Background
Adverse outcomes associated with transfusion of blood products have been reported in a number of observational studies1–6. The cause of these adverse outcomes are thought to be due to structural or biochemical changes that blood undergoes while in storage and has been collectively referred to as a “storage lesion”7,8. Furthermore, the release of microparticles (MP) from stored blood has also been described as potential culprits. Studies on MP have become the forefront in medical research in a number of pathologic diseases and disorders such as cancer, atherosclerosis and venous thromboembolism9–12. These subcellular structures are released from the phospholipid bilayer of red cells, leukocytes and platelets. Recently, they have been described as being bioactive in nature by presenting on their surface negatively charged proteins such as phosphatidylserine13–15. In theory these negatively charged proteins act as a binding site for cofactors and enzymes involved in the coagulation pathway. In fact recent studies have isolated large numbers of procoagulant MPs from stored blood and have demonstrated the ability of these MPs to generate thrombin in the absence of TF by a factor XIa mediated pathway16,17.
Only recently have MP been studied in trauma patients; a pilot study by Park and colleagues describes increased levels of procoagulant MP in the blood of trauma patients soon after injury18. Currently, there is a paucity of data that directly quantifies the levels of procoagulant MP in the blood of trauma patients who receive stored blood products. We hypothesized that the transfusion of stored red blood cells (RBC) products increase the levels of procoagulant MPs present in the blood of traumatically injured patients.
Methods
Data were prospectively collected from February 2011 to January 2013 and included the most severely injured trauma patients based on physiological criteria. The patients were transported to the Mayo Clinic Emergency Department(ED) by ambulance or air transport. Exclusion criteria were: age < 18 years, active treatment for anticoagulation (e.g., heparin, warfarin) or antithrombotic therapy (excluding aspirin or non-steroidal anti-inflammatory drugs), preexisting coagulopathy, more than 12 hours from time of injury, any transfusion of blood products prior to blood sample collection, active malignancy, sepsis or renal failure, or burn injuries. The time of injury (TOI) was assessed by the pre-hospital crew based on information at the scene. If the time of injury was unclear, the pre-hospital crew estimated the time and relayed this information to the emergency communication center. A trauma alert page is then sent to the hospital and laboratory staff as to the TOI. Demographic data collected included: injury severity score (ISS), age, sex and overall mortality. During the same time period, blood samples were collected for MP reference (control) analysis from 27 even non-injured subjects with no prior history of thrombosis (i.e., stroke, myocardial infarction or venous thromboembolism who were being seen at the Mayo Clinic. These subjects had not received any anticoagulation (heparin or warfarin) or taking antithrombotic (e.g., thienopyridine; including aspirin or non-steroidal anti-inflammatory drugs) within 7 days prior to blood sample collection. This study was approved by the Mayo Clinic IRB (#10-001889).
Sample Collection and Processing
Blood samples were collected in the ED within 2 hours from time of injury, 6 hours from time of injury and 24 hours from time of injury. Blood samples collected in the ED (within 2 hours from time of injury) or at 6 hours from the time of injury were defined as pre-transfusion samples. Blood samples collected at 24 hours from the time of injury were defined as post-transfusion. The transfusion of blood products occurred at varying times within these defined collection times. When patients were unable to provide consent at the time of the trauma, consent was obtained from the patient or legal guardian prior to patient discharge; otherwise the study sample was appropriately discarded.
A total of 18ml of blood was collected by antecubital venipuncture into an anticoagulant containing sodium citrate (3.2%) for MP analysis. Multiple aliquots of platelet poor plasma (PPP) were prepared by two centrifugations (3000g, 15 minutes) and then frozen at −80 degrees Celsius until analysis.
