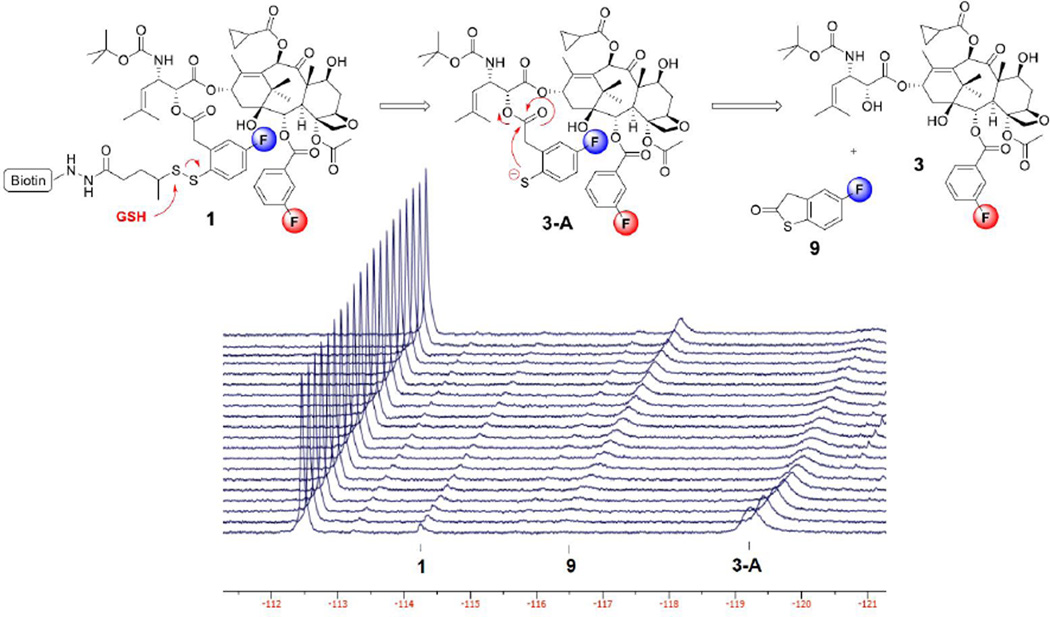
The chemical shifts (ppm) of the two fluorine signals in probe 1 were first examined in aqueous DMSO solutions (30% DMSO, 70% D2O, 25 °C) of (a) SB-T-12145 (3), (b) probe 1, (c) fluorobenzothiolactone (9), and (d) a mixture of the three compounds (see Supplemental Information). The fluorine signal of the linker unit appeared at −114.1 ppm and is clearly distinct from that of thiolactone 9 (−116.3 ppm). There was no appreciable difference in chemical shift (−112.5 ppm) between the signal arising from the fluorine in free taxoid 3 and that in probe 1. This may indicate that the fluorine at the meta-position of benzoate at C2 in taxoid unit in probe 1 is not spatially close to the phenylacetate moiety in the linker unit in this solvent system. In turn, this signal can be used as the internal standard.
The time-resolved 19F NMR spectra of disulfide linker cleavage in probe 1 in the same solution, starting from 1 hour after the addition of GSH (6 eq, 15 mM) at 25 °C with 15 minute intervals, is shown in Figure 4. The reference signal of the fluorine atom o\in the C2-benzoate group of 3 and the taxoid moiety in 1 was clearly identified at −112.5 ppm, i.e., no appreciable chemical shift change during the process under the conditions employed. As Figure 4 shows, most of probe 1 (at −114.1 ppm) was consumed after incubation with GSH for 1 hour. However, the disappearance of 1 did not directly correspond to the increase in the amount of thiolactone 9 (at −116.3 ppm). This result makes a sharp contrast to the observation for the model system shown in Figure 1. Instead of the immediate formation of 9, the 19F NMR analysis detected a substantial formation of 2’-fluoro(sulfhydryl)phenylacetyltaxoid 3-A (at −119.2 ppm) at the beginning of the monitoring. The observed 5 ppm upfield shift in this fluorine signal strongly indicates the likely formation of thiolate at para to the position of fluorine in the phenylacetate moiety. Then, the thiolactonization of 3-A forms 3. This correlation is apparent from the observed decrease in the 3-A signal and the increase in 3 signal over the time course.
Figure 4.
Time-resolved 19F NMR spectra for the disulfide linker cleavage and thiolactonization process of probe 1 (2.5 mM) in 30% DMSO in D2O beginning at 1 hour after the addition of 6 equivalents of GSH at 25 °C with 15 min intervals (128 scans/spectrum).
This 19F NMR experiment disclosed that the “self-immolation” of the disulfide linker proceeded in two steps, generating the mechanistically anticipated thiolate 3-A as a detectable transient species/intermediate, prior to thiolactonization to release a drug. It also suggests that the introduction of a fluorine para to a disulfide linkage had a profound effect on the rate of linker cleavage as well as thiolactonization. Thus, the para-fluorine can stabilize the thiolate being formed, which may contribute to faster disulfide cleavage in the thiol-disulfide exchange process, as well as slower thiolactonization by reducing the nucleophilicity of the thiolate species. It should also be noted that the 2-step self-immolation process was not observed in the model system mentioned above (Figure 1), which may indicate that the steric and/or conformational factors in the microenvironment of probe 1, a biotin-linker-taxoid conjugate, are substantially different from those of the model system. Consequently, probe 1 has provided unique and very useful information for the mechanism of “self-immolation”, which would not have been possible by other means.
Attempts to utilize probe 1 as a 19F NMR probe in cell culture media or blood plasma (10% DMSO, 20% D2O, 70% plasma/media) were hampered by the poor solubility and low signal intensity of the probe. At the concentrations required for 19F NMR detection of single fluorine (1.0–2.5 mM), the conjugate was insoluble and precipitated from most aqueous solutions, including cell culture media and blood plasma. However, the use of Solutol HS15 as an excipient (5% Solutol HS15, 5% EtOH, 20% D2O, 70% saline) was found to solubilize the conjugate effectively at these concentrations, and quantification of the 19F NMR signals from probe 1 was readily achieved. The addition of 6 equivalents of GSH (15 mM) to probe 1 in this formulation showed only ca. 20% linker cleavage after 10 hours (see Figure S2 in the Supporting Information), as compared to the nearly-quantitative cleavage within 1 hour in DMSO/D2O (see Figure S4 in the Supporting Information). The results clearly indicate that excipients used for in vivo drug efficacy studies have a profound effect on the stability of drug conjugates or TTDDSs by protecting disulfide linkers from GSH-mediated cleavage. To further investigate the effects of excipients on the stability and reactivity of the self-immolative disulfide linker unit of a TTDDS in relevant biological media, a double “3-FAB” probe 2 was designed and synthesized.
2.3. Design and Synthesis of Probe 2
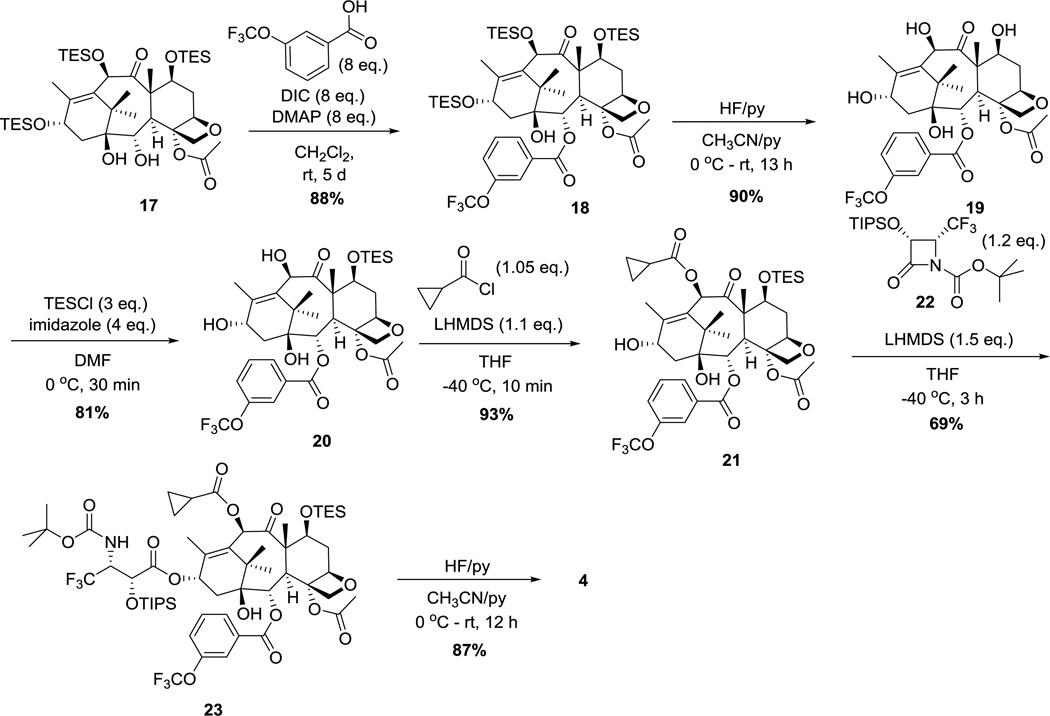
Probe 2 was designed as a more sensitive 19F NMR probe with higher solubility in aqueous media than probe 1. The aqueous solubility of probe 2 was increased by incorporating a triethylene glycol moiety in the linker unit, and the sensitivity was heightened by using CF3 and CF3O groups as reporter signals. The CF3 group at C3’ of the taxoid moiety is located adjacent to the site of linker attachment and designed to be used as the reporter signal for linker cleavage, while the meta-CF3O group of benzoate at C2 was designed to be used as an internal standard signal for quantification (Figure 3). This design relies on a significant difference in chemical shift between the CF3 group in taxoid and the taxoid moiety in probe 2 so that the peaks may be integrated for quantitative assessment of linker stability and reactivity. For probe 2, we designed and synthesized fluorotaxoid SB-T-12822-5 (4) as the warhead, placing a CF3 group at C3’ and a CF3O group at the meta position of benzoate at C2.
Synthesis of fluorotaxoid 4 is illustrated in Scheme 4. Esterification of 2-debenzoyl-tri-TES-baccatin 17 with 3-(trifluoromethoxy)benzoic acid in the presence of DIC and DMAP afforded tri-TES-fluorobaccatin 18 in 88% yield. Deprotection of TES groups with HF-pyridine gave fluorobaccatin 19, which was protected with a TES group at the hydroxyl group at C7 to afford 7-TES-fluorobaccatin 20 in 73% yield for 2 steps. Acylation of the hydroxyl group at C10 with cyclopropanecarbonyl chloride in the presence of LHMDS gave fluorobaccatin 21 in 93% yield. The Ojima-Holton coupling of 21 with (3R,4R)-3-TIPSO-4-CF3-β-lactam 22 [43] in the presence of LHMDS afforded 2’-TIPS-7-TES-taxoid 23. The subsequent deprotection of the silyl groups with HF-pyridine afforded SB-T-12822-5 (4) in 60% for 2 steps.
Scheme 4.
Synthesis of SB-T-12822-5 (4).
