Abstract
Background
Using clinical guidelines in the management of patients with multimorbidity can lead to the prescription of multiple and sometimes conflicting medications.
Aim
To explore how GPs make decisions when prescribing for multimorbid patients, with a view to informing intervention design.
Design and setting
In-depth qualitative interviews incorporating chart-stimulated recall with purposively sampled GPs in the Republic of Ireland.
Method
Grounded theory analysis with iterative theory development.
Results
Twenty GPs were interviewed about 51 multimorbid cases. In these cases, GPs integrated information from multiple sources including the patient, specialists, and evidence-based medicine. Difficulties arose when recommendations or preferences conflicted, to which GPs responded by ‘satisficing’: accepting care that they deemed satisfactory and sufficient for a particular patient. Satisficing was manifest as relaxing targets for disease control, negotiating compromise with the patient, or making ‘best guesses’ about the most appropriate course of action to take. In multimorbid patients perceived as stable, GPs preferred to ‘maintain the status quo’ rather than rationalise medications, even in cases with significant polypharmacy. Proactive changes in medications were facilitated by continuity of care, sufficient consultation time, and open lines of communication with the patient, other healthcare professionals, and other GPs.
Conclusion
GPs respond to conflicts in the management of multimorbid patients by making compromises between patient-centred and evidence-based care. These findings will be used to inform interventions that aim to care in multimorbidity.
Keywords: chronic disease, decision making, general practice, medication therapy management, physician’s practice patterns, qualitative research
INTRODUCTION
Multimorbidity, the co-occurrence of two or more chronic diseases, affects over 50% of patients with chronic disease in primary care and leads to increased mortality, higher rates of disability, declines in functional status, and lower quality of life.1,2 For healthcare systems, multimorbidity leads to higher rates of healthcare utilisation, especially high-cost services such as hospitalisations and emergency department visits;3–5 and this burden is increasing as a result of the ageing demographic. Thus optimising the care of multimorbidity is of concern for healthcare research, policy, and education.6
Multimorbid patients are also more likely to experience polypharmacy and potentially inappropriate prescribing than patients with single diseases.7,8 However, prescribing ‘appropriately’ in multimorbidity is not always straightforward.9,10 Guidelines exist for most common chronic diseases and offer benefits associated with the best available evidence, but adhering to guidelines in the management of a patient with multimorbidity almost invariably leads to multiple medications, resulting in increased risk of drug interactions, adverse effects, and poor adherence.11,12 Furthermore, most guidelines do not consider patient preferences, quality of life, or the expected time to benefit.13 Thus prescribing in multimorbidity poses a dilemma: to prescribe a recommended medication that may, via polypharmacy, lead to adverse effects, or not to prescribe a medication that may have potential benefits.14
Despite the prevalence of multimorbidity, there have been few professional-oriented interventions developed to improve patient outcomes in this field.15 Prescribing behaviour appears to be a worthy candidate for such an intervention. It is known that GPs question the usefulness of single-disease guidelines in multidisease patients;16 however, little is known about how GPs choose what to do when faced with guidelines that indicate that multiple and sometimes conflicting medicines should be prescribed. An important first step in intervention design is to gain a thorough understanding of existing behaviour.17,18 Thus this study aimed at exploring how and why GPs make decisions when prescribing for multimorbid patients, with a view to informing the design interventions to assist prescribing and multimorbidity care.
METHOD
Design
A qualitative study was conducted using a grounded theory approach. In-depth interviews were performed with GPs using chart-stimulated recall (CSR), a clinical assessment tool that uses a medical chart to stimulate a physician’s recall of a case and its management.19,20
How this fits in
Prescribing for patients with multimorbidity poses dilemmas for GPs, related to polypharmacy, treatment burden, and potentially inappropriate medications. To develop interventions to improve prescribing for multimorbid patients, a greater understanding of how and why GPs prescribe in multimorbidity is required. This study uses case-specific information to reveal the compromises between patient-centred and evidence-based care made by GPs in multimorbidity, in a process facilitated by continuity of care, sufficient time within the consultation, and open lines of communication with the patient, other healthcare professionals, and other GPs. These novel findings will better inform the development of interventions to assist and improve prescribing and multimorbidity care.
Setting
The study was conducted in the Republic of Ireland, where GPs play a gatekeeping role in the healthcare system. Most GPs in Ireland are private practitioners, but most also provide public health services to people with the means-tested medical card, which allows free GP care at the point of access.21
Sampling
A purposive sample of GPs was selected from attendees at two regional continuing professional development meetings and supplemented by snowball sampling where necessary to gain representation of GPs by: length of time qualified (>10 or <10 years); practice location (rural or urban); and practice size (single or group practice).