MP Analyses
The flow cytometric assay to measure plasma MPs was adapted from the method of Ayers et. al.19 and by personal correspondence with Paul Harrison, Oxford Haemophilia and Thrombosis Centre, Churchhill Hospital, Oxford, UK. Labeling of MP of platelet poor plasma was performed using Annexin-V-FITC (BD Pharmingen, 556420), which binds to procoagulant phosphatidylserine. Since not all MP expose phosphatidylserine on their surface, both AnnV (procoagulant) and AnnV negative MP were measured. The stained MP were counted with a FACSCanto II flow cytometer (BD Biosciences, San Jose CA), with use of an internal standard of microbeads. Suitable MP gate on a flow cytometry plot of forward and scatter (FSc) vs. side scatter (SSc) as previously published was used to distinguish MP from small platelets 20. Additionally, we used a commercially available reference plasma, Cryocheck (Precision Biologic, Dartmouth N.S.), which was used with every carousel of patient samples to ensure that our technique for MP analysis was consistent. Between two experienced research technologists, the coeffcient of variation (CV) using our reference plasma has consistently been in the 13 – 15% range.
Red Blood Cell (Blood) Transfusion Criteria
We reviewed the number of transfusion of packed red blood cells (RBCs) given during the first 24 hours after injury. The RBC was categorized as being <14 or ≥ 14 days old. A second category used was RBC < 28 or ≥ 28 days old. The standard operating procedure concerning RBC transfusion was established by the Laboratory Medicine and Pathology at the Mayo Clinic, Rochester and there was no change in institutional transfusion policy during this study.
Statistical Analyses
Statistical analysis was performed using SAS, version 9.3 (SAS Institute Inc, Cary, North Carolina) and SPSS 10.1 (SPSS Inc, Chicago, Illinois). A comparison of continuous variables between patients and volunteers (controls) was performed using Wilcoxan rank sum test or Students t-test. Matched paired t- test was also performed as indicated. Our data is presented as mean ± standard deviation or median values (interquartile range); p < 0.05 considered to be significant.
Results
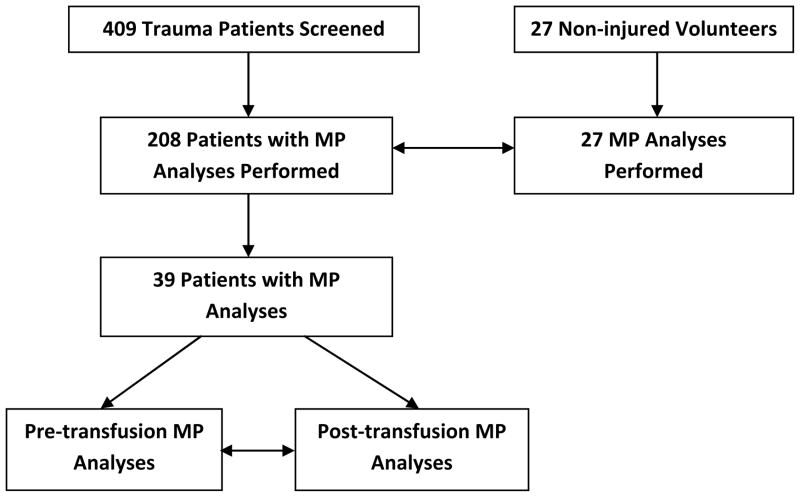
A total of 409 patients were enrolled during the study period (Table 1, Diagram 1). Sixty eight percent of our patients were men with a median age of 48(29, 62) years and injury severity score (ISS) of 12 (15, 19). The overall mortality was 3% with 90% patients having suffered blunt injuries (including closed head injury). We then analyzed the blood samples collected in the ED of 208 patients and found that the total number of procoagulant MP were greater in our trauma patients 758 (405, 1627) as compared to our non-injured control subjects 232 (125, 372), p < 0.0001(Table 2). This difference remained significant even after we adjusted for age and sex, p < 0.0001. Of the 208 patients, 39 patients received RBC transfusions and had their blood analyzed. These 39 patients received RBC transfusions and had their blood analyzed for MP before (within 6 hours of injury) and after (at 24 hours after injury) RBC transfusions. When we compared the procoagulant MP counts in the 39 patients before and after their transfusion of RBC, the procoagulant MP levels did not increase, p = 0.07(Table 3). The median number of RBC given to the 39 patients was 3 units (2, 5) and the median length of storage time of these units was 17 (15, 24) days old.
Table 1.