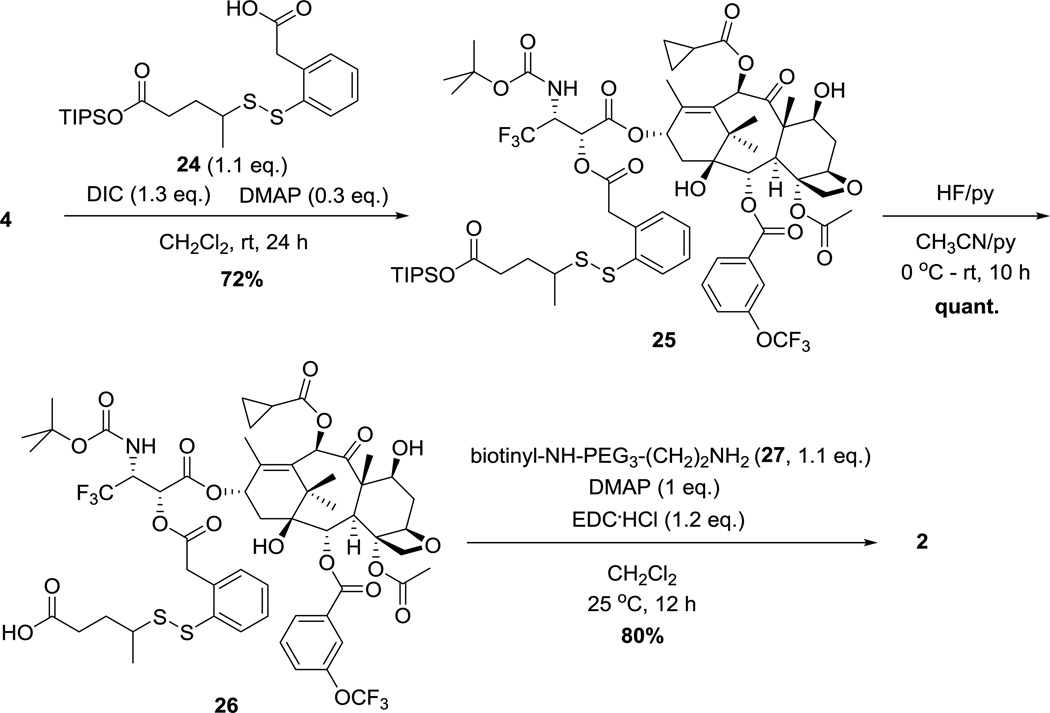
Synthesis of probe 2 is illustrated in Scheme 5. Ester coupling of taxoid 4 with self-immolative disulfide linker TIPS ester 24 [5] in the presence of DIC and DMAP gave taxoid-linker conjugate 25 in 72% yield. Deprotection of TIPS with HF-pyridine followed by amide coupling with biotinyl-NH(PEG)3(CH2)2NH2 (27) gave probe 2 in 80% yield.
Scheme 5.
Synthesis of BLT-S-F6 probe (2).
2.4. Effects of Solvent Systems and Drug Formulations on the 19F NMR chemical shifts of the CF3 Groups at the 3’ Position of Taxoid 4 and the Taxoid Moiety in Probe 2
The 3’-trifluoromethyl and m-trifluoromethoxy groups of fluorotaxoid 4 and probe 2 were used as quantitative 19F NMR reporter signals to assess the stability and reactivity of probe 2. The 19F NMR chemical shift changes and differences between probe 2 and free taxoid 4 were measured on a 400 MHz NMR spectrometer in various solvent and formulation systems. Results are summarized in Table 1. The corresponding 19F NMR spectra are shown as Figure S3 – S12 in the Supporting Information.
Table 1.
19F NMR chemical shifts (ppm) of probe 2 and taxoid 4 in various formulationsa
| Entry | Formulation | Ratio |
m-OCF3 taxoid 4 |
m-OCF3 probe 2 |
|Δ m- OCF3| |
CF3 taxoid 4 |
CF3 probe 2 |
|Δ CF3| |
|---|---|---|---|---|---|---|---|---|
| 1a | CDCl3 | 100 | −57.961 | −57.923 | 0.038 | −73.404 | −73.116 | 0.288 |
| 2 | blood plasma:D2O: EtOH:polysorbate 80 |
84:10:4:2 | −58.074 | −58.074 | 0 | −72.838 | −73.048 | 0.210 |
| 3 | RPMI-1640:D2O: EtOH:polysorbate 80 |
84:10:4:2 | −57.906 | −57.906 | 0 | −72.705 | −72.911 | 0.206 |
| 4 | D2O:EtOH:polysorbate 80 | 94:4:2 | −57.888 | −57.888 | 0 | −72.702 | −72.889 | 0.187 |
| 5 | saline:EtOH:D2O | 50:40:10 | −58.419 | −58.436 | 0.017 | −73.342 | −73.412 | 0.070 |
| 6 | PBS:EtOH:D2O | 50:40:10 | −58.413 | −58.431 | 0.018 | −73.339 | −73.404 | 0.065 |
| 7 | DMSO:D2O | 70:30 | −56.459 | −56.431 | 0.028 | −71.115 | −71.068 | 0.047 |
| 8 | D2O:EtOH | 60:40 | −58.417 | −58.436 | 0.019 | −73.361 | −73.407 | 0.046 |
| 9 | D2O:Solutol HS15:EtOH | 84:8:8 | −58.047 | −58.047 | 0 | −72.910 | −72.910 | 0 |
19F NMR spectra were obtained for 200 µM solutions of 2, 4, and a 1:1 mixture of 2 and 4 in various formulations with or without excipients with 10% D2O as the NMR solvent (>256 scans). The difference in the chemical shifts between each respective 2-m-OCF3 and 3’-CF3 was calculated. Spectral overlays are provided in Supporting Information.
The difference in chemical shifts between the 3’-CF3 and 2’-m-CF3O groups was first examined in chloroform-d. A chemical shift difference of nearly 0.3 ppm with baseline resolution was observed between the 3’-CF3 groups of 2 (−73.116 ppm) and 4 (−73.404 ppm), respectively. In contrast, but as anticipated, the 2-m-OCF3 groups of 2 (−57.923 ppm) and 4 (−57.961 ppm) showed a much smaller difference (Entry 1). Then, samples of fluorotaxoid 4 and probe 2 were prepared in various biologically relevant aqueous media with or without excipient, and the 19F NMR chemical shift differences were measured. The most significant difference in chemical shift between the 3’-CF3 signals of 2 and 4 was observed when polysorbate 80 was used as a surfactant (Entries 2–4) with as large as 0.21 ppm difference with baseline resolution in blood plasma solution (Entry 2). In contrast, no appreciable change in chemical shift was detected when Solutol HS15 was used as the excipient (Entry 9). The results revealed that different excipients would make different microenvironments to probe 2, which should also be applicable to various drugs and drug conjugates. In the absence of excipient, the 19F NMR chemical shift differences between the 3’-CF3 of probe 2 and fluorotaxoid 4 were 0.070–0.065 ppm in (saline or PBS)/EtOH/D2O solutions (Entries 5 and 6) and 0.047–0.046 ppm in aqueous DMSO and EtOH solutions (Entries 7 and 8). For the 2-m-CF3O signals, no chemical shift difference was observed between probe 2 and fluorotaxoid 4 in the presence of an excipient. However, in the absence of excipient, small changes in chemical shift were detected ranging from 0.02 to 0.04 ppm.
2.5. Assessment of the Stability and Reactivity of Probe 2 in Human Blood Plasma by 19F NMR
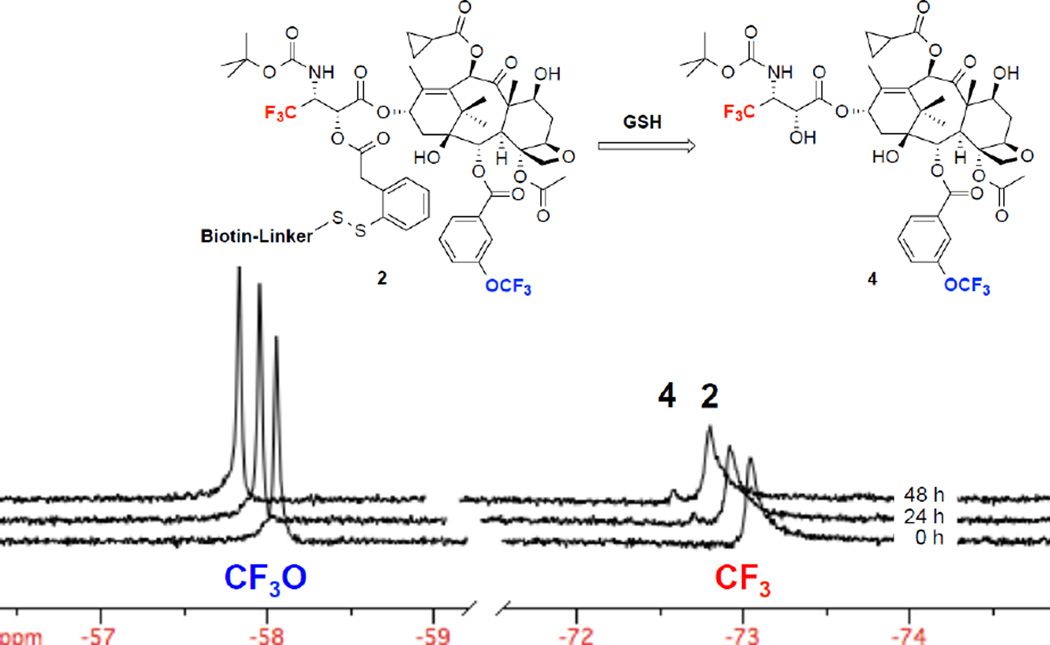
The stability and reactivity of probe 2 in human blood plasma at 37 °C was evaluated by 19F NMR using the 3’-CF3 signals of probe 2 (−73.048 ppm) and released fluorotaxoid 4 (−72.838 ppm) as key reporters. In 86% blood plasma, 10% D2O, 2% EtOH, and 2% polysorbate 80, probe 2 remained stable with a very little release (<10%) of fluorotaxoid 4 via linker cleavage and thiolactonization for 48 hours at 37 °C (Figure 5). The result implies that the putative half-life of drug conjugates and TTDDSs using this self-immolative linker unit and taxoids as warheads in this formulation would be longer than one week in human blood plasma.
Figure 5.
Time-resolved 19F NMR spectra for the drug release of probe 2 (200 µM) in 86% blood plasma, 10% D2O, 2% ethanol, and 2% polysorbate 80 at 37 °C without supplemental GSH at 0, 24, and 48 h (2048 scans/spectrum). The signals of 2-m-OCF3(left) and 3’-CF3(right) for 2 and free taxoid 4 are shown, which indicates minimal drug release after 48 h.
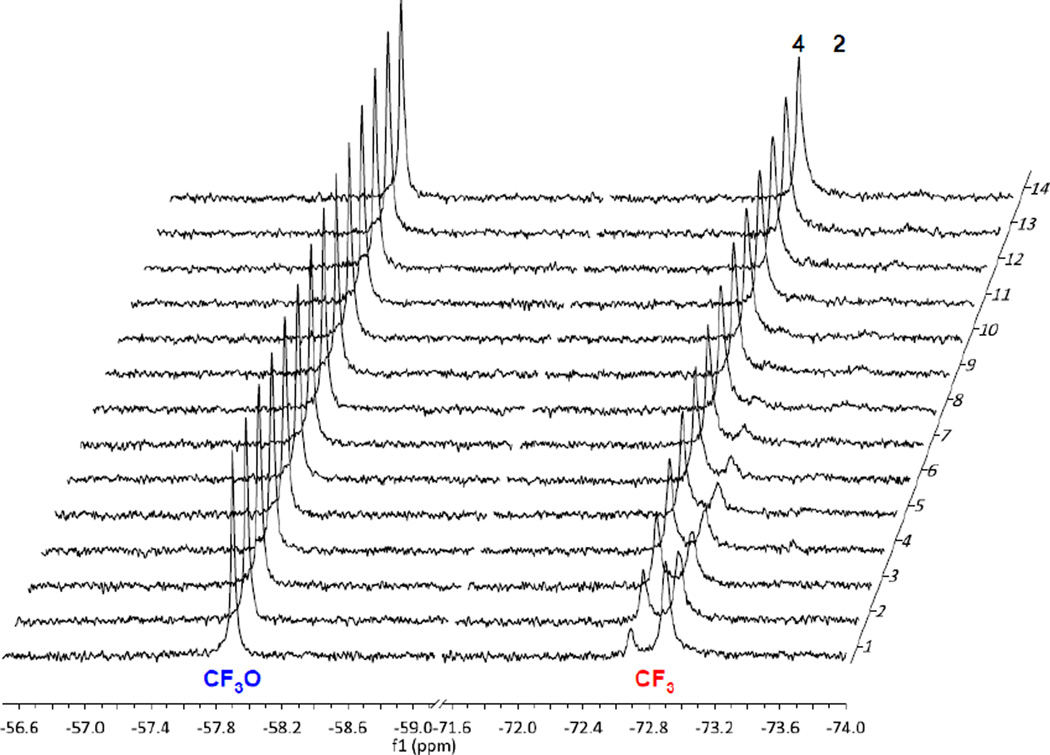
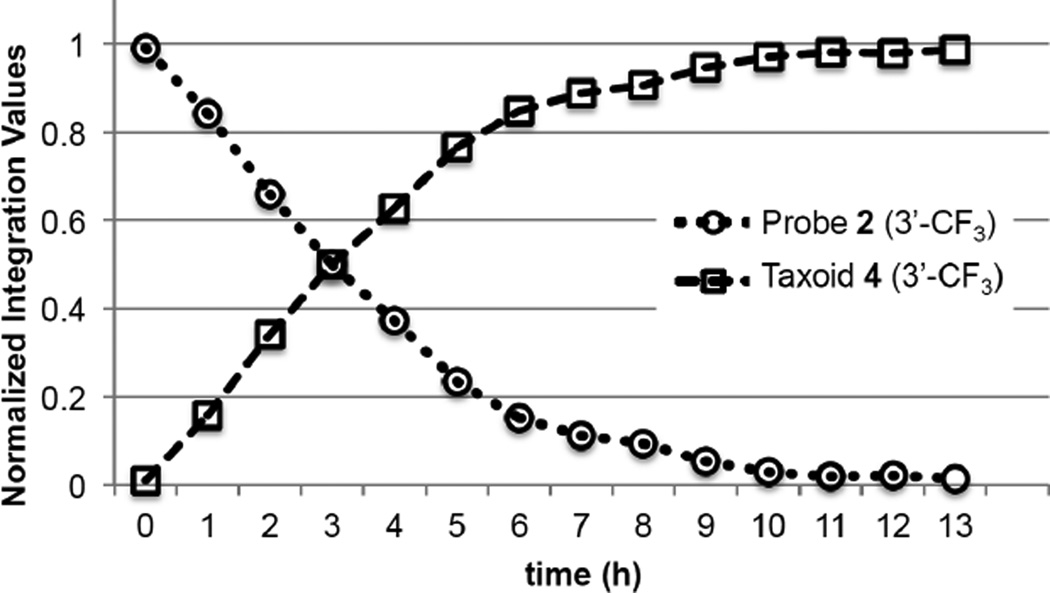
To validate the results, supplemental GSH (100 equiv., 20 mM concentration) was added to probe 2 and analyzed by time-resolved 19F NMR spectroscopy with 1 hour intervals. As Figure 6 shows, >98% of probe 2 disappeared and the corresponding amount of fluorotaxoid 4 appeared within 10 hour at 37 °C. The half-life of probe 2 with 100 equivalents of GSH in blood plasma was calculated to be ~3 h, which was determined by plotting the changes in integration of the C3’-CF3 signals of probe 2 and free fluorotaxoid 4 over time, as shown in Figure 7. As the intracellular concentration of glutathione is known to be at the 10 mM level [44], this experiment allows us to estimate the half-life of this self-immolative disulfide linker in the cytosolic compartments following RME.