Data collection
Interviews took place in participants’ clinics between February 2013 and November 2013. Prior to the interview, GPs were asked to choose patients from their practice who had three or more chronic diseases, and were prescribed five or more chronic disease medications for the purpose of CSR. Where feasible, GPs were asked to choose patients seen on the day of or the day preceding the interview, to maximise their recollection of the case details. During the interview, the GP was asked to give a summary of each case including demographics, diagnoses, and prescribed medications, before describing the patient’s recent consultations using the medical notes as an aide-memoire. The interview followed the participant’s description of a chosen case’s sequential consultations as far as possible. A topic guide, derived from a systematic literature review on the challenges experienced by GPs in the management of multimorbid patients,16 was referred to during interviews and included prompts on the use of clinical guidelines, goals of care, and shared decision making. The topic guide was modified after each interview to pursue emergent themes (examples of topic guides are available on request from the authors). All interviews were conducted by a single interviewer, audiotaped, and transcribed in full.
Analysis
Coding was data-driven according to the grounded theory approach described by Charmaz.22 The first stage comprised open coding of GPs’ actions in multimorbidity, and the causes, conditions, and consequences of these actions. The second stage of coding comprised categorisation of the coded data based on conceptual similarity. Divergent cases were actively sought. This approach to coding was agreed a priori by team consensus. The first three transcripts were read, coded, and compared by two researchers, focusing on interviewing technique and the development of preliminary codes. The next three interviews were coded and compared by two researchers, and all remaining interviews were coded by one researcher as they took place, adhering to the tenets of constant comparison. Once data collection was complete, other members of the team independently coded an additional three randomly assigned interviews. Field notes, memos, coding, and theoretical development were discussed at regular team meetings. NVivo 10 was used for data management (version 10). Demographic and chronic disease information of the cases discussed were analysed descriptively using Microsoft Excel. The consolidated criteria for reporting qualitative research (COREQ) statement was used to inform reporting of the findings (available from the authors on request).
RESULTS
Twenty GPs were interviewed. Characteristics of participating GPs are shown in Table 1. A total of 51 patients with multimorbidity were discussed during the 20 interviews. The median patient age was 75 years (range 39–92 years) and 55% were female. Patients had an average of 8.3 chronic diseases and were prescribed an average of 10.6 regular medications (An overview of the cases are available on request from the authors). Interviews lasted on average 42 minutes (range 32–65 minutes). Conceptual data saturation occurred at interview 18, as subsequent interviews did not contribute to the development of new themes.
Table 1.
Characteristics of GP participants (N = 20)
| Participants, % (n) | |
|---|---|
| Practice location | |
| Rural | 45 (9) |
| Urban | 35 (7) |
| Mixed | 20 (4) |
|
| |
| Type of practice | |
| Single handed | 30 (6) |
| Group practice | 70 (14) |
|
| |
| Length qualified | |
| <10 years in practice | 30 (6) |
| >10 years in practice | 70 (14) |
Participant quotations representative of typical responses have been selected to illustrate qualitative findings, supplemented with relevant case details where applicable.
Factors influencing decisions in multimorbidity
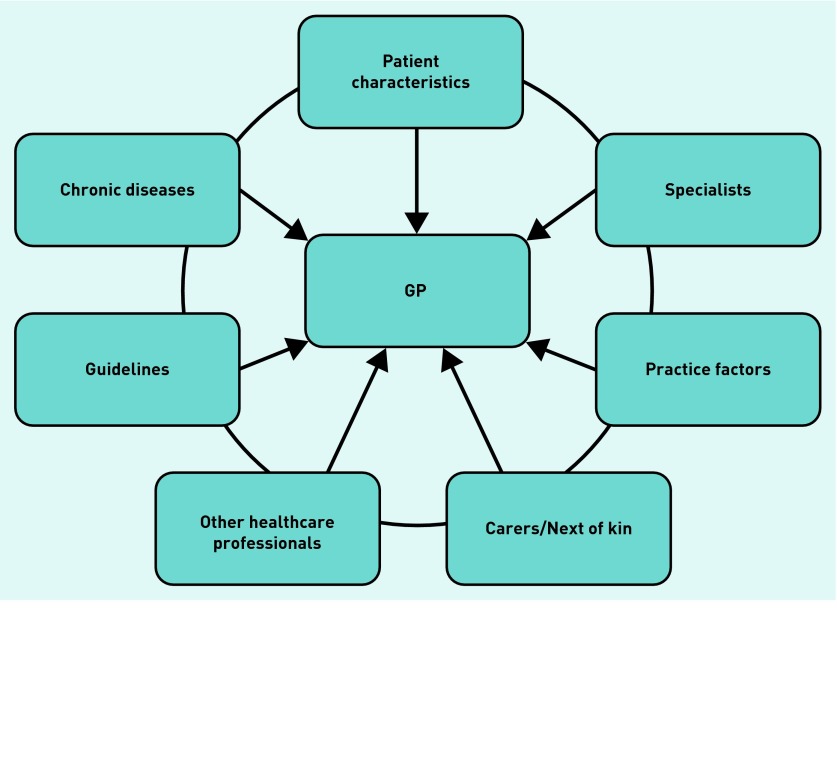
Figure 1 shows the diverse range of influences on GPs’ decisions in multimorbidity. GPs considered and integrated the factors deemed relevant to a particular case to make an appropriate decision for that patient. Multiple chronic diseases did not always lead to difficult decisions, even when multiple medications and complex combinations were present:
‘I have a lot of patients with hypertension, lipid disorder, and thyroid disease but I wouldn’t classify those as multimorbid. They are most of the time fairly straight forward. It is only when you add something else into the mix that it gets complicated.’