Demographic Data
| Median Injury Severity Score * | 12 (15–19) |
| Transfused (%) | 12 |
| Median Age* | 48 (29–62) |
| Male (%) | 68 |
| Female (%) | 32 |
| Overall Mortality (%) | 3 |
| Median Units Transfused * | 3 (2–5) |
| Median Age of Transfused RBCs Days * | 17 (16–21) |
| Age Range of Units Transfused Days | 4–40 |
Data are presented as median with interquartile range, n=409
Table 2.
Procoagulant MP Levels in Trauma Patients Compared to Control Subjects
| Trauma Patients (n = 409) | Control Subjects (n = 27) | p value | |
|---|---|---|---|
| Total Procoagulant MP (#/μl)* | 758 (405, 1627) | 232(125, 372) | < 0.0001 |
Data are presented as median with interquartile range; (#/μl: number of MP per μl of platelet poor plasma)
Table 3.
Pre-transfusion and Post-transfusion MP Levels of 39 Patients:
| Pre-transfusion Baseline (ED or 6hours) | Post-transfusion (24 hour) | p value | |
|---|---|---|---|
| Total Procoagulant MP levels (#/μ/l)* | 392 (237, 1057) | 406 (187, 643) | 0.07 |
Data are presented as median with interquartile range. (#/μl: number of MP per μl of platelet poor plasma)
To determine if the length of storage leads to an increase in number of procoagulant MP, the RBC unit with the maximal age in days was used for each of the 39 patients; the age (days) of each RBC unit transfused in these patients is outlined in Table 4. Only 4 patients received RBC that was less than 14 days old and 29 patients received blood that was less than 28 days old. We determined that patients who were transfused with RBC that were ≥14 days old did not have increased procoagulant MP levels when compared to those who received blood that was < 14 days old, p = 0.5(Table 5). This was also true for those who received blood that was ≥ 28 days old when compared to those that received blood that was < 28 days old, p = 0.84(Table 6). The median number of RBC < 14 days and ≥14 days were 2.0 (1.5, 3.5) and 4.0 (2.0, 5.0) units, respectively with p = 0.38. The median number of RBC < 28 days and ≥ 28 days were 3.0 (2, 5) and 3.5 (2, 4) units, respectively with p = 0.90. The median ISS of RBC < 14 days and ≥ 14 days were 20 (9, 26) and 25 (11, 34), respectively with p = 0.3. The median ISS of RBC < 28 days and ≥ 28 days were 25 (11, 34) and 25 (17, 33), respectively with p =0.98.
Table 4.
Age of RBC Units in Days
| Patient | Age of Units of RBC (days) | Oldest Unit Blood days |
|---|---|---|
| 1 | 21,21 | 21 |
| 2 | 27,27,27 | 27 |
| 3 | 36,36 | 36 |
| 4 | 12,11,11,12,11 | 12 |
| 5 | 16 | 16 |
| 6 | 10,22 | 22 |
| 7 | 16,16,25,25 | 25 |
| 8 | 17 | 17 |
| 9 | 33,7,7,9 | 33 |
| 10 | 4 | 4 |
| 11 | 39,40 | 40 |
| 12 | 16, 15 | 16 |
| 13 | 21,24,21,24 | 24 |
| 14 | 16,16,17,17,16,15,15,16 | 17 |
| 15 | 29,29,29,29 | 29 |
| 16 | 20,27,14,14,20 | 27 |
| 17 | 17,30,29,17,17,8,8,9,9 | 30 |
| 18 | 31,31,31,31,31,31,31,31,31,31,27,23,23,31,31 | 31 |
| 19 | 16,18,18,18,16 | 18 |
| 20 | 26,26,26 | 26 |
| 21 | 21,21,21,24,21,21,21,21,21,9,9,9,9,9,9,22,24,22,22,18,10,16,11,16 | 24 |
| 22 | 21,21 | 21 |
| 23 | 20,20,20,23 | 20 |
| 24 | 16 | 16 |
| 25 | 24,22 | 24 |
| 26 | 33,33 | 33 |
| 27 | 9,9,22 | 22 |
| 28 | 31,31,31 | 31 |
| 29 | 8,8,21,9,9,9,9 | 21 |
| 30 | 28,29,30,28 | 30 |
| 31 | 21,21 | 21 |
| 32 | 16,16 | 16 |
| 33 | 7,24,7,7,24,24 | 24 |
| 34 | 15,19,17,15,14,15 | 19 |
| 35 | 19,18,18, | 19 |
| 36 | 10,10 | 10 |
| 37 | 13,13 | 13 |
| 38 | 17,17,17,17,16,16,16,16,17,17,17,17,17,17,17,16,17,17,16,16,22,16,16,16,16,16,17.17,15,17 | 22 |
| 39 | 12,17,17,18,17,17,26,17,18 | 26 |
Table 5.