Figure 6.
Time-resolved 19F NMR spectra for the drug release of probe 2 (200 µM) in 86% blood plasma, 2% ethanol, and 2% polysorbate 80 in D2O at 30 min after the addition of 100 equivalents of GSH at 37 °C with 1 h intervals (1024 scans/spectrum) for 13 h. The signals of 2-m-OCF3(left) and the 3’-CF3(right) are shown, which indicate full drug release after 13.5 h.
Figure 7.
Normalized changes in integration of 3’-CF3 peaks of probe 2 with 100 equiv. of GSH in 86% blood plasma, 2% ethanol, 2% polysorbate 80, 10% D2O and released taxoid 4. Experimental data and normalized values are shown in Table S1 in the Supporting Information.
It should be noted that the linker cleavage and drug release of probe 2 with 100 equivalents of supplemental GSH in 96% D2O, 2% EtOH, 2% polysorbate 80 at 37 °C was significantly slower than that under the same conditions in blood plasma, wherein only 50% drug release was observed at approximately 4 days as determined by 19F NMR analysis (Figure S11 in the Supporting Information). This result serves to recapitulate the observation made during the NMR analysis of probe 1 that the presence of an excipient significantly reduces the rate of disulfide cleavage (Solutol HS15 for 1 and polysorbate 80 for 2). However, blood plasma contains many proteins which are not present in the D2O or saline/PBS formulations and likely to interact with the excipient, which leads to its dissociation from the drug conjugate, rendering the disulfide linkage more exposed to the attack of the supplemental GSH.
3. Conclusions
We have successfully synthesized two novel 19F NMR probes 1 (BLT-F2 conjugate) and 2 (BLT-S-F6 conjugate), bearing biotin as the tumor-targeting module, a self-immolative linker unit, and a fluorotaxoid as the warhead for the assessment of stability and reactivity of those drug conjugates by means of 19F NMR spectroscopy. Fluorine atoms or CF3 groups were strategically incorporated into the conjugates to investigate the mechanism of linker cleavage and factors that influence their plasma and metabolic stability by real-time monitoring with 19F NMR. Time-resolved 19F NMR study on probe 1 disclosed a stepwise mechanism for the release of a fluorotaxoid, which might not be detected by other analytical methods, as well as indicated a profound effect of the fluorine para to the disulfide group of the phenylacetate moiety on the rate of linker cleavage and thiolactonization.
It was also found that the stability of probe 1 was dramatically enhanced when 5% Solutol HS15 was used as excipient. Thus, the effects of excipients on the stability and reactivity of drug conjugates bearing a self-immolative disulfide linker unit were further studied by using probe 2. Probe 2 was designed to be more water-soluble and have enhanced sensitivity by the introduction of a polyethylene glycol oligomer to the linker unit and CF3 groups to the taxoid warhead. Since the clean analysis of the linker stability and reactivity of drug conjugates in blood plasma or cell culture media by HPLC and 1H NMR is very challenging, the use of 19F NMR would provide a practical solution to this problem. Indeed, it has been shown that probe 2 is very useful to study the stability and reactivity of the self-immolative disulfide linker system in human blood plasma by 19F NMR. The use of polysorbate 80 as excipient for the formulation of probe 2 was found to dramatically increase the stability of the disulfide linker system. Thus, the half-life of probe 2 in human blood plasma is estimated to be longer than one week. Nevertheless, probe 2 releases the taxoid warhead smoothly (t1/2 ~3 hours) in the presence of 100 equivalents of supplemental GSH (20 mM), which is equivalent to the level of GSH in tumors and thus mimics the drug release in cancer cells. Further studies on the applications of fluorine-probes for the tumor-targeting drug conjugates as well as drug delivery systems by means of 19F NMR spectroscopy are actively in progress in our laboratory.
4. Experimental
4.1 General Methods
1H, 13C and 19F NMR spectra were measured and recorded on a Varian 300, 400, or 500 MHz NMR spectrometer or a Brüker 300, 400, 500 MHz NMR spectrometer. Hexafluorobenzene was used as an external reference for 19F NMR. Melting points were measured on a Thomas-Hoover capillary melting point apparatus and are uncorrected. TLC was performed on Sorbent technologies aluminum-backed Silica G TLC plates (Sorbent Technologies, 200 µm, 20 × 20 cm), and column chromatography was carried out on silica gel 50 (Merck, 230–400 mesh ASTM). Low resolution mass spectrometry analysis was carried out on an Agilent LC-MSD mass spectrometer at the Institute of Chemical Biology and Drug Discovery, Stony Brook, NY. High resolution mass spectrometry (HRMS) analysis was carried out on an Agilent LC-UV-TOF mass spectrometer at the Institute of Chemical Biology and Drug Discovery, Stony Brook, NY or at the Mass Spectrometry Laboratory, University of Illinois at Urbana-Champaign, Urbana, IL.
4.2 Materials
All reagents were purchased from Sigma-Aldrich, Fisher Scientific, and VWR International, and used as received or purified before use by standard methods. Tetrahydrofuran was freshly distilled from sodium and benzophenone. Dichloromethane was also distilled immediately prior to use under nitrogen from calcium hydride. 10-Deacetylbaccatin III was obtained from Indena, SpA, Italy. 10-Cyclopropanecarbonyl-10-deacetyl-2-debenzoyl-2-(3-fluorobenzonyl)-7-triethylsilylbaccatin III (5) [35], (3R,4S)-1-tert-butoxycarbonyl-3-triisoproylsilyloxy-4-(2-methyl-1-propenyl)-2-azetidinone (6) [39], 5-fluoro-3H–benzo[b]thiophene (8) [40], triisopropylsilyl 4-(pyridine-2-yldisulfanyl)pentanoate (11) [5], biotinylhydrazine (16) [42], 10-deacetyl-2-debenzoyl-7,10,13-tris(triethylsilyl)baccatin III (17) [35], (3R,4R)-1-(tert-butoxycarbonyl)-3-triisopropylsiloxy-4-trifluoromethylazetidin-2-one (22) [43], 2-(5-oxo-triisopropylsiloxypentan-2-yl)disulfanylphenylacetic acid (24) [5], and biotinyl-NH(PEG)3(CH2)2-NH2 (27) [45] were prepared according to literature methods. Human peripheral blood plasma (HemaCare), 0.9% sodium chloride injection saline with 10% dextrose (Baxter Healthcare Corp.), RPMI cell culture medium, and phosphate-buffered saline (PBS) (Gibco) were used as received for formulation and NMR-based assays.
4.3 Synthesis of SB-T-12145 (3)
4.3.1. 2-Debenzoyl-2-(3-fluorobenzoyl)-3'-dephenyl)-3'-(2-methyl-2-propenyl)-10-(cyclopropanecarbonyl)docetaxel [SB-T-12145] (3)
To a cooled solution of 5 (185 mg, 0.25 mmol) and (+)−6 (124 mg, 0.31 mmol) in THF (10 mL) at −40 °C was added a [1 M] solution of LHMDS in tert-butyl methyl ether (0.36 mL), and the mixture was allowed to react for 1.5 h at −40 °C with stirring. The reaction was quenched with saturated NH4Cl (5 mL), and the reaction mixture was allowed to warm to room temperature, diluted with H2O (30 mL), and extracted with ethyl acetate (2 × 30 mL). The combined organic layers were washed with brine (3 × 30 mL), dried over MgSO4, and concentrated in vacuo. Purification of the crude product by column chromatography on silica gel with hexanes/ethyl acetate (4:1) as eluent gave 7 as a white solid (236 mg, 83% yield): 1H NMR (300 MHz, CDCl3): δ 0.46–0.64 (m, 6H), 0.82–0.98 (m, 13H), 1.04–1.14 (m, 21H), 1.19 (s, 3H), 1.27 (s, 3H), 1.34 (s, 9H), 1.68 (s, 3H), 1.75 (s, 3H), 1.78 (s, 3H), 1.82–1.96 (m, 1H), 2.01 (s, 3H), 2.20–2.32 (m, 2H), 2.36 (s, 3H), 2.42–2.58 (m, 1H), 3.85 (d, J = 7.2 Hz, 1H), 4.17 (d, J = 8.4 Hz, 1H), 4.30 (d, J = 8.4 Hz, 1H), 4.40–4.52 (m, 2H), 4.70–4.86 (m, 2H), 4.95 (d, J = 8.1 Hz, 1H), 5.33 (d, J = 8.7 Hz, 1H), 5.65 (d, J = 7.5 Hz, 1H), 6.06 (t, 1H), 6.48 (s, 1H), 7.31 (m, 1H), 7.45 (m, 1H), 7.79 (d, J = 10.2 Hz, 1H), 7.90 (d, J = 7.8 Hz, 1H). MS (ESI) m/z: Calcd. for C60H93FNO15Si2+: 1142.6; Found: 1142.6.