(GP15)
Figure 1.
Influences on GPs’ decision making in multimorbidity.
Satisficing: an approach to decision making in multimorbidity
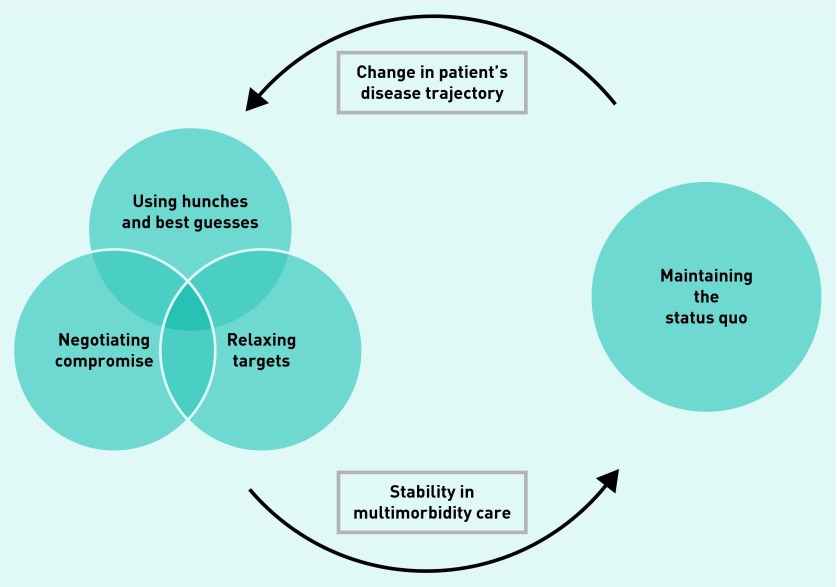
Conflicts arose in cases because of potential interactions between diseases and medications; discrepancies between the patients’ preferences and best practice recommendations; or lack of an evidence base relevant to multimorbidity. In response GPs tried to find a balance between optimal disease management and patient-centred care in a process of satisficing: settling for chronic disease management that was satisfactory and sufficient, given the particular circumstances of that patient. Figure 2 shows the different manifestations of satisficing, which depended on the patient’s disease trajectory or level of stability.
Figure 2.
Approaches to decision making in multimorbidity.
Relaxing targets
Satisficing meant that GPs accepted less stringent levels of disease control than were advised by guidelines. This was seen in cases where the management of one disease was prioritised over others because of severity or symptoms:
‘I’m not aiming for very tight control — I’m happy if his sugars are running a little higher than normal. I mean he has got cardiac failure as well, his life expectancy isn’t brilliant — so long term I think, I don’t think it’s his type 2 diabetes that’s going to kill him.’
(GP7 discussing 77-year-old male with nine chronic diseases prescribed 16 regular medications)
Suboptimal targets were also accepted in patients with poor adherence in whom GPs felt that, because of the impact of multiple medications, disease ‘control is as good as he [patient] will allow it to be, he’s not madly compliant’ (GP17). When patients developed side effects from guideline-recommended medications, GPs considered other factors before deciding whether to relax disease targets or continue the drug:
‘If we increase her drugs for her cardiac failure and she is getting more dizzy, then we will always go back to the last stage before she had symptoms …’
(GP20 discussing her decision to prioritise patient comfort in a 71-year-old female with cardiac failure, orthostatic hypotension, seven other chronic diseases, and nine regular medications)
‘I think, I suppose, at the end of it his cardiac and renal function are what are going to kill him, not getting up at night to pee.’
(GP17 discussing his decision to prioritise disease control in a 64-year-old male with 10 chronic diseases and 13 regular medications, whose urinary symptoms are exacerbated by diuretics)
Negotiating compromise
Conflicts sometimes arose between what the GP thought best for a patient and the patient’s requests or a specialist’s recommendations. Here, GPs negotiated to find a satisfactory compromise, using techniques such as concessions over drug dose or duration, gradual weaning off medications, or substitution with lower-risk alternatives:
‘Well it wouldn’t be “my way or the highway”; you need to negotiate it, because as you know people have all sorts of kind of fixed ideas about things really and it can be difficult to dislodge them.’