MP Levels After < 14 Day Old and ≥ 14 Day Old RBC Transfusion:
| <14 day (n = 4) | ≥ 14 day (n = 35) | p value | |
|---|---|---|---|
| Total Procoagulant MP levels (#/μ/l)* | 207(179,2255) | 369(151,578) | 0.50 |
Data are presented as median with interquartile range. (#/μl: number of MP per μl of platelet poor plasma)
Table 6.
MP levels after < 28 day old and ≥ 28 day old RBC transfusion:
| < 28 days (n = 29) | ≥ 28 days(n = 10) | p value | |
|---|---|---|---|
| Total Procoagulant MP levels (#/μ/l)* | 321(153,583) | 408 (245, 512) | 0.84 |
Data are presented as median with interquartile range. (#/μl: number of MP per μl of platelet poor plasma)
Discussion
This study presents quantification of MP in peripheral blood of trauma patients and their values before and after RBC transfusion. This is a first of such study of its kind. Previous studies published comprise mainly of in-vitro analysis of stored blood products6,9,11,12,16,17,28. Elevated levels of procoagulant MP are evident in the blood of traumatically injured patients; however, we found that transfusion of blood did not significantly affect the number of total procoagulant MP.
The debate continues in regards to transfusion of “old” versus “young” blood. There are studies that show poorer outcomes with transfusion of older blood1–6 while others report no significant difference in outcomes with the transfusion of older blood21–24. The exact mechanism associated with the risk of transfusion of old blood is poorly understood. Current research has focused on the role of procoagulant MP in stored blood and its immunomodulatory effects in critically ill and injured patients1,5,16,17,25,26.
Spinella and colleagues have observed that trauma patients who were transfused 5 units or more of ≥ 28 day old RBC had significant increase in deep vein thrombosis and was associated with multiple organ failure (MOF)1. Another study by Koch and colleagues demonstrated that when cardiac surgical patients were transfused with RBC > 14 days old, an increased rates of sepsis, renal failure and mortality were observed 2. However, these patients in the study who received blood > 14 days old received 6 or more units of blood. Increased volume of blood transfusion is an independent predictor of mortality. In our particular study, the volume of blood transfused did not differ significantly within our 14 and 28 day analysis groups.
The non-leukocyte reduced blood has been shown to contain activated platelets that interact with white blood cells and result in procoagulant activity27. Our study used exclusively pre-storage leukocyte reduced blood which has been shown in current literature to reduce the number of circulating procoagulant MP in stored blood28. Recent literature also reports a correlation between ISS and procoagulant MP levels that could provide a potential confounding variable18. In our particular study, there was no significant difference in injury severity scores within the 14 day and 28 day transfusion analysis groups.
Our study has several limitations. First, the data presented in this study were derived from a parent study, which is a prospective case-cohort study designed to assess the role of MP in the development of venous thromboembolism (VTE). This is a significant limitation as we did not time the MP analyses just before and after transfusions. Secondly, we did not analyze blood products other than RBC and it is possible that even though the absolute total MP count may not increase after transfusion, certain MP from different cells may increase in number. Such changes may have an effect on the development of transfusion related complications such as ARDS, MOF and VTE. Third, due to the low number of patients who had MP analyzed before and after receiving RBC transfusions, we were unable to use the cutoff age of < 14 or < 28 days to assess the impact of age of RBC on clinical outcome. Rather, we looked at the oldest (maximal) unit transfused instead of the mean age. Studies that use mean age of RBC to define patients who received fresh or old RBCs fail to represent the contribution of the oldest unit. It is believed that the youngest units of RBCs tend to balance out the contribution from the oldest unit transfused. Therefore, we used the parameter of maximal age of RBC transfused for our analyses. The median age of transfused blood in our study was 17 days old and 75% received blood that was < 28 days old. The results of our study, which did not reveal an increase in total MP count after transfusion may be related to two reasons: The use pre-storage leukocyte reduced RBC and decreased age of transfused RBC.