To a cooled solution of 7 (230 mg, 0.20 mmol) in CH3CN-pyridine (1:1) (16 mL) at 0 °C was added HF-pyridine (2.4 mL), and the mixture was allowed to warm from 0 °C to room temperature and react for 12 h with stirring. The reaction was quenched with 10% citric acid (5 mL), and the reaction mixture was neutralized with saturated NaHCO3 (30 mL) and extracted with ethyl acetate (2 × 30 mL). The combined organic layers were washed with saturated CuSO4 solution (3 × 30 mL), H2O (2 × 30 mL), and brine (3 × 30 mL), and then dried over MgSO4, and concentrated in vacuo. Purification of the crude product by column chromatography on silica gel with hexanes/ethyl acetate (1:2) as eluent gave SB-T-12145 (3) as a white solid (159 mg, 91%): mp 153–155 °C; 1H NMR (500 MHz, CDCl3): δ 1.02–1.20 (m, 5H), 1.22 (s, 3H), 1.33 (s, 3H), 1.42 (s, 9H), 1.60–1.72 (m, 3H), 1.74 (s, 3H), 1.83 (s, 3H), 1.833 (s, 3H), 1.97 (s, 3H), 2.11 (s, 1H), 2.28–2.50 (m, 3H), 2.42 (s, 3H), 2.54–2.72 (m, 1H), 3.88 (d, J = 7.2 Hz, 1H), 4.21 (d, J = 8.4 Hz, 1H), 4.27 (d, J = 8.4 Hz, 1H), 4.48 (dd, J = 6.6, 10.5 Hz, 1H), 4.74–4.88 (m, 2H), 5.04 (d, J = 8.1 Hz, 1H), 5.39 (d, J = 6.0 Hz, 1H), 5.70 (d, J = 7.2 Hz, 1H), 6.23 (t, J = 9.0 Hz, 1H), 6.37 (s, 1H), 7.38 (t, J = 8.4 Hz, 1H), 7.53 (m, 1H), 7.86 (d, J = 9.0 Hz, 1H), 7.97 (d, J = 7.8 Hz, 1H); 13C NMR (125 MHz, CDCl3): δ 9.25, 9.51, 13.05, 15.03, 18.54, 21.97, 22.35, 25.73, 26.66, 28.22, 35.49, 35.55, 43.15, 45.65, 51.60, 58.52, 72.23, 72.39, 73.71, 75.42, 75.52, 77.04, 77.30, 76.31, 79.25, 81.04, 84.49, 116.85, 117.05, 120.54, 120.72, 120.89, 126.01, 130.34, 130.40, 131.42 (d, J = 7.5 Hz), 132.78, 142.80, 155.46, 161.56, 163.51, 165.70, 170.03, 175.17, 202.87; 19F NMR (282 MHz, CDCl3) δ −111.90; HRMS (TOF): Calcd. for C45H59FNO15+: 872.3863; Found: 872.3864 (Δ = 0.1 ppm).
4.4 Synthesis of 19F-labeled self-immolative disulfide linker (12)
4.4.1. 5-Fluoro-3H–benzo[b]thiophen-2-one (9)
To a solution of 5-fluoro-3H–benzo[b]thiophene (8) (350 mg, 2.3 mmol) in Et2O (5 mL) was added n-BuLi (1.4 mL, 1.6 M in hexanes) dropwise at −40 °C, and the mixture was allowed to stir for 45 min at −40 °C. To the cooled solution was added B(OBu)3 (635 mg, 2.76 mmol) in Et2O (1 mL), and the mixture was allowed to warm from −40 °C to 0 °C and react for 1 h with stirring. The reaction was quenched with 2 N HCl (10 mL), and the reaction mixture was diluted with H2O (20 mL) and extracted with Et2O (3 × 30 mL). The combined organic layers were extracted with 2 M NaOH (3 × 30 mL), and the combined aqueous layers were acidified to pH 1 with concentrated HCl and extracted again with Et2O (3 × 25 mL). The second ether extract (75 mL) was dried over MgSO4 and concentrated in vacuo to give 2,4,6-tri(5-benzo[b]thiophen-2-yl)-1,3,5-trioxa-2,4,6-triborinane as an off-white solid.
To a solution of crude triborinane (330 g, 0.62 mmol) in EtOH (4 mL) was added 30% H2O2 (1.3 mL, 10 mmol), and the mixture was allowed to react for 12 h at room temperature with stirring. The reaction mixture was diluted with H2O (10 mL) and extracted with CH2Cl2 (2 × 20 mL). The combined organic layers were washed with brine (2 × 30 mL), dried over MgSO4, and concentrated in vacuo to afford a brown oil. Purification of the crude product by column chromatography on silica gel with hexanes/ethyl acetate (5:1) as eluent gave 9 as a pale yellow solid (172 mg, 46% for 2 steps): mp 101–102 °C; 1H NMR (300 MHz, CDCl3): δ 3.98 (s, 2H), 7.01–7.07 (m, 1H), 7.05 (d, J = 8.7 Hz, 1H), 7.29 (dd, J = 4.8, 9.6 Hz, 1H); 19F NMR (282 MHz, DCl3): δ −116.24 (s, 1F). HRMS (TOF): Calcd. for C8H6FOS+: 169.0118; Found: 169.0115 (Δ = −1.8 ppm).
4.4.2. 2-Sulfhydryl-5-fluorophenyl acetic acid (10)
To a solution of 9 (165 mg, 0.98 mmol) in THF (2 mL) and heated at 60 °C was added LiOH .H2O (300 mg, 7.8 mmol) in H2O (2 mL), and the mixture was allowed to react for 6 h at 60 °C with stirring. The reaction mixture was allowed to cool to room temperature, and the mixture was acidified to pH 2 using 1 M HCl and extracted with ethyl acetate (3 × 15 mL). The combined organic layers were washed with brine (2 × 15 mL), dried over MgSO4, and concentrated in vacuo to afford a yellow solid. Purification of the crude product by column chromatography on silica gel with hexanes/ethyl acetate (3:1) as eluent gave 10 as an off-white solid (171 mg, 94%): 1H NMR (300 MHz, CDCl3): δ 3.40 (bs, 1H), 3.84 (s, 2H), 6.92 (dt, J = 2.7, 8.4 Hz, 1H), 7.01 (dd, J = 2.7, 9.0 Hz, 1H), 7.43 (dd, J = 5.4, 8.4 Hz, 1H); 19F NMR (282 MHz, CDCl3): δ −113.05 (s, 1F). MS (ESI): Calcd. for C8H6FO2S−: 185.0; Found: 185.0.
4.4.3. 2-(1-Methyl-4-oxo-5-triisopropylsilyloxybutyldisulfanyl)-5-fluorophenylacetic acid (12)
To a solution of 10 (165 mg, 0.89 mmol) in THF (2 mL) at 0 °C was added a cooled solution of 11 (354 mg, 0.89 mmol) in THF (2 mL) at 0 °C, and the mixture was allowed to react for 30 min at 0 °C with stirring. The reaction mixture was allowed to warm to room temperature and was directly concentrated in vacuo to afford a pink oil. Purification of the crude product by column chromatography on silica gel with hexanes/ethyl acetate (3:1) as eluent gave 12 as a colorless oil (142 mg, 34%): 1H NMR (300 MHz, CDCl3): δ 1.00–1.10 (m, 18H), 1.20–1.32 (m, 3H), 1.26 (d, J = 6.9 Hz, 3H), 1.81 (m, J = 8.1 Hz, 1H), 1.94 (m, J = 8.1 Hz, 1H), 2.39 (dt, J = 2.7, 7.8 Hz, 2H), 2.89 (m, J = 6.9 Hz, 1H), 3.89 (s, 2H), 6.94 −7.02 (m, 1H), 6.97 (d, J = 8.4 Hz, 1H), 7.74 (dd, J = 6.0, 8.4 Hz, 1H); 19F NMR (282 MHz, CDCl3): δ −113.13 (s, 1F). MS (ESI): Calcd. for C22H35FO4S2Si−: 473.2; Found: 473.2.
4.5. Synthesis of BLT-F2 (1)
4.5.1. SB-T-12145-(SS-F-Linker)-CO2TIPS (13)
To a cooled solution of SB-T-12145 (3) (140 mg, 0.16 mmol) and DMAP (4 mg, 0.03 mmol) in CH2Cl2 (4 mL) at −10 °C was added DIC (0.024 mL, 0.13 mmol) followed by the dropwise addition of a solution of 12 (61 mg, 0.13 mmol) in CH2Cl2 (4 mL), and the mixture was allowed to warm from −10 °C to room temperature and react for 4 h with stirring. The reaction mixture was directly concentrated in vacuo to give a yellow oil. Purification of the crude product by column chromatography on silica gel with hexanes/ethyl acetate (3:1) as eluent gave 13 as a white solid (132 mg, 77%): 1H NMR (300 MHz, CDCl3): δ 1.02–1.20 (m, 22H), 1.20–1.32 (m, 3H), 1.23 (s, 3H), 1.24–1.28 (m, 3H), 1.33 (s, 3H), 1.42 (s, 9H), 1.60–1.72 (m, 1H), 1.74 (s, 3H), 1.81 (m, 1H), 1.83 (s, 3H), 1.84 (s, 3H), 1.92 (m, 1H), 1.97 (s, 3H), 2.11 (s, 1H), 2.24–2.52 (m, 4H), 2.42 (s, 3H), 2.54–2.72 (m, 1H), 2.87 (m, 1H), 3.88 (d, J = 7.2 Hz, 1H), 3.93 (s, 2H), 4.21 (d, J = 8.4 Hz, 1H), 4.27 (d, J = 8.4 Hz, 1H), 4.48 (dd, J = 6.6, 10.5 Hz, 1H), 4.74–4.88 (m, 2H), 5.04 (d, J = 8.1 Hz, 1H), 5.39 (d, J = 6.0 Hz, 1H), 5.70 (d, J = 7.2 Hz, 1H), 6.23 (t, J = 9.0 Hz, 1H), 6.37 (s, 1H), 6.94 −7.02 (m, 2H), 7.38 (t, J = 8.4 Hz, 1H), 7.53 (m, 1H), 7.75 (dd, J = 6.0, 8.4 Hz, 1H), 7.86 (d, J = 9.0 Hz, 1H), 7.97 (d, J = 7.8 Hz, 1H); 19F NMR (282 MHz, CDCl3): δ −112.87 (s, 1F), −111.93 (s, 1F). MS (ESI): Calcd. for C67H92F2NO18S2Si+: 1328.5; Found: 1328.5.
4.5.2. SB-T-12145-(SS-F-Linker)-CO2H (14)
To a cooled solution of 13 (132 mg, 0.10 mmol) in CH3CN-pyridine (1:1) (8 mL) at 0 °C was added HF-pyridine (1.3 mL), and the mixture was allowed to warm from 0 °C to room temperature and react for 12 h with stirring. The reaction was quenched with 10% aqueous citric acid (5 mL), and the reaction mixture was neutralized with saturated NaHCO3 (20 mL) and extracted with ethyl acetate (2 × 40 mL). The combined organic layers were washed with saturated CuSO4 (2 × 50 mL), H2O (50 mL) and brine (3 × 50 mL), dried over MgSO4, and concentrated in vacuo to afford a pale yellow solid. Purification of the crude product by column chromatography on silica gel with hexanes/ethyl acetate (1:1) as eluent gave 14 as a white solid (113 mg, 97%): 1H NMR (300 MHz, CDCl3): δ 1.03–1.24 (m, 4H), 1.23 (s, 3H), 1.24–1.28 (m, 3H), 1.33 (s, 3H), 1.42 (s, 9H), 1.60–1.72 (m, 1H), 1.74 (s, 3H), 1.81 (m, 1H), 1.83 (s, 3H), 1.84 (s, 3H), 1.92 (m, 1H), 1.97 (s, 3H), 2.11 (s, 1H), 2.24–2.52 (m, 4H), 2.42 (s, 3H), 2.54–2.72 (m, 1H), 2.87 (m, 1H), 3.88 (d, J = 7.2 Hz, 1H), 3.93 (s, 2H), 4.21 (d, J = 8.4 Hz, 1H), 4.27 (d, J = 8.4 Hz, 1H), 4.48 (dd, J = 6.6, 10.5 Hz, 1H), 4.74–4.88 (m, 2H), 5.05 (d, J = 8.1 Hz, 1H), 5.39 (d, J = 6.0 Hz, 1H), 5.70 (d, J = 7.2 Hz, 1H), 6.23 (t, J = 9.0 Hz, 1H), 6.37 (s, 1H), 6.94 −7.02 (m, 2H), 7.38 (t, J = 8.4 Hz, 1H), 7.53 (m, 1H), 7.75 (dd, J = 6.0, 8.4 Hz, 1H), 7.86 (d, J = 9.0 Hz, 1H), 7.97 (d, J = 7.8 Hz); 19F NMR (282 MHz, CDCl3): δ −113.41 (d, 1F), −111.86 (s, 1F). MS (ESI): Calcd for C58H71F2NO18S2+ (M+H+): 1172.4; Found 1172.4.