(GP14 on an 81-year-old male with a recent myocardial infarction and hypertension who requested anti-inflammatories for increasing joint pain)
Hunches and best guesses
When presented with a range of options, none of which were clearly right or wrong, many GPs used a ‘hunch’ or made a ‘best guess’ as to which option to take. This occurred in situations where the reason for a patient’s symptoms was unclear, potentially attributable to many of the patient’s existing diagnoses:
‘He has lots of reasons to be short of breath — so his pulmonary emboli can do it; his anaemia can do it; his lobectomy can do it; his CCF could do it; and his COPD could do it; so ah, it’s basically a case of trying to figure out and sort them out. I know him quite well, and what his baseline is, so it’s a case of trying to figure out what is the major cause each time he comes in ... we generally try and make a best guess at it.’
(GP7 discussing 77-year-old male with nine chronic diseases and 16 regular medications)
Best guesses were also required because ‘you don’t have guidelines for every situation — there are times when you just have to make a decision as best you can’ (GP6). GPs relied heavily on their prior knowledge and experience of the patient in this process.
Maintaining the status quo
Once a multimorbid patient appeared to be stable, GPs’ default approach was to ‘maintain the status quo’ (GP1) rather than interfere with drug regimens, unless they saw clear evidence of adverse drug effects:
‘... really didn’t entertain changing them because why stir things up?’
(GP19)
‘... look she’s on it, she’s fine, it doesn’t bother her, its suiting her fine.’
(GP12)
‘... like he is very stable on them all but it does seem like an awful lot.’
(GP2)
‘... she’s doing better than she has in a long time — I’m not going to rock the boat at all.’
(GP11)
Although concerned about polypharmacy, GPs had a greater fear of medico-legal repercussions or negative responses from the patient or their next of kin if rationalising medications led to clinical events:
‘I think litigation is a huge issue: as I say the wife is on the ball; okay I say “look let’s get rid of his aspirin and his statin — he has no ischaemic heart disease”. And then say, he gets a myocardial infarction in four months’ time and you say “should I have left him on the statin?”.’
(GP6 discussing 84-year-old male with hypertension, hyperlipidaemia, osteoarthritis, recent deep venous thrombosis, prostate cancer, osteoporosis, and constipation on 13 medications)
GPs were reassured that the ongoing use of some medications was ‘justified’ (GP7) because they were commenced by a specialist or recommended in best practice guidelines, in many cases years before:
‘There is very little we can get away with in terms of manoeuvring with her. She has a lot of pathology and she probably needs virtually everything she is on there.’
(GP9 discussing an 86-year-old female with anxiety, osteoporosis, stage 3 kidney disease, hypothyroidism, coronary artery disease, atrial fibrillation, cardiac failure, osteoarthritis, stress urinary incontinence, COPD, diverticular disease, aortic stenosis, and constipation on 14 medications)
Resources to assist decision making in multimorbidity
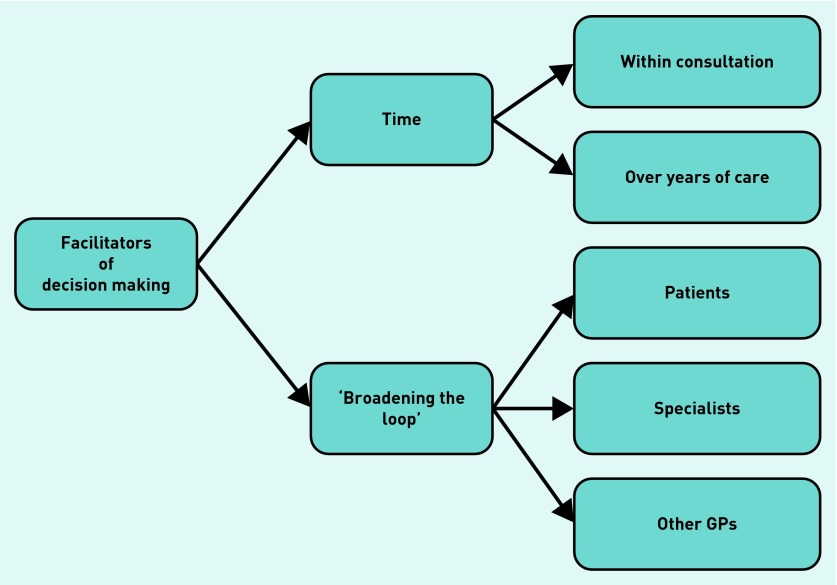
Figure 3 shows the key facilitators to resolving conflicts in prescribing decisions: ‘broadening the loop’ of communication to involve others in the decision-making process and the availability of time. Deficiencies in these processes were common, which left GPs less comfortable with their decisions.
Figure 3.
Facilitators of decision making in multimorbidity.
Broadening the loop to patients
GPs believed that many multimorbid patients preferred not to be involved in decisions, where ‘the more complex their needs, the more they rely on you to be the final arbitrator or the overseer’ (GP19). Some GPs felt that patients would be unable to understand the various conflicts and uncertainties faced, and so would ‘just worry about it myself … rather than imparting a huge amount of knowledge’ (GP16). This contrasted with cases in which the GP shared the uncertainty and responsibility for a decision with the patient, evident in situations involving younger GPs or those with a shorter professional relationship with the patient:
‘You have to go “this is your life, your decision” and then give them my advice but they have to make the decision for themselves.’