Conclusion
We have demonstrated that total number of procoagulant MP is significantly greater in trauma patients as compared to volunteers. However, the transfusion of RBC did not lead to increased levels of procoagulant MP. This finding is likely due to the use of pre-storage leukocyte reduced blood and decreased age of transfused RBC. Currently, a prospective case-cohort study to characterize the profiles of the procoagulant MP over time will enhance our understanding of their role in the hypercoagulable state after injury.
Figure 1.
Selection of Patients Who Had MP Measured Before and After Initial RBC Transfusion
Acknowledgments
The authors deeply thank the research coordinators Melissa M. Kuntz, Teron T. Cox, Michael J. Ferrara and Debbie A. Dixon). We thank the Clinical Research Unit (CRU) of the Center for Translational Science Activities (CTSA) for their 24 hour support in sample collection. We are grateful for the technical assistance received from Timothy M. Halling and the Hematology Research Laboratory, Mayo Clinic, Rochester, MN for sample analysis. We also thank Dr. Camille M. van Buskirk and Karafa S. Badjie with the department of Laboratory Medicine and Pathology for providing information on the blood products that were transfused in this study.
Footnotes
Conflicts of Interest: No conflicts of interest
Author Contributions:
Myung S. Park: Study design, literature search, data collection and analysis, data interpretation, manuscript preparation and manuscript critical revision.
Satbir K. Dhillon: Literature search, data collection, data interpretation, manuscript preparation.
Mindy L. Houck: Data collection, manuscript preparation.
Donald H. Jenkins: Manuscript preparation.
Jordan K. Rosedahl: Data analysis, manuscript preparation.
William S. Harmsen: Study Design, Data analysis, Data interpretation, manuscript preparation.
Timothy M. Halling: Data collection, Data interpretation, manuscript preparation.
Disclosures of funding: This project described was supported by Grant Number K08 GM093133-04 (MSP) from the National Institute of General Medical Sciences (NIGMS), W81XWH-10-2-2010 (MSP) from the Telemedicine and Advanced Technology Research Center (TATRC) of the Department of Defense, 1 UL1 RR024150 from the National Center for Research Resources (NCRR)< a component of the National Institutes of Health (NIH), the NIH Roadmap for Medical Research and by Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH and the Department of Defense. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/
Contributor Information
Satbir K. Dhillion, Email: dhillon.satbir@mayo.edu.
Mindy L. Houck, Email: houck.mindy@mayo.edu.
Donald H. Jenkins, Email: jenkins.donald@mayo.edu.
Jordan K. Rosedahl, Email: rosedahl.jordan@mayo.edu.
William S. Harmsen, Email: harmsen.william@mayo.edu.
Timothy M. Halling, Email: halling.timothy@mayo.edu.