4.5.3. SB-T-12145-(SS-F-Linker)-OSu (15)
To a cooled solution of 14 (113 mg, 0.097 mmol) and HOSu (20 mg, 0.174 mmol) in CH2Cl2 (3 mL) at 0 °C was added DIC (0.016 mL, 0.104 mmol), and the mixture was allowed to warm from 0 °C to room temperature and react for 6 h with stirring. The reaction mixture was directly concentrated in vacuo to afford a yellow solid. Purification of the crude product by column chromatography on silica gel with hexanes/ethyl acetate (1:2) as eluent gave 15 as a white solid (91 mg, 79%): 1H NMR (300 MHz, CDCl3): δ 1.03–1.24 (m, 4H), 1.23 (s, 3H), 1.24–1.28 (m, 3H), 1.33 (s, 3H), 1.42 (s, 9H), 1.60–1.72 (m, 1H), 1.74 (s, 3H), 1.81 (m, 1H), 1.83 (s, 3H), 1.84 (s, 3H), 1.92 (m, 1H), 1.97 (s, 3H), 2.11 (s, 1H), 2.24–2.52 (m, 4H), 2.42 (s, 3H), 2.54–2.72 (m, 1H), 2.83 (bs, 4H), 2.87 (m, 1H), 3.88 (d, J = 7.2 Hz, 1H), 3.93 (s, 2H), 4.21 (d, J = 8.4 Hz, 1H), 4.27 (d, J = 8.4 Hz, 1H), 4.48 (dd, J = 6.6, 10.5 Hz, 1H), 4.74–4.88 (m, 2H), 5.05 (d, J = 8.1 Hz, 1H), 5.39 (d, J = 6.0 Hz, 1H), 5.70 (d, J = 7.2 Hz, 1H), 6.23 (t, J = 9.0 Hz, 1H), 6.37 (s, 1H), 6.94 −7.02 (m, 2H), 7.381 (t, J = 8.4 Hz, 1H), 7.53 (m, 1H), 7.75 (dd, J = 6.0, 8.4 Hz, 1H), 7.86 (d, J = 9.0 Hz, 1H), 7.97 (d, J = 7.8 Hz, 1H); 19F NMR (282 MHz, CDCl3): δ −112.87 (s, 1F), −111.93 (s, 1F); MS (ESI) for C62H75F2N2O20S2+ calcd: 1269.4; Found 1269.4.
4.5.4. Biotin-(SS-F-Linker)-SB-T-12145 [BLT-F2] (1)
To a solution of 15 (87 mg, 0.069 mmol) and 16 (28 mg, 0.11 mmol) in DMSO (0.5 mL) at 0 °C was added pyridine (0.15 mL), and the mixture was allowed to warm from 0 °C to room temperature and react for 72 h with stirring. The reaction mixture was directly concentrated in vacuo to afford a yellow-green oil. Purification of the crude product by column chromatography on silica gel with 6% CH3OH in CH2Cl2 as eluent gave BLT-F2 (1) as a white solid (62 mg, 77%): mp 129–131 °C; 1H NMR (500 MHz, CD3OD): δ 1.03–1.24 (m, 4H), 1.23 (s, 3H), 1.24–1.32 (m, 5H), 1.38–1.70 (m, 4H), 1.33 (s, 3H), 1.42 (s, 9H), 1.60–1.72 (m, 1H), 1.74 (s, 3H), 1.81 (m, 1H), 1.83 (s, 3H), 1.84 (s, 3H), 1.92 (m, 1H), 1.97 (s, 3H), 2.11 (m, 3H), 2.24–2.52 (m, 4H), 2.42 (s, 3H), 2.54–2.72 (m, 2H), 2.83 (br s, 4H), 2.87 (m, 2H), 3.22 (m, 1H), 3.88 (d, J = 7.2 Hz, 1H), 3.93 (s, 2H), 4.21 (d, J = 8.4 Hz, 1H), 4.27 (d, J = 8.4 Hz, 1H), 4.48 (dd, J = 6.6, 10.5 Hz, 1H), 4.74–4.32 (m, 1H), 4.49 (m, 1H), 4.88 (m, 2H), 5.05 (d, J = 8.1 Hz, 1H), 5.39 (d, J = 6.0 Hz, 1H), 5.70 (d, J = 7.2 Hz, 1H), 6.23 (t, J = 9.0 Hz, 1H), 6.37 (s, 1H), 6.94 −7.02 (m, 2H), 7.381 (t, J = 8.4 Hz, 1H), 7.53 (m, 1H), 7.75 (dd, J = 6.0, 8.4 Hz, 1H), 7.86 (d, J = 9.0 Hz, 1H), 7.97 (d, J = 7.8 Hz, 1H); 13C NMR (125 MHz, CD3OD): δ 7.78, 8.99, 12.38, 13.59, 17.22, 19.41, 19.49, 20.93, 24.96, 25.55, 27.36, 27.94, 29.35, 30.53, 30.90, 32.96, 35.35, 36.11, 38.15, 39.03, 39.64, 43.16, 45.72, 46.67, 49.30, 55.51, 57. 86, 60.26, 61.84, 70.15, 70.91, 71.59, 75.32, 75.44, 75.94, 77.69, 79.11 80.96, 84.45, 114.87, 115.09, 116.11, 116.34, 117.68, 117.91, 119.78, 119.93, 120.15, 125.68, 130.29, 130.37, 132.32, 132.39, 132.97, 133.36, 133.45, 136.67, 137.30, 141.25, 156.09, 161.16, 161.37, 163.60, 163.81, 164.74, 164.88, 168.82, 169.98; 19F NMR (376 MHz, CD3OD): δ −112.95 (s, 1F), −111.88 (s, 1F). HRMS (TOF): Calcd. for C68H88F2N5O19S3+: 1412.5204: Found: 1412.5159 (Δ = −3.2 ppm).
4.6. Synthesis of SB-T-12822-5 (4)
4.6.1. 10-Deacetyl-2-debenzoyl-2-(3-trifluoromethoxybenzoyl)-7,10,13-tris(triethylsilyl)baccatin III (18)
To a solution of 17 (0.398 g, 0.508 mmol), 3-(trifluoromethoxy)benzoic acid (0.838 g, 4.07 mmol), and DMAP (0.497 g, 4.07 mmol) in CH2Cl2 (10 mL) was added DIC (0.63 mL, 4.07 mmol), and the mixture was allowed to react for 10 h at room temperature with stirring. The reaction was quenched with saturated NH4Cl (10 mL), and the reaction mixture was diluted with H2O (10 mL) and extracted with ethyl acetate (3 × 30 mL). The combined organic layers were washed with brine (3 × 30 mL), dried over MgSO4, and concentrated in vacuo to give a yellow solid. Purification of the crude product by column chromatography on silica gel with hexanes/ethyl acetate (6:1) as eluent gave 18 as a white solid (0.435 g, 88%): 1H NMR (500 MHz, CDCl3): δ 0.64 (m, 18H), 0.99 (m, 27H), 1.12 (s, 3H), 1.19 (s, 3H), 1.50 (s, 1H), 1.65 (s, 3H), 1.88 (t, J = 10.6 Hz, 1H), 1.98 (s, 3H), 2.10 (m, 1H), 2.19 (m, 1H), 2.27 (s, 3H), 2.53 (m, 1H), 3.86 (d, J = 7.0 Hz, 1H), 4.13 (d, J = 8.2 Hz, 1H), 4.25 (d, J = 8.2 Hz, 1H), 4.40 (dd, J = 6.6, 10.3 Hz, 1H), 4.94 (m, 2H), 5.19 (s, 1H), 5.59 (d, J = 7.2 Hz, 1H), 7.43 (d, J = 9.0 Hz, 1H), 7.52 (t, J = 8.0 Hz, 1H), 7.98 (s, 1H), 8.02 (d, J = 7.8 Hz, 1H); 13C NMR (125 MHz, CDCl3): δ 4.80, 5.21, 5.94, 6.85, 6.95, 10.40, 14.59, 20.55, 22.17, 26.29, 37.23, 39.71, 42.93, 46.88, 58.19, 68.22, 72.61, 75.74, 76.09, 76.46, 79.63, 80.72, 84.01, 122.03, 126.03, 128.53, 130.17, 131.62, 135.61, 139.55, 149.21, 165.64, 169.99, 205.64; 19F NMR (282 MHz, CDCl3): δ −58.48 (s, 3F); HRMS (TOF): Calcd. for C48H78F3O11Si3+: 971.4799: Found: 971.4806 (Δ = 0.7 ppm).
4.6.2. 10-Deacetyl-2-debenzoyl-2-(3-trifluoromethoxybenzoyl)baccatin III (19)
To a cooled solution of 18 (0.573 g, 0.590 mmol) in CH3CN-pyridine (1:1) (40 mL) was added HF-pyridine (5.7 mL), and the mixture was allowed to warm from 0 °C to room temperature and react for 13 h with stirring. The reaction was quenched with 10% citric acid (15 mL), and the reaction mixture was diluted with H2O (10 mL) and extracted with ethyl acetate (3 × 30 mL). The combined organic layers were washed with saturated CuSO4 (3 × 30 mL) and brine (3 × 30 mL), dried over MgSO4, and concentrated in vacuo to give 19 as a white solid (0.333 g, 90%): 1H NMR (500 MHz, DMSO-d6): δ 0.97 (s, 3H), 0.98 (s, 3H), 1.57 (s, 3H), 1.69 (m, 1H), 1.94 (s, 3H), 2.18 (d, J = 8.3 Hz, 1H), 2.21 (s, 3H), 2.32 (m, 1H), 3.86 (d, J = 7.1 Hz, 1H), 4.06 (s, 2H), 4.13 (m, 1H), 4.52 (s, 1H), 4.66 (m, 1H), 4.97 (d, J = 8.3 Hz, 1H), 5.05 (d, J = 7.1 Hz, 1H), 5.17 (d, J = 2.6 Hz, 1H), 5.28 (d, J = 4.6 Hz, 1H), 5.43 (d, J = 7.1 Hz, 1H), 7.76 (s, 1H), 7.77 (t, J = 8.1 Hz, 1H), 7.96 (s, 1H), 8.06 (dt, J = 1.7, 7.0 Hz, 1H); 13C NMR (125 MHz, DMSO-d6): δ 10.16, 15.34, 20.58, 22.53, 27.22, 37.04, 42.88, 46.96, 57.49, 66.47, 71.41, 74.87, 75.88, 76.06, 77.40, 80.56, 84.18, 121.91, 126.52, 129.06, 131.62, 132.86, 134.89, 142.07, 148.79, 164.27, 169.89, 210.70; 19F NMR (376 MHz, DMSO-d6): δ −56.92 (s, 3F). HRMS (TOF): Calcd. for C30H36F3O11+: 629.2204; Found: 629.2207 (Δ = 0.5 ppm).
4.6.3. 10-Deacetyl-2-debenzoyl-2-(3-trifluoromethoxybenzoyl)-7-triethylsilylbaccatin III (20)
To a cooled solution of 19 (0.333 g, 0.530 mmol) and imidazole (0.144 g, 2.12 mmol) in DMF (5.3 mL) was added chlorotriethylsilane (0.3 mL, 1.59 mmol), and the mixture was allowed to react for 30 min at 0 °C with stirring. The reaction was quenched with saturated NH4Cl (10 mL), and the reaction mixture was diluted with H2O (10 mL) and extracted with ethyl acetate (3 × 30 mL). The combined organic layers were washed with brine (3 × 30 mL), dried over MgSO4, and concentrated in vacuo to give a colorless oil. Purification of the crude product by column chromatography on silica gel with hexanes/ethyl acetate (3:2) as eluent gave 20 as a white solid (0.320 g, 81%): 1H NMR (500 MHz, CDCl3): δ 0.54 (m, 6H), 0.93 (t, J = 8.0 Hz, 9H), 1.07 (s, 6H), 1.72 (s, 3H), 1.89 (dt, J = 2.1, 12.6 Hz, 1H), 2.07 (s, 3H), 2.08 (t, J = 5.3 Hz, 1H), 2.25 (t, J = 8.1 Hz, 2H), 2.26 (s, 3H), 2.47 (m, 1H), 3.95 (d, J = 6.9 Hz, 1H), 4.14 (d, J = 8.2 Hz, 1H), 4.24 (d, J = 2.1 Hz, 1H), 4.28 (d, J = 8.2 Hz, 1H), 4.41 (dd, J = 7.0, 10.6 Hz, 1H), 4.86 (m, 1H), 4.96 (d, J = 7.9 Hz, 1H), 5.16 (d, J = 2.1 Hz, 1H), 5.57 (d, J = 7.0 Hz, 1H), 7.45 (d, J = 8.1 Hz, 1H), 7.52 (t, J = 7.9 Hz, 1H), 7.98 (s, 1H), 8.03 (dt, J = 1.1, 7.7 Hz, 1H); 13C NMR (125 MHz, CDCl3): δ 5.13, 6.72, 9.86, 15.19, 19.43, 22.39, 26.83, 37.17, 38.44, 42.61, 46.96, 57.89, 67.85, 72.91, 74.64, 75.34, 78.85, 80.63, 84.23, 122.12, 126.10, 128.50, 130.21, 131.41, 134.98, 141.89, 149.22, 149.24, 165.54, 170.77, 210.24; 19F NMR (376 MHz, CDCl3): δ −57.86 (s, 3F). HRMS (TOF): Calcd. for C36H50F3O11Si+: 743.3069; Found: 743.3072 (Δ = 0.4 ppm).