(GP3 discussing primary prevention in a 54-year-old male with six chronic diseases and six regular medications)
GPs had specific difficulties talking to multimorbid patients about stopping medications; they feared this could be interpreted by the patient as a withdrawal of care and potentially damage the doctor–patient relationship:
‘What you are saying by stopping it [a statin] is “I’m stopping this now because really now you are so old so if you get a heart attack at this stage … whatever”.’
(GP5 discussing the message he feared he would give by stopping a statin in an 84-year-old female with seven chronic diseases on 18 medications)
Broadening the loop to other healthcare professionals
GPs liked to ‘share the onus of responsibility’ (GP16) with specialists and pharmacists in complicated multimorbid patients ‘rather than flying solo on it’ (GP14). The usefulness of specialist input was limited, however, by a lack of timely access to and communication from specialists or by their single disease rather than generalist approach to the patient:
‘... in fairness to them, all their letters were bang on ... for COPD: do the sputum, give him the azithromycin, he has the home oxygen — tell him to use that. Everything was according to guidelines. Renal the same, trial this — if this doesn’t work this is what we’re doing — push this as far as we can, nephro-protection and all this, and it’s all bang on target. The same for cardiology. But when you put it in the clinical setting it isn’t working …’
(GP2 discussing 51-year-old male with eight chronic diseases on 13 medications)
Broadening the loop to fellow GPs
When faced with difficult decisions, many GPs elected to ‘have a practice discussion about it I think, it won’t take very long’ (GP18). They found that ‘to bounce [ideas] off your colleagues just helps, even if it is just something like “what in the name of God am I going to do about this”, it’s really important.’ (GP8). Single-handed GPs struggled in this regard, although some used continuing medical education, especially small group meetings, as a forum for discussing complicated cases with other GPs.
Time over multiple consultations
Return consultations were an opportunity to re-evaluate the patient, thereby reassuring the GP and patient, giving clarity on the best approach to take, and facilitating the management of multiple competing demands:
‘We checked her blood pressure; upped her medications; had a chat about her knees; I encouraged her to go back to the Weight Watchers. I’m going to follow her up in a month’s time; she hasn’t had her bloods done for a bit, so she’ll have that done before she gets back. I chatted to her about the antidepressant — she was keen on cutting it down but I’ve known her for years and winter is her bad time … so, I said “Look ... how about waiting until the spring again. We can have a chat about it then and just see?” and maybe if she loses a bit of weight, she might find that she is feeling a little bit better in herself and it might be a more appropriate time to do it.’
(GP11 discussing a 52-year-old female with depression, anxiety, hypertension, ANA positive arthritis, prior cauda equina syndrome, osteoarthritis, obesity, and acne on six medications)
A lack of relational continuity of care could adversely affect management, especially in some of the larger practices where ‘... you have different people making a clinical judgement on him based on how he is from week to week which is difficult.’
(GP2)
Time within the consultation
GPs reported that rationalising medications ‘is time consuming, you definitely want to have your wits about you, and without it (extra time) the potential for making mistakes is very much increased’. (GP14). Thus, lack of time pushed GPs towards ‘maintaining the status quo’, rather than active attempts to change management, especially if considering changing ‘something that you have been giving them for the last 15 years — and now you’re suddenly saying the evidence is saying that we shouldn’t be giving you aspirin anymore — it takes time, time to explain that to them’. (GP6)
DISCUSSION
Summary
This qualitative study demonstrates the range of influences on GPs’ prescribing decisions in multimorbid patients. When conflicts arise between these factors, GPs take an approach of satisficing — providing care they feel is satisfactory and sufficient for a particular patient. With changing chronic disease trajectories, satisficing means accepting trade-offs among drugs, diseases, and best practice recommendations. In stable multimorbidity and in the absence of nuanced communication techniques, GPs act to preserve the doctor–patient relationship ahead of medication rationalisation.
Strengths and limitations
The credibility of the present findings was enhanced by using chart-stimulated recall (CSR), which has been shown to be a valid way of assessing clinical decision making through improving recall of actual rather than perceived behaviour. CSR also allowed probing into why certain decisions were made, which was necessary for the purpose of identifying targets for professional interventions.19,20,23 By combining CSR and grounded theory, substantive issues for GPs emerged from the data that are additive to existing qualitative research with GPs on multimorbidity, much of which is based on case vignettes or focus groups.24–27 Although purposive and snowball sampling were both used to recruit a sample that was representative of the national GP profile, those who participated may have had a greater interest in or a particular agenda relating to the study question.28 The sample size was likely sufficient, however, given that data saturation was achieved.29 Clinician researchers have been shown to get richer data from GP participants than non-clinical researchers, but they can introduce clinical biases into data collection and interpretation.30 This risk of professional bias was reduced by the diverse professional backgrounds of the research team.31
Comparison with existing literature
Satisficing, a portmanteau of the words satisfy and suffice, was initially described by Simon in 1956 as human decision making that is limited by ‘uncertainty about the consequences that would follow from each alternative, incomplete information about the set of alternatives and complexity that prevents necessary computations from being carried out’.32 Satisficing is evaluation of the options available only until an acceptable one is found and was evident in this study, where GPs were unable to evaluate the risk–benefit of all potential options for a multimorbid patient given the deficiencies in evidence-based medicine and the time available for making decisions.