S. Myung, Email: park.myung@mayo.edu.
References
- 1.Spinella PC, Carroll CL, Staff I, Gross R, Mc Quay J, Keibel L, Charles EW, Holcomb JB. Duration of red blood cell storage is associated with increased incidence of deep vein thrombosis and in hospital mortality in patients with traumatic injuries. Critical Care. 2009;13:R151. doi: 10.1186/cc8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koch CG, Khandwala F, Li L, Estafanous FG, Loop FD, Blackstone EH. Persistent effect of red cell transfusion on health related quality of life after cardiac surgery. Ann Thorac Surg. 2006;82:13–20. doi: 10.1016/j.athoracsur.2005.07.075. [DOI] [PubMed] [Google Scholar]
- 3.Zallen Gm, Offner PJ, Moore EE, Blackwell K, Ciesla DJ, Gabriel J, Denny C, Silliman CC. Age of transfused blood is an independent risk factor for post injury multiple organ failure. Am J Surg. 1999;178(6):570–572. doi: 10.1016/s0002-9610(99)00239-1. [DOI] [PubMed] [Google Scholar]
- 4.Offner PJ, Moore EE, Biffl WL, Johnson JL, Silliman CC. Increased rate of infection associated with transfusion of old blood after severe injury. Arch Surg. 2002;137(6):711–716. doi: 10.1001/archsurg.137.6.711. [DOI] [PubMed] [Google Scholar]
- 5.Purdy FR, Tweeddale MG, Merrick PM. Association of mortality with age of blood transfused in septic ICU patients. Can J Anaesth. 1997;44(12):1256–1261. doi: 10.1007/BF03012772. [DOI] [PubMed] [Google Scholar]
- 6.Belizaire RM, Prakash PS, Richter JR, Robinson BR, Edwards M, Caldwell CC, Lentsch AB, Pritts TA. Microparticles from Stored Red Blood Cells Activate Neutrophils and Cause Lung Injury after Hemorrhage and Resuscitation. J Am Coll Surg. 2012;214:648–657. doi: 10.1016/j.jamcollsurg.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim-Shapiro DB, Lee J, Gladwin MT. Storage lesion. Role of red cell breakdown. Transfusion. 2011;51(4):844–851. doi: 10.1111/j.1537-2995.2011.03100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almizraq R, Tchir JDR, Holovati JL, Acker JP. Storage of red blood cells affects membrane composition, microvesiculation, and in vitro quality. Transfusion. 2013;53:2258–2267. doi: 10.1111/trf.12080. [DOI] [PubMed] [Google Scholar]
- 9.Mallat Z, Hugel B, Ohan J, Leseche G, Freyssinet J, Tedgui A. Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques: A role for apoptosis in plaque thrombogenicity. Circulation. 1999;99:348–353. doi: 10.1161/01.cir.99.3.348. [DOI] [PubMed] [Google Scholar]
- 10.Thaler CA, Mackman N, Bertina M, Kaider A, Marosi C, Key S, Barcel DA, Scheithauer W, Kornek G, Zielinski C, Pabinger I. Microparticle-associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients. J Thromb Haemost. 2012;10:1363–70. doi: 10.1111/j.1538-7836.2012.04754.x. [DOI] [PubMed] [Google Scholar]
- 11.Van Doormal FF, Kleinjan A, Berckmans RJ, Mackman N, Manly D, Kamphulsen PW, Rchel DJ, Buller HR, Sturk A, Nieuwland R. Coagulation activation and microparticle-associated coagulant activity in cancer patients. Thromb Haemost. 2012;108:160–165. doi: 10.1160/TH12-02-0099. [DOI] [PubMed] [Google Scholar]
- 12.Bucciarelli P, Martinelli I, Artoni A, Passamonti M, Previtali E, Merati G, Tripodi A, Mannuccio Mannucci P. Circulating microparticles and risk of venous thromboembolism. Thrombosis Research. 2012;129:591–597. doi: 10.1016/j.thromres.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 13.Tinmouth A, Chin-Yee L. The clinical consequence of the red cell storage lesion. Transfus Med Rev. 2001;15:91–107. doi: 10.1053/tmrv.2001.22613. [DOI] [PubMed] [Google Scholar]
- 14.Chung SM, Bae ON, Lim KM, Noh JY, Lee MY, Jung YS, Chung JH. Lysophosphatidic acid induces thrombogenic activity through phosphatidylserine exposure and procoagulant microvesicle generation in human erythrocytes. Arterioscler Thromb Vasc Biol. 2007;27:414–21. doi: 10.1161/01.ATV.0000252898.48084.6a. [DOI] [PubMed] [Google Scholar]