4.6.4. 10-(Cyclopropanecarbonyl)-10-deacetyl-2-debenzoyl-2-(3-trifluoromethoxybenzoyl)-7-triethylsilylbaccatin III (21)
To a cooled solution of 20 (0.240 g, 0.323 mmol) in THF (3.2 mL) was added 1 M LHMDS in THF (0.35 mL, 0.355 mmol) followed by cyclopropanecarbonyl chloride (31 µL, 0.340 mmol), and the mixture was allowed to react for 10 min at −40 °C with stirring. The reaction was quenched with saturated NH4Cl (10 mL), and the reaction mixture was diluted with H2O (10 mL) and extracted with ethyl acetate (3 × 30 mL). The combined organic layers were washed with brine (3 × 30 mL), dried over MgSO4, and concentrated in vacuo to give a crude yellow oil. Purification of the crude product by column chromatography on silica gel with hexanes/ethyl acetate (3:2) as eluent gave 21 as a white solid (0.245 g, 93%): 1H NMR (500 MHz, CDCl3): δ 0.57 (m, 6H), 0.90 (m, 2H), 0.91 (t, J = 8.0 Hz, 9H), 1.05 (s, 3H), 1.19 (s, 3H), 1.67 (s, 3H), 1.75 (m, 1H), 1.87 (dt, J = 2.0, 12.3 Hz, 1H), 2.08 (m, 1H), 2.19 (s, 3H), 2.24 (m, 2H), 2.26 (s, 3H), 2.53 (m, 1H), 3.89 (d, J = 7.0 Hz, 1H), 4.12 (d, J = 8.0 Hz, 1H), 4.27 (d, J = 8.0 Hz, 1H), 4.47 (dd, J = 6.7, 10.3 Hz, 1H), 4.84 (m, 1H), 4.96 (d, J = 8.2 Hz, 1H), 5.61 (d, J = 7.1 Hz, 1H), 6.46 (s, 1H), 7.45 (d, J = 8.1 Hz, 1H), 7.53 (t, J = 8.0 Hz, 1H), 8.00 (s, 1H), 8.05 (dt, J = 1.2, 7.8 Hz, 1H); 13C NMR (125 MHz, CDCl3): δ 5.23, 6.74, 8.57, 8.71, 9.87, 12.98, 14.17, 14.93, 20.07, 21.04, 22.44, 26.80, 37.15, 38.07, 42.68, 47.23, 53.40, 58.55, 60.39, 67.88, 72.31, 75.27, 75.47, 76.35, 78.84, 80.76, 84.21, 122.15, 126.12, 128.52, 130.21, 131.40, 132.60, 143.99, 149.22, 165.60, 170.72, 173.17, 202.24. 19F NMR (376 MHz, CDCl3): δ −57.86 (s, 3F); HRMS (TOF): Calcd. for C40H54F3O12Si+: 811.3331; Found: 811.3336 (Δ = 0.6 ppm).
4.6.5. 2-Debenzoyl-3’-dephenyl-10-(cyclopropanecarbonyl)-2-(3-trifluoromethoxybenzoyl)-7-triethylsilyl-2’-triisopropylsilyl-3’-trifluoromethyldocetaxel (23)
To a cooled solution of 21 (0.245 g, 0.302 mmol) and 22 (0.155 g, 0.369 mmol) in THF (5 mL) was added 1 M LHMDS in THF (0.4 mL, 0.4 mmol), and the mixture was allowed to react for 3 h at −40 °C with stirring. The reaction was quenched with NH4Cl (10 mL), and the reaction mixture was diluted with H2O (10 mL) and extracted with ethyl acetate (3 × 30 mL). The combined organic layers were washed with brine (3 × 30 mL), dried over MgSO4, and concentrated in vacuo to give a yellow oil. Purification of the crude product by column chromatography on silica gel with hexanes/ethyl acetate (4:1) as eluent gave 23 as a white solid (0.254 g, 69%): 1H NMR (500 MHz, CDCl3): δ 0.55 (m, 6H), 0.90 (t, J = 8.0 Hz, 9H), 0.92 (m, 2H), 1.11 (m, 21H), 1.17 (s, 3H), 1.22 (s, 3H), 1.31 (s, 9H), 1.58 (m, 3H), 1.68 (s, 3H), 1.75 (m, 1H), 1.88 (m, 1H), 2.01 (s, 3H), 2.30 (m, 1H), 2.32 (s, 3H), 2.50 (m, 1H), 3.84 (d, J = 7.1 Hz, 1H), 4.17 (d, J = 8.7 Hz, 1H), 4.29 (d, J = 8.4 Hz, 1H), 4.45 (dd, J = 6.7, 10.5 Hz, 1H), 4.65 (m, 1H), 4.91 (s, 1H), 4.93 (t, J = 16.6 Hz, 1H), 5.17 (d, J = 10.6 Hz, 1H), 5.65 (d, J = 7.6 Hz, 1H), 6.07 (t, J = 8.5 Hz, 1H), 6.47 (s, 1H), 7.45 (d, J = 7.5 Hz, 1H), 7.55 (t, J = 8.0 Hz, 1H), 8.01 (s, 1H), 8.06 (d, J = 7.8 Hz, 1H); 13C NMR (125 MHz, CDCl3): δ 5.27, 6.73, 8.70, 8.83, 10.02, 12.70, 12.93, 14.18, 14.25, 17.79, 17.92, 21.29, 22.22, 26.50, 27.92, 35.19, 37.09, 43.23, 46.71, 58.33, 70.01, 72.18, 72.44, 72.66, 75.48, 76.36, 78.84, 81.12, 84.26, 122.20, 126.11, 128.57, 130.32, 131.26, 133.59, 140.25, 149.26, 155.12, 165.54, 169.87, 170.61, 173.14, 201.81; 19F NMR (376 MHz, CDCl3): δ −57.98 (s, 3F), −72.55 (s, 3F). HRMS (TOF): Calcd. for C58H86F6NO16Si2+: 1222.5384; Found: 1222.5383 (Δ = 0.1 ppm).
4.6.6. 2-Debenzoyl-3’-dephenyl-10-(cyclopropanecarbonyl)-2-(3-trifluoromethoxybenzoyl)-3’-trifluoromethyldocetaxel [SB-T-12822-5] (4)
To a cooled solution of 23 (0.190 g, 0.155 mmol) in CH3CN-pyridine (1:1) (10 mL) was added HF-pyridine (1.9 mL), and the mixture was allowed to warm from 0 °C to room temperature and react for 12 h with stirring. The reaction was quenched with 10% citric acid (10 mL), and the reaction mixture was diluted with H2O (10 mL) and extracted with ethyl acetate (3 × 30 mL). The combined organic layers were washed with saturated CuSO4 (3 × 30 mL) and brine (3 × 30 mL), dried over MgSO4, and concentrated in vacuo to give a colorless oil. Purification of the crude product by column chromatography on silica gel with hexanes/ethyl acetate (3:7) as eluent gave SB-T-12822-5 (4) as a white solid (0.128 g, 87%): mp 141–142 °C; 1H NMR (500 MHz, CDCl3): δ 1.00 (m, 2H), 1.14 (m, 2H), 1.15 (s, 3H), 1.24 (s, 3H), 1.31 (s, 9H), 1.67 (s, 3H), 1.78 (m, 1H), 1.85 (m, 1H), 1.88 (s, 3H), 2.32 (t, J = 12.7 Hz, 2H), 2.35 (s, 3H), 2.54 (m, 1H), 2.60 (d, J = 4.1 Hz, 1H), 3.43 (d, J = 4.6 Hz, 1H), 3.81 (d, J = 7.0 Hz, 1H), 4.16 (d, J = 8.4 Hz, 1H), 4.28 (d, J = 8.4 Hz, 1H), 4.40 (m, 1H), 4.69 (d, J = 4.0 Hz, 1H), 4.74 (m, 1H), 4.95 (d, J = 7.9 Hz, 1H), 5.22 (d, J = 7.9 Hz, 1H), 5.22 (d, J = 10.8 Hz, 1H), 5.63 (d, J = 7.1 Hz, 1H), 6.21 (t, J = 8.0 Hz, 1H), 6.29 (s, 1H), 7.46 (d, J = 7.4 Hz, 1H), 7.56 (t, J = 8.0 Hz, 1H), 8.00 (s, 1H), 8.06 (d, J = 7.8 Hz, 1H); 13C NMR (125 MHz, CDCl3): δ 9.30, 9.54, 13.03, 13.73, 14.22, 14.89, 19.14, 21.05, 21.09, 22.00, 22.14, 26.70, 27.93, 30.65, 35.34, 35.48, 43.20, 45.66, 53.46, 58.55, 60.45, 64.42, 68.06, 72.19, 73.46, 75.25, 75.63, 76.32, 79.17, 81.15, 81.34, 84.50, 122.34, 126.28, 128.60, 130.43, 131.15, 133.34, 141.82, 149.33, 154.65, 165.65, 170.22, 171.88, 175.16, 203.65; 19F NMR (470 MHz, CDCl3): δ −57.96 (s, 3F), −73.39 (d, J = 8.0 Hz, 3F). HRMS (TOF): Calcd. for C43H52F6NO16+: 952.3185. Found: 952.3199 (Δ = 1.5 ppm).
4.7. Synthesis of BLT-S-F6 (2)
4.7.1. SB-T-12822-5-(Me-SS-Linker)-OTIPS (25)
To a solution of SB-T-12822-5 (4) (0.200 g, 0.210 mmol), 24 (0.116 g, 0.230 mmol), and DMAP (0.012 g, 0.062 mmol) in CH2Cl2 (10 mL) was added DIC (36 µL, 0.230 mmol), and the mixture was allowed to react for 12 h at room temperature with stirring. The reaction was quenched with saturated NH4Cl (10 mL), and the reaction mixture was diluted with H2O (10 mL) and extracted with CH2Cl2 (3 × 30 mL). The combined organic layers were washed with brine (3 × 30 mL), dried over MgSO4, and concentrated in vacuo to afford a yellow oil. The diisopropylurea byproduct was removed by filtration with diethyl ether. Purification of the crude product by column chromatography on silica gel with hexanes/ethyl acetate (2:1) as eluent gave 25 as a white solid (0.210, 72%): 1H NMR (500 MHz, CDCl3): δ 0.97 (m, 2H), 1.08 (d, J = 7.5 Hz, 18H), 1.25 (m, 3H), 1.26 (s, 3H), 1.31 (d, J = 7.5 Hz, 3H), 1.36 (s, 9H), 1.69 (s, 3H), 1.80 (m, 1H), 1.92 (s, 3H), 2.30 (m, 1H), 2.35 (s, 3H), 2.46 (m, 2H), 2.57 (m, 1H), 2.65 (d, J = 3.5 Hz, 1H), 2.97 (m, 1H), 3.87 (m, 4H), 4.01 (bs, 2H), 4.09 (m, 1H), 4.18 (d, J = 8.0 Hz, 1H), 4.31 (d, J = 8.0 Hz, 1H), 4.45 (m, 1H), 4.92 (m, 1H), 4.99 (d, J = 9.0 Hz, 1H), 5.22 (d, J = 10.0 Hz, 1H), 5.37 (s, 1H), 5.66 (d, J = 7.0 Hz, 1H), 6.22 (t, J = 9.5 Hz, 1H), 6.30 (s, 1H), 7.21 (m, 3H), 7.48 (d, J = 8.0 Hz, 1H), 7.59 (t, J = 8.0 Hz, 1H), 7.84 (d, J = 7.5 Hz, 1H), 8.04 (s, 1H), 8.09 (d, J = 7.5 Hz, 1H); 13C NMR (125 MHz, CDCl3): δ 9.23, 9.47, 9.55, 11.88, 12.99, 14.79, 15.30, 17.79, 19.14, 20.52, 21.99, 22.21, 23.36, 24.70, 26.64, 27.92, 30.95, 33.00, 35.26, 35.38, 36.64, 38.48, 43.15, 45.61, 46.03, 58.47, 64.40, 65.89, 69.30, 72.15, 72.37, 75.30, 75.76, 76.24, 79.36, 81.00, 81.58, 84.54, 122.31, 126.21, 127.71, 128.49, 128.55, 130.19, 130.89, 130.72, 131.21, 132.38, 132.76, 137.51, 142.77, 149.31, 154.67, 156.83, 165.65, 166.21, 169.39, 169.67, 173.00, 175.11, 203.85; 19F NMR (470 MHz, CDCl3): δ −57.95 (s, 3F), −73.31 (d, J = 8.0 Hz, 3F). HRMS (TOF): Calcd. for C65H86F6NO19S2Si+: 1390.4903; Found: 1390.4882 (Δ= −1.5 ppm).