In a focus group study, Smith et al described GPs’ and pharmacists’ views on polypharmacy in multimorbid patients as resulting from the appropriate prescribing of risk-reducing medications indicated by single-disease guidelines.24 The current study moves beyond this concept to describe the strategies used by GPs to manage multiple medications where conflicting guidance exists. Some of the approaches to satisficing, such as relaxing targets for disease control, may have arisen because of the relative autonomy experienced by healthcare practitioners in the Irish healthcare system with respect to chronic disease management. This contrasts with the findings of Bower et al who, in a UK-based study with GPs and practice nurses, found that greater tensions between disease-focused and patient-centred care occurs for GPs striving to meet the demands of the Quality and Outcomes Framework.25
Processes similar to satisficing were also evident in large quantitative studies in multimorbidity. For example, studies from the US show that patients with discordant multimorbidity are less likely to have guideline-consistent hyperlipidaemia management.33,34 In Switzerland, trends for preventive care are lower in multimorbid patients with dementia.35 There is increasing recognition that improving adherence to guidelines may not be the best management strategy for patients with multiple medical problems.14,36
Implications for research and practice
Although GPs in this study provided logical reasons for their decisions, the potential negative outcomes associated with both polypharmacy and suboptimal disease management must be remembered and ways to support GPs’ prescribing in multimorbidity are thus required.9 In hospital specialties, there is an increasing trend towards multidisciplinary team (MDT) meetings, which operationalise collaborative decision making to deliver evidence-based yet patient-centred care. The potential for multidisciplinary review in primary care has been evaluated in trials such as PINCER, a pharmacist-led information technology intervention that reduced medication errors in general practice.37 Some ‘errors’ were over-ruled by GPs, however, on the basis of clinical experience of the patient, and there were also concerns about the long-term feasibility of pharmacists working in general practice.38 Participants in the present study undertook informal case reviews of complicated multimorbid patients with their fellow GPs. Even without the rigorous processes of the MDT, participants benefited from the close proximity, ready availability, and generalist perspective of their colleagues. Collaborative decision making between GPs deserves further exploration as a potential option for intervention in this field.39
Regarding shared decision making, previous work has shown that, although patients like to hear about the management options available to them, most will seek and accept their GPs’ advice on the best option to take.40 This implies that GPs must have the knowledge and confidence to offer patients specific recommendations.41,42 Although attempts are under way to improve the attentiveness of guidelines to multimorbidity, they will not be able to cover all eventualities in multimorbidity and some professional judgement will always be required.43,44 Relational continuity of care was an essential feature of how such judgements were made in this study, and should be prioritised in interventions that aim to promote shared decision making with multimorbid patients.
Lastly, competing demands in multimorbidity lead to greater demands on GPs’ time and less proactive management of medications. A number of trials are already dealing with the issue of time as part of a multifaceted intervention in multimorbidity and the results are keenly awaited.45,46
In conclusion, the Cochrane review group suggested that future multimorbidity interventions should be embedded with inter-professional collaboration and integrated into existing healthcare systems.15 The present results suggest that interventions to support prescribing in multimorbidity should also prioritise relational continuity of care, facilitate communication with patients on available and preferred options, and provide GPs with a means of collaborative decision making and treatment planning. These findings will help inform the design of interventions that aim at improving medication management and patient-centred care in multimorbidity.
Acknowledgments
The authors gratefully acknowledge the time provided by the GPs who participated in this study.
Funding
Carol Sinnott is on an academic fellowship programme sponsored by the Health Research Board and the Health Service Executive, Ireland (HRB/NSAFP/2011/3). Sheena Mc Hugh is a post-doctoral research fellow funded by the Health Research Board Interdisciplinary Capacity Enhancement Awards (HRB/ICE/2012/12). Maria Boyce is a researcher/PhD student on the Health Research Board Scholars and SIREN projects (HRB/PHD/2007/16). The sponsors of the study had no role in the study design, data collection, data analysis, data interpretation, writing of the report, or in the decision to submit the paper.