- 15.Andrews DA, Low PS. Role of red blood cells in thrombosis. Curr Opin Hematol. 1999;6:76–82. doi: 10.1097/00062752-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Rubin O, Delobel J, Prudent M, Lion N, Kohl K, Tucker E, Tissot J, Angelillo-Scherrer A. Red Blood cell-derived microparticles isolated from blood units initiate and propagate thrombin generation. Transfusion. 2013;53:1744–1754. doi: 10.1111/trf.12008. [DOI] [PubMed] [Google Scholar]
- 17.Gao Y, Lv L, LIU S, Su M. Elevated levels of thrombin-generating microparticles in stored red blood cells. Vox Sanguinis. 2013;105:11–17. doi: 10.1111/vox.12014. [DOI] [PubMed] [Google Scholar]
- 18.Park MS, Owen BA, Ballinger BA, Sarr MG, Schiller HJ, Zietlow SP, Jenkins DH, Ereth MH, Owen WG, Heit JA. Quantification of hypercoagulable state after blunt trauma: Microparticle and thrombin generation are increased relative to injury severity, while standard markers are not. Surgery. 2012;151:831–6. doi: 10.1016/j.surg.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ayers L, Kohler M, Harrison P, et al. Measurement of Circulating Cell-derived Microparticles by Flow Cytometry: Sources of Variability Within the Assay. Thromb Res. 201;127:370–377. doi: 10.1016/j.thromres.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Jayachandran M, Litwillwe RD, Owen WG, Heit JA, Behrenbeck T, Mulvagh SL, Araoz PA, Budoff MJ, Harman SM, Miller VM. Characterization of blood borne microparticles as markers of premature coronary calcification in newly menopausal women. Am J Physiol Heart Circ. 2008;295:H931–8. doi: 10.1152/ajpheart.00193.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Straten AH, Soliman Hamad MA, van Zundert AA, Martens EJ, ter Woorst JF, de Wold AM, Scharnhorst V. Effect of duration of red blood cell storage on early and late mortality after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2011;141:231–7. doi: 10.1016/j.jtcvs.2010.02.059. [DOI] [PubMed] [Google Scholar]
- 22.Robinson SD, Janssen C, Fretz EB, Berry B, Chase AJ, Siega AD, Carere RG, Fung A, Simkus G, Klinke WP, Hilton JD. Red blood cell storage duration and mortality in patients undergoing percutaneous coronary intervention. Am Heart J. 2010;159:876–81. doi: 10.1016/j.ahj.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 23.van de watering l. Red cell storage and prognosis. Vox Sang. 2011;100:36–45. doi: 10.1111/j.1423-0410.2010.01441.x. [DOI] [PubMed] [Google Scholar]
- 24.Zimrin AB, Hess JR. Current issues relating to the transfusion of stored red blood cells. Vox Sang. 2009;96:93–103. doi: 10.1111/j.1423-0410.2008.01117.x. [DOI] [PubMed] [Google Scholar]
- 25.Gauvin F, Spinella PC, Lacroix J, Chocker G, Ducruet T, Karam O, Hebert PC, Hutchison JS, Hume HA, Tucci M Canadian Critical Care Trials Group and the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. Association between length of storage of transfused red blood cells and multiple organ dysfunction syndrome in pediatric intensive care patients. Transfusion. 2010;50:1902–12. doi: 10.1111/j.1537-2995.2010.02661.x. [DOI] [PubMed] [Google Scholar]
- 26.Karam O, Tucci M, Bateman ST, Ducruet T, Spinella PC, Randolph AG, Lacroix K. Association between length of storage of red blood cell units and outcome of critically ill children; a prospective observational study. Crit Care. 2010;14:R57. doi: 10.1186/cc8953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keating FK, Butenas S, Fung MK, Schneider DJ. Platelet-white blood cell (WBC) interaction, WBC apoptosis, and procoagulant activity in stored red blood cells. Transfusion. 2011;51:1086–1095. doi: 10.1111/j.1537-2995.2010.02950.x. [DOI] [PubMed] [Google Scholar]
- 28.Sugawara A, Nollet KE, Yajima K, Saito S, Ohto H. Preventing platelet-derived microparticle formation and possible side effects with prestorage leukofiltration of whole blood. Arch Pathol Lab Med. 2010;134:771–775. doi: 10.5858/134.5.771. [DOI] [PubMed] [Google Scholar]