4.7.2. SB-T-12822-5-(Me-SS-Linker)-COOH (26)
To a cooled solution of 25 (0.210 g, 0.151 mmol) in CH3CN-pyridine (1:1) (45 mL) was added HF-pyridine (2.1 mL), and the mixture was allowed to warm from 0 °C to room temperature and react for 10 h with stirring. The reaction was quenched with 10% citric acid (10 mL), and the reaction mixture was diluted with H2O (20 mL) and extracted with ethyl acetate (3 × 20 mL). The combined organic layers were washed with saturated CuSO4 (3 × 20 mL) and brine (3 × 20 mL), dried over MgSO4, and concentrated in vacuo to afford 26 as a white solid (0.189 g, quant.): 1H NMR (500 MHz, CDCl3): (1:1 mixture of two diastereomers) δ 0.91 (m, 2H), 1.11 (m, 3H), 1.26 (s, 3H), 1.28 (m, 3H), 1.35 (s, 4.5H), 1.36 (s, 4.5H), 1.79 (m, 4H), 1.90 (s, 1.5H), 1.91 (s, 1.5H), 2.30 (m, 3H), [2.39 (s, 1.5H), 2.42 (s, 1.5H)], 2.65 (m, 2H), [2.94 (m, 0.5 H), 3.02 (m, 0.5 H)], 4.00 (dd, J = 9.0, 17.0 Hz, 1H), 4.08 (d, J = 7.0 Hz, 1H), 4.15 (d, J = 7.0 Hz, 1H), 4.31 (d, J = 8.0 Hz, 2H), 4.43 (m, 1H), 4.93 (m, 1H), 5.00 (d, J = 9.0 Hz, 1H), 5.35 (dd, J = 10.5, 16.0 Hz, 1H), [5.48 (s, 0.5 H), 5.52 (s, 0.5 H)], 5.66 (d, J = 7.0 Hz, 1H), 6.24 (m, 1H), [6.29 (s, 0.5H), 6.30 (s, 0.5H)], 7.26 (m, 3H), 7.35 (m, 1H), 7.48 (d, J = 8.0 Hz, 1H), 7.59 (t, J = 8.0 Hz, 1H), 7.84 (d, J = 7.5 Hz, 1H), 8.03 (s, 1H), 8.08 (d, J = 8.0 Hz, 1H); 13C NMR (125 MHz, CDCl3): δ 9.25, 9.47, 9.56, 11.75, 11.86, 13.01, 13.73, 14.15, 14.22, 14.73, 14.74, 17.22, 19.14, 20.11, 20.21, 21.09, 21.91, 22.12, 22.16, 22.68, 22.72, 26.61, 26.65, 27.92, 29.39, 29.72, 30.65, 31.61, 31.95, 35.26, 35.42, 38.45, 43.13, 45.38, 45.75, 45.87, 52.36, 52.61, 53.22, 55.95, 58.45, 60.44, 64.40, 69.41, 69.48, 72.14, 79.29, 81.21, 81.28, 81.57, 81.61, 84.42, 122.32, 126.22, 127.57, 127.63, 127.92, 128.53, 128.80, 129.75, 130.04, 130.40, 130.89, 131.22, 132.32, 132.76, 137.20, 137.59, 142.73, 149.32, 154.70, 154.78, 157.21, 165.63, 166.45, 169.55, 170.73, 175.14, 175.18, 195.28, 203.75; 19F NMR (470 MHz, CDCl3): δ −57.95 (s, 3F), −73.09 [(d, J = 8 Hz, 1.5H), −73.15 (d, J = 1.5H)]. HRMS (TOF): Calcd. for C56H69F6N2O19S2+ [M+NH4+]: 1251.3835; Found: 1251.3820 (Δ= −1.2 ppm).
4.7.3. Biotin-PEG-(SS-Linker)-SB-T-12822-5 [BLT-S-F6] (2)
To a solution of 26 (0.189 g, 0.151 mmol), 27 (0.094 g, 0.226 mmol), and DMAP (0.019 g, 0.151 mmol) in CH2Cl2 (10 mL) was added EDC˙HCl (0.035 g, 0.181 mmol), and the mixture was allowed to react for 12 h at room temperature with stirring. The reaction mixture was directly concentrated in vacuo to give a yellow oil. Purification of the crude produt by column chromatography on silica gel with 8% CH3OH in CH2Cl2 as eluent gave BLT-S-F6 (2) as a white solid (0.165 g, 80%): mp 104–106 °C; 1H NMR (500 MHz, CDCl3): δ 1.02 (t, J = 8.0 Hz, 2H), 1.18 (s, 3H), 1.28 (s, 3H), 1.30 (d, J = 6.8 Hz, 3H), 1.37 (s, 9H), 1.48 (m, 4H), 1.73 (m, 9H), 1.91 (m, 4H), 1.92 (s, 3H), 2.34 (m, 4H), 2.35 (s, 3H), 2.36 (m, 1H), 2.48 (t, J = 7.5 Hz, 2H), 2.55 (m, 1H), 2.75 (d, J = 12.5 Hz, 1H), 2.92 (m, 1H), 2.93 (dd, J = 4.5, 12.5 Hz, 1H), 3.20 (m, 2H), 3.46 (m, 4H), 3.59 (t, J = 5.0 Hz, 2H), 3.66 (m, 10H), 3.83 (d, J = 7.0 Hz, 1H), 4.01 (m, 2H), 4.09 (dd, J = 3.5, 6.5 Hz, 1H), 4.19 (d, J = 8.5 Hz, 1H), 4.35 (m, 5H), 4.54 (m, 1H), 4.94 (m, 1H), 4.99 (d, J = 9.0 Hz, 1H), 5.20 (s, 2H), 5.39 (s, 1H), 5.57 (t, J = 11.0 Hz, 1H), 5.66 (d, J = 7.0 Hz, 1H), 5.78 (s, 1H), 6.17 (s, 1H), 6.20 (t, J = 8.0 Hz, 1H), 6.33 (s, 1H), 6.51 (m, 1H), 6.75 (bt, 1H), 7.28 (m, 2H), 7.35 (m, 1H), 7.48 (d, J = 8.0 Hz, 1H), 7.59 (t, J = 7.5 Hz, 1H), 7.83 (d, J = 7.5 Hz, 1H), 8.03 (s, 1H), 8.08 (d, J = 8.0 Hz, 1H); 13C NMR (125 MHz, CDCl3): δ 9.21, 9.39, 9.62, 13.04, 14.74, 20.62, 20.97, 22.03, 22.52, 25.14, 22.52, 25.14, 25.54, 26.59, 27.96, 28.05, 28.12, 28.27, 28.31, 29.73, 31.31, 33.42, 33.50, 35.32, 35.64, 35.84, 38.44, 39.10, 30.15, 40.52, 42.86, 43.18, 45.85, 46.33, 47.85, 53.46, 55.32, 55.32, 55.50, 58.38, 60.19, 61.78, 69.41, 69.94, 70.00, 70.08, 70.36, 71.92, 72.47, 75.30, 75.71, 76.25, 79.20, 81.08, 81.52, 84.53, 122.29, 126.18. 127.79, 128.54, 130.40, 130.50, 130.55, 130.86, 131.26, 132.64, 132.92, 137.59, 142.29, 149.30, 153.96, 154.82, 163.42, 163.64, 165.59, 166.44, 170.00, 172.43, 173.33, 173.33, 174.04, 174.85, 203.75; 19F NMR (470 MHz, CDCl3): δ −57.92 (s, 3F), −73.11 (d, J = 7.5 Hz, 3F). HRMS (TOF): Calcd. for C74H98F6N5O23S3+: 1634.5713; Found: 1634.5698 (Δ= −0.9 ppm).
4.8 19F NMR Experiments
19F NMR experiments were performed on a Brüker Nanobay 400 MHz NMR spectrometer operating at a 19F Larmor frequency of 376 MHz with a BBFOPLUS 5 mm probe (1H –19F) at 25 °C or 37 °C. 19F NMR spectra were recorded using a pulse sequence of proton decoupling with a spectral width of 89,285 Hz (237 ppm for normal runs and 15,040 Hz (40 ppm) for kinetic runs, an acquisition time of 0.8 s, and a relaxation delay of 1.0 s. The obtained spectra were analyzed with TOPSPIN 3.0 (Brüker).
4.8.1. Time-resolved 19F NMR for probe 2
Fluorotaxoid 3 and probe 1 were first each dissolved in DMSO (150 µL), and then diluted with D2O (350 µL) to final concentrations of 2.5 mM (30% DMSO in D2O). Glutathione (reduced form, GSH) (six equivalents) was added to the solution, which was immediately shaken vigorously and added to a NMR tube. Time-resolved 19F NMR spectra, representing disulfide bond cleavage and thiolactonization indicating drug release, were recorded in real-time at 25 °C, beginning at 1 h following the GSH addition by measuring 1 spectrum every 15 min (128 scans/spectrum) over a 6 h period (total of 20 spectra).
4.8.2. 19F NMR study on probe 1 in a formulation with Solutol HS15
To test the linker cleavage in an excipient formulation, probe 1 was dissolved in a 1:1 mixture of ethanol and Solutol HS15 to the final concentration of 25 mM. An aliquot of this stock was then diluted with 7:2 saline:D2O, such that the final concentration was 2.5 mM with thorough vortexing throughout to maintain complete solubility. Formulation was performed with a 500 µL sample volume in an NMR tube with D2O as the NMR reference solvent. Time-resolved 19F NMR spectra, representing disulfide bond cleavage and thiolactonization indicating drug release, were recorded in real-time at 25 °C beginning at 1 h after the GSH addition (15 mM) by measuring 1 spectrum every 15 min (128 scans/spectrum) over a 10 h period (total of 40 spectra). Disulfide cleavage was quantified by the comparison of the integrations with the linker-F peak at −113.7 ppm normalized to the fluorotaxoid signal at −112.23 ppm.
4.8.3. Formulations of probe 2
Stock solutions of SB-T-12145 (3) and BLT-S-F6 (2) were prepared by dissolving each compound in ethanol to the final concentration of 10 mM. In each experiment, an aliquot of the prepared stock solution (10 µL) was diluted with various volumes of aqueous media (PBS, saline, cell culture medium or blood plasma), 0–8% ethanol and/or excipient (solutol HS15 or polysorbate 80), and D2O to the final volume of 500 µL. Formulations with excipients were performed with a 500 µL sample volume in an NMR tube with D2O as the NMR reference solvent. Chemical shift differences were determined by comparison of the recorded chemical shifts of the 2-m-OCF3 and 3’-CF3 groups for mixtures of 2 and 4 in each formulation conditions.
4.8.4. Time-resolved 19F NMR analysis of probe 2
For linker stability and reactivity studies in human blood plasma, supplemental glutathione (100 equivalents) was dissolved in 200 µM solutions of 2 with 86% blood plasma, 10% D2O, 2% ethanol, 2% polysorbate 80 (500 µL). Time-resolved 19F NMR spectra, representing disulfide bond cleavage and drug release, were recorded in real-time at 37 °C beginning at 30 min after the GSH addition by measuring one spectrum every 1 h (1024 scans/spectrum) over a 13 h period (total of 13 spectra). The rate of drug release was monitored by measuring the integration ratio of the 3’-CF3 peaks of probe 2 and free taxoid 4. The normalized integration ratios indicating drug release were plotted as a function of time.