Ethical approval
Ethical approval was granted by the clinical research ethics committee of the Cork University Teaching Hospitals (reference ECM 4(t) 12/6/12) and from the research ethics committee of the Irish College of General Practitioners.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 2.Salisbury C, Johnson L, Purdy S, et al. Epidemiology and impact of multimorbidity in primary care: a retrospective cohort study. Br J Gen Pract. 2011 doi: 10.3399/bjgp11X548929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marengoni A, Angleman S, Melis R, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10:430–439. doi: 10.1016/j.arr.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Glynn LG, Valderas JM, Healy P, et al. The prevalence of multimorbidity in primary care and its effect on health care utilization and cost. Fam Pract. 2011;28:516–523. doi: 10.1093/fampra/cmr013. [DOI] [PubMed] [Google Scholar]
- 5.Lochner KA, Cox CS. Prevalence of multiple chronic conditions among Medicare beneficiaries, United States, 2010. Prev Chronic Dis. 2013 doi: 10.5888/pcd10.120137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward BW, Schiller JS. Prevalence of multiple chronic conditions among US adults: estimates from the National Health Interview Survey, 2010. Prev Chronic Dis. 2013 doi: 10.5888/pcd10.120203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Payne RA, Avery AJ, Duerden M, et al. Prevalence of polypharmacy in a Scottish primary care population. Eur J Clin Pharmacol. 2014;70:575–581. doi: 10.1007/s00228-013-1639-9. [DOI] [PubMed] [Google Scholar]
- 8.Galvin R, Moriarty F, Cousins G, et al. Prevalence of potentially inappropriate prescribing and prescribing omissions in older Irish adults: findings from The Irish LongituDinal Study on Ageing study (TILDA) Eur J Clin Pharmacol. 2014;70:599–606. doi: 10.1007/s00228-014-1651-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan C, O’Mahony D, Kennedy J, et al. Potentially inappropriate prescribing in an Irish elderly population in primary care. Br J Clin Pharmacol. 2009;68:936–947. doi: 10.1111/j.1365-2125.2009.03531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Payne RA, Abel GA, Avery AJ, et al. Is polypharmacy always hazardous? A retrospective cohort analysis using linked electronic health records from primary and secondary care. Br J Clin Pharmacol. 2014;77(6):1073–1082. doi: 10.1111/bcp.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pronovost PJ. Enhancing physicians’ use of clinical guidelines. JAMA. 2013;310:2501–2502. doi: 10.1001/jama.2013.281334. [DOI] [PubMed] [Google Scholar]
- 12.Patterson SM, Hughes C, Kerse N, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev. 2012:CD008165. doi: 10.1002/14651858.CD008165.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Bayliss EA, Bonds DE, Boyd CM, et al. Understanding the context of health for persons with multiple chronic conditions: moving from what is the matter to what matters. Ann Fam Med. 2014;12:260–269. doi: 10.1370/afm.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyd CM, Leff B. Will running the numbers first violate the principles of patient-centered care? Ann Intern Med. 2008;149:838–839. doi: 10.7326/0003-4819-149-11-200812020-00018. [DOI] [PubMed] [Google Scholar]
- 15.Smith SM, Soubhi H, Fortin M, et al. Managing patients with multimorbidity: systematic review of interventions in primary care and community settings. BMJ. 2012 doi: 10.1136/bmj.e5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinnott C, Mc Hugh S, Browne J, Bradley C. GPs’ perspectives on the management of patients with multimorbidity: systematic review and synthesis of qualitative research. BMJ Open. 2013 doi: 10.1136/bmjopen-2013-003610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6:42. doi: 10.1186/1748-5908-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008 doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goulet F, Jacques A, Gagnon R, et al. Assessment of family physicians’ performance using patient charts: interrater reliability and concordance with chart-stimulated recall interview. Eval Health Prof. 2007;30:376–392. doi: 10.1177/0163278707307924. [DOI] [PubMed] [Google Scholar]
- 20.Guerra CE, Jacobs SE, Holmes JH, Shea JA. Are physicians discussing prostate cancer screening with their patients and why or why not? A pilot study. J Gen Intern Med. 2007;22:901–907. doi: 10.1007/s11606-007-0142-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Health Service Executive Primary Care Reimbursement Service. Statistical analysis of claims and payments. 2012. http://www.hse.ie/eng/staff/PCRS/PCRS_Publications/PCRSannreport12.pdf (accessed 14 Dec 2014)
- 22.Charmaz K. Constructing grounded theory. A practical guide through qualitative analysis. 1st edn. London: Sage; 2006. [Google Scholar]