Supplementary Material
Highlights.
-
➢
Two novel tumor-targeting biotin conjugates, BLT-F2 (1) and BLT-S-F6 (2) were designed as 19F NMR probes.
-
➢
Rapid disulfide exchange of BLT-F2 (2) with GSH, followed by slow thiolactonization and drug release.
-
➢
para-Fluoro group accelerates rate of disulfide cleavage, thiolactonization, and drug release.
-
➢
Linker cleavage was slowed with an excipient, indicating a strong “stabilizing effect.”
-
➢
BLT-S-F6 (2) is stable in blood plasma, yet releases the drug when exposed to cytosolic levels of GSH.
Acknowledgments
This research was supported by a grant from the National Institute of Health (CA 103314 to I.O.). Generous support from Indena SpA, is gratefully acknowledged. The authors are grateful to Dr. James Marecek and Dr. Francis Picart for their valuable help with NMR experiments. The authors would also like to thank Dr. Béla Ruzsicska for his helpful discussions on mass spectrometric analyses.
Abbreviations
- 3-FABS
three fluorine atoms for biochemical screening
- ADC
antibody drug conjugate
- BLT
biotin-linker-taxoid
- CFM
confocal fluorescence microscopy
- DIC
N,N’-diisopropylcarbodiimide
- EDC
1-ethyl-3-(3-(dimethylamino)propyl)-carbodiimide
- GSH
glutathione
- HOSu
N-hydroxysuccinimide
- PEG
polyethylene glycol
- RME
receptor-mediated endocytosis
- SMDC
small-molecule drug conjugate
- SWNT
single-walled carbon nanotube
- TES
triethylsilyl
- TTDDS
tumor-targeted drug delivery system
- TTM
tumor-targeting module
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supporting Information
Supporting information contains 19F NMR spectra of probes 1 and 2 as well as fluorotaxoids 3 and 4 in various formulations; Table of experimental and normalized integration data for time-resolved 19F NMR spectra for examining the stability and reactivity of probe 2 in blood plasma-D2O-ethanol-polysorbate 80 formulation; Time resolved 19F NMR spectra for examining the stability and reactivity of probe 2 in polysorbate-ethanol-D2O formulation.
References
- 1.Ojima I. Acc. Chem. Res. 2008;41:108–119. doi: 10.1021/ar700093f. [DOI] [PubMed] [Google Scholar]
- 2.Ojima I, Zuniga ES, Berger WT, Seitz JD. Future Med. Chem. 2012;4:33–50. doi: 10.4155/fmc.11.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaracz S, Chen J, Kuznetsova LV, Ojima I. Bioorg. Med. Chem. 2005;13:5043–5054. doi: 10.1016/j.bmc.2005.04.084. [DOI] [PubMed] [Google Scholar]
- 4.Chen S, Zhao X, Chen J, Kuznetsova L, Wong SS, Ojima I. Bioconjug. Chem. 2010;21:979–987. doi: 10.1021/bc9005656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vineberg JG, Zuniga ES, Kamath A, Chen YJ, Seitz JD, Ojima I. J. Med. Chem. 2014;57:5777–5791. doi: 10.1021/jm500631u. (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vlahov IR, Leamon CP. Bioconjug. Chem. 2012;23:1357–1369. doi: 10.1021/bc2005522. [DOI] [PubMed] [Google Scholar]
- 7.Leamon CP. Curr. Opin. Invest. Drugs. 2008;9:1277–1286. [PubMed] [Google Scholar]
- 8.Chen J, Jaracz S, Zhao X, Chen S, Ojima I. Exp. Opin. Drug Deliv. 2005;2:873–890. doi: 10.1517/17425247.2.5.873. [DOI] [PubMed] [Google Scholar]
- 9.Ojima I, Geng X, Wu X, Qu C, Borella CP, Xie H, Wilhelm SD, Leece BA, Bartle LM, Goldmacher VS, Chari RV. J. Med. Chem. 2002;45:5620–5623. doi: 10.1021/jm025540g. [DOI] [PubMed] [Google Scholar]
- 10.Zolot RS, Basu S, Million RP. Nature Rev. Drug Discov. 2013;12:259–260. doi: 10.1038/nrd3980. [DOI] [PubMed] [Google Scholar]
- 11.Ducry L, Stump B. Bioconjug. Chem. 2010;21:5–13. doi: 10.1021/bc9002019. [DOI] [PubMed] [Google Scholar]
- 12.Carter PJ, Senter PD. Cancer J. 2008;14:154–169. doi: 10.1097/PPO.0b013e318172d704. [DOI] [PubMed] [Google Scholar]
- 13.Ojima I. ChemBioChem. 2004;5:628–635. doi: 10.1002/cbic.200300844. [DOI] [PubMed] [Google Scholar]
- 14.Ojima I. Acc. Chem. Res. 2008;41:108–119. doi: 10.1021/ar700093f. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Chen S, Zhao X, Kuznetsova LV, Wong SS, Ojima I. J. Am. Chem. Soc. 2008;130:16778–16785. doi: 10.1021/ja805570f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ojima I. Fluorine in Medicinal Chemistry and Chemical Biology. Chichester: Wiley-Blackwell; 2009. [Google Scholar]
- 17.Gerig JT. Methods Enzymol. 1989;177:3–23. doi: 10.1016/0076-6879(89)77003-8. [DOI] [PubMed] [Google Scholar]
- 18.Dalvit C, Ardini E, Flocco M, Fogliatto GP, Mongelli N, Veronesi M. J. Am. Chem. Soc. 2003;125:14620–14625. doi: 10.1021/ja038128e. [DOI] [PubMed] [Google Scholar]
- 19.Dalvit C. Prog. Nucl. Magn. Res. Spect. 2007;51:243–271. [Google Scholar]
- 20.Keita M, Kaffy J, Troufflard C, Morvan E, Crousse B, Ongeri S. Org. Biomol. Chem. 2014;12:4576–4581. doi: 10.1039/c4ob00962b. [DOI] [PubMed] [Google Scholar]
- 21.Ojima I, Kuduk SD, Chakravarty S, Ourevitch M, Bégué J-P. J. Am. Chem. Soc. 1997;119:5519–5527. [Google Scholar]
- 22.Ojima I, Inoue T, Slater JC, Lin S, Kuduk SC, Chakravarty S, Walsh JJ, Gilchrist L, McDermott AE, Cresteil T, Monsarrat B, Pera P, Bernacki RJ. Synthesis of Enatiopure F-Containing Taxoids and Their Use as Anticancer Agents as well as Probes for Biomedical Problems. In: Ramachandran PV, editor. Asymmetric Fluoroorganic Chemistry: Synthesis, Application, and Future Directions. ACS Symp. Ser. 746, American Chemical Society: Washington, D. C; 1999. pp. 158–181. [Google Scholar]
- 23.Ojima I, Inoue T, Chakravarty S. J. Fluor. Chem. 1999;97:3–10. [Google Scholar]
- 24.Geney R, Sun L, Pera P, Bernacki Ralph J, Xia S, Horwitz Susan B, Simmerling Carlos L, Ojima I. Chem. Biol. 2005;12:339–348. doi: 10.1016/j.chembiol.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Sun L, Simmerling C, Ojima I. ChemMedChem. 2009;4:719–731. doi: 10.1002/cmdc.200900044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ojima I. J. Org. Chem. 2013;78:6358–6383. doi: 10.1021/jo400301u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ojima I. ChemBioChem. 2004;5:628–635. doi: 10.1002/cbic.200300844. [DOI] [PubMed] [Google Scholar]
- 28.Kigawa J, Minagawa Y, Kanamori Y, Itamochi H, Cheng X, Okada M, Oisho T, Terakawa N. Cancer. 1998;82:697–702. doi: 10.1002/(sici)1097-0142(19980215)82:4<697::aid-cncr12>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 29.Zheng ZB, Zhu G, Tak H, Joseph E, Eiseman JL, Creighton DJ. Bioconjug. Chem. 2005;16:598–607. doi: 10.1021/bc0499634. [DOI] [PubMed] [Google Scholar]
- 30.Ojima I, Slater JC, Michaud E, Kuduk SD, Bounaud PY, Vrignaud P, Bissery MC, Veith JM, Pera P, Bernacki RJ. J. Med. Chem. 1996;39:3889–3896. doi: 10.1021/jm9604080. [DOI] [PubMed] [Google Scholar]
- 31.Ojima I, Slater JC, Kuduk SD, Takeuchi CS, Gimi RH, Sun CM, Park YH, Pera P, Veith JM, Bernacki RJ. J. Med. Chem. 1997;40:267–278. doi: 10.1021/jm960563e. [DOI] [PubMed] [Google Scholar]
- 32.Ojima I, Wang T, Miller ML, Lin S, Borella C, Geng X, Pera P, Bernacki RJ. Bioorg. Med. Chem. Lett. 1999;9:3423–3428. doi: 10.1016/s0960-894x(99)00629-0. [DOI] [PubMed] [Google Scholar]
- 33.Ojima I, Das M. J. Nat. Prod. 2009;72:554–565. doi: 10.1021/np8006556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuznetsova L, Sun L, Chen J, Zhao X, Seitz J, Das M, Li Y, Veith JM, Pera P, Bernacki RJ, Xia S, Horwitz SB, Ojima I. J. Fluor. Chem. 2012;143:177–188. doi: 10.1016/j.jfluchem.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ojima I, Chen J, Sun L, Borella CP, Wang T, Miller ML, Lin S, Geng X, Kuznetsova L, Qu C, Gallager D, Zhao X, Zanardi I, Xia S, Horwitz SB, Mallen-St Clair J, Guerriero JL, Bar-Sagi D, Veith JM, Pera P, Bernacki RJ. J. Med. Chem. 2008;51:3203–3221. doi: 10.1021/jm800086e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ojima I, Slater J, Michaud E, Kuduk S, Bounaud P, Vrignaud P, Bissery M, Veith J, Pera P, Bernacki R. J. Med. Chem. 1996;39:3889–3896. doi: 10.1021/jm9604080. [DOI] [PubMed] [Google Scholar]
- 37.Botchkina GI, Zuniga ES, Das M, Wang Y, Wang H, Zhu S, Savitt AG, Rowehl RA, Leyfman Y, Ju J, Shroyer K, Ojima I. Mol. Cancer. 2010;9:192–204. doi: 10.1186/1476-4598-9-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Botchkina GI, Zuniga ES, Rowehl RH, Park R, Bhalla R, Bialkowska AB, Johnson F, Golub LM, Zhang Y, Ojima I, Shroyer K. PLoS ONE. 2013;8:e60884. doi: 10.1371/journal.pone.0069884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ojima I, Fumero-Oderda CL, Kuduk SD, Ma Z, Kirikae F, Kirikae T. Bioorg. Med. Chem. 2003;11:2867–2888. doi: 10.1016/s0968-0896(03)00181-0. [DOI] [PubMed] [Google Scholar]
- 40.Perez-Silanes S, Martinez-Esparza J, Oficialdegui AM, Villanueva H, Orus L, Monge A. J. Heterocyclic Chem. 2001;38:1025–1030. [Google Scholar]
- 41.Banerjee PS, Zuniga ES, Ojima I, Carrico IS. Bioorg. Med. Chem. Lett. 2011;21:4985–4988. doi: 10.1016/j.bmcl.2011.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilchek M, Bayer EA. Methods Enzymol. 1990;184:123–138. doi: 10.1016/0076-6879(90)84267-k. [DOI] [PubMed] [Google Scholar]
- 43.Kuznetsova LU, IM, Pepe A, Zanardi I, Wu X, Ojima I. J. Fluor. Chem. 2004;125:487–500. [Google Scholar]
- 44.Appenzeller-Herzog C. J. Cell. Sci. 2011;124:847–855. doi: 10.1242/jcs.080895. [DOI] [PubMed] [Google Scholar]
- 45.Borcard F, Godinat A, Staedler D, Blanco HC, Dumont AL, Chapuis-Bernasconi C, Scaletta C, Applegate LA, Juillerat FK, Gonzenbach UT, Gerber-Lemaire S, Juillerat-Jeanneret L. Bioconjug. Chem. 2011;22:1422–1432. doi: 10.1021/bc200147m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.