- 23.Rochefort CM, Morlec J, Tamblyn RM. What differentiates primary care physicians who predominantly prescribe diuretics for treating mild to moderate hypertension from those who do not? A comparative qualitative study. BMC Fam Pract. 2012;13:9. doi: 10.1186/1471-2296-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith SM, O’Kelly S, O’Dowd T. GPs’ and pharmacists’ experiences of managing multimorbidity: a ‘Pandora’s box’. Br J Gen Pract. 2010 doi: 10.3399/bjgp10X514756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bower P, Macdonald W, Harkness E, et al. Multimorbidity, service organization and clinical decision making in primary care: a qualitative study. Fam Pract. 2011;28:579–587. doi: 10.1093/fampra/cmr018. [DOI] [PubMed] [Google Scholar]
- 26.Luijks HD, Loeffen MJW, Lagro-Janssen AL, et al. GPs’ considerations in multimorbidity management: a qualitative study. Br J Gen Pract. 2012 doi: 10.3399/bjgp12X652373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuling J, Gebben H, Veehof LJ, et al. Deprescribing medication in very elderly patients with multimorbidity: the view of Dutch GPs. A qualitative study. BMC Fam Pract. 2012;13:56. doi: 10.1186/1471-2296-13-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kavanagh KE, O’Brien N, Glynn LG, et al. WestREN: a description of an Irish academic general practice research network. BMC Fam Pract. 2010;11:74. doi: 10.1186/1471-2296-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Francis JJ, Johnston M, Robertson C, et al. What is an adequate sample size? Operationalising data saturation for theory-based interview studies. Psychol Health. 2010;25:1229–1245. doi: 10.1080/08870440903194015. [DOI] [PubMed] [Google Scholar]
- 30.Chew-Graham CA, May CR, Perry MS. Qualitative research and the problem of judgement: lessons from interviewing fellow professionals. Fam Pract. 2002;19:285–289. doi: 10.1093/fampra/19.3.285. [DOI] [PubMed] [Google Scholar]
- 31.Barry CA, Britten N, Barber N, et al. Using reflexivity to optimize teamwork in qualitative research. Qual Health Res. 1999;9:26–44. doi: 10.1177/104973299129121677. [DOI] [PubMed] [Google Scholar]
- 32.Simon HA. Theories of bounded rationality. In: McGuire CB, editor. Decision and organization : a volume in honor of Jacob Marschak. Amsterdam: North Holland Publishing Company; 1972. pp. 161–176. [Google Scholar]
- 33.Druss BG, Rosenheck RA, Desai MM, Perlin JB. Quality of preventive medical care for patients with mental disorders. Med Care. 2002;40:129–136. doi: 10.1097/00005650-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Lagu T, Weiner MG, Hollenbeak CS, et al. The impact of concordant and discordant conditions on the quality of care for hyperlipidemia. J Gen Intern Med. 2008;23:1208–1213. doi: 10.1007/s11606-008-0647-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Streit S, da Costa BR, Bauer DC, et al. Multimorbidity and quality of preventive care in Swiss university primary care cohorts. PLoS One. 2014 doi: 10.1371/journal.pone.0096142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Covinsky KE. Multimorbidity, guidelines, and clinical inertia. JAMA Int Med. 2014;174:819. doi: 10.1001/jamainternmed.2013.14406. [DOI] [PubMed] [Google Scholar]
- 37.Avery AJ, Rodgers S, Cantrill JA, et al. A pharmacist-led information technology intervention for medication errors (PINCER): a multicentre, cluster randomised, controlled trial and cost-effectiveness analysis. Lancet. 2012;379:1310–1319. doi: 10.1016/S0140-6736(11)61817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cresswell KM, Sadler S, Rodgers S, et al. An embedded longitudinal multi-faceted qualitative evaluation of a complex cluster randomized controlled trial aiming to reduce clinically important errors in medicines management in general practice. Trials. 2012;13:78. doi: 10.1186/1745-6215-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen DA, Levy M, Cohen Castel O, Karkabi K. The influence of a professional physician network on clinical decision making. Patient Educ Couns. 2013;93:496–503. doi: 10.1016/j.pec.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 40.Levinson W, Kao A, Kuby A, Thisted RA. Not all patients want to participate in decision making. A national study of public preferences. J Gen Intern Med. 2005;20:531–535. doi: 10.1111/j.1525-1497.2005.04101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elwyn G, Edwards A, Gwyn R, Grol R. Towards a feasible model for shared decision making: focus group study with general practice registrars. BMJ. 1999;319:753–756. doi: 10.1136/bmj.319.7212.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60:301–312. doi: 10.1016/j.pec.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Guthrie B, Payne K, Alderson P, et al. Adapting clinical guidelines to take account of multimorbidity. BMJ. 2012 doi: 10.1136/bmj.e6341. [DOI] [PubMed] [Google Scholar]
- 44.Goodman RA, Boyd C, Tinetti ME, et al. IOM and DHHS meeting on making clinical practice guidelines appropriate for patients with multiple chronic conditions. Ann Fam Med. 2014;12:256–259. doi: 10.1370/afm.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.ISRCTN Registry Living well with multiple morbidity: The development and evaluation of a primary care-based complex intervention to support patients with multiple morbidities. 2013. http://www.controlled-trials.com/ISRCTN34092919/mindfulness (accessed 14 Dec 2014)
- 46.ISRCTN Registry The 3D Study: improving whole person care. 2014. http://www.controlled-trials.com/ISRCTN06180958/ (accessed 16 Jan 2015)