Abstract
Joint instability creates a clinical and economic burden in the health care system. Injuries and disorders that directly damage the joint structure or lead to joint instability are highly associated with osteoarthritis (OA). Thus, understanding the physiology of joint stability and the mechanisms of joint instability-induced OA is of clinical significance. The first section of this review discusses the structure and function of major joint tissues, including periarticular muscles, which play a significant role in joint stability. Because the knee, ankle, and shoulder joints demonstrate a high incidence of ligament injury and joint instability, the second section summarizes the mechanisms of ligament injury-associated joint instability of these joints. The final section highlights the recent advances in the understanding of the mechanical and biological mechanisms of joint instability-induced OA. These advances may lead to new opportunities for clinical intervention in the prevention and early treatment of OA.
Keywords: joint stability, joint instability, joint injury, ligament injury, osteoarthritis
Introduction
Osteoarthritis (OA) is the most common form of joint disease that is characterized by loss of articular cartilage, remodeling of subchondral bone, osteophyte formation, ligamentous laxity, weakening of periarticular muscles, and thickening of the joint capsule.1,2 It is thought to be the most prevalent of all musculoskeletal pathologies, affecting an estimated 10% of the world’s population over the age of 60 years.1 As a disease that is highly correlated with aging, its prevalence is expected to continue to rise as global life expectancy continues to increase. OA is a progressive, and often debilitating, disease that creates a profound societal and economic burden and carries significant physical and psychological consequences for the affected individual.2
A significant association between previous joint injury and resultant joint instability with the development of subsequent OA has been clearly established in the literature.3–6 This disease process is commonly referred to as posttraumatic osteoarthritis (PTOA) and its occurrence is most prevalent in highly active populations such as elite athletes and military personnel.7,8 It is estimated that more than 40% of individuals who sustain significant ligamentous, meniscal, or articular surface injuries will develop PTOA3 Correlatively, approximately 12% of the overall prevalence of lower extremity OA is attributable to previous trauma.4 This review will explore in detail the structures that compose synovial joints, injury to these structures, resultant joint instability, and its association with OA.
Definition and Structure of Major Joint Tissues
Articular cartilage
Articular cartilage is a highly specialized viscoelastic connective tissue that is found overlying the bone ends in synovial joints.9,10 Injury to articular cartilage presents a very difficult challenge for both patients and health care providers. The Scottish physician William Hunter famously stated in his address to the Royal Society in 1743, “From Hippocrates to the present age, it is universally allowed that ulcerated cartilage is a troublesome thing that, once destroyed, is not repaired.”11
The major components of articular cartilage include water, extracellular matrix (ECM), and chondrocytes. Up to 80% of the wet weight of articular cartilage is supplied by water. The flow of water through the articular cartilage plays a pivotal role in providing nutrients by diffusion to the chondrocytes and assists in the lubrication of the joint surface.9 The ECM provides resistance to the flow of water. The ECM accounts for approximately 95% of the dry weight of articular cartilage.10 The primary macromolecule found in articular cartilage is collagen, with type II collagen representing 90%–95% of the total collagen content and types I, IV, V, VI, IX, and XI accounting for the remainder.9 The second largest group of macromolecules present is proteoglycans, which consist of a protein core with covalently linked glycosaminoglycan chains. The main proteoglycans found in articular cartilage include aggrecan, decorin, biglycan, and fibromodulin, with aggrecan being the most abundant.9 Negatively charged carboxyl and sulfate groups found on these glycosaminoglycan chains, namely keratin sulfate and chondroitin sulfate, have a high affinity for water. The primary cellular component of articular cartilage is the chondrocyte. These cells are largely anaerobic, have limited cell-to-cell contact and have a low potential for replication. These characteristics are responsible for the limited healing potential of articular cartilage.
Articular cartilage is a highly specialized and structured tissue that is devoid of blood vessels, nerves, or lymphatics. Its structure can be divided into four zones, each with unique properties. The superficial tangential zone contains a layer of tightly packed collagen fibers (primarily types I and IX) oriented parallel to the articular surface known as “lamina splendens,” as well as a layer of flattened chondrocytes.10 This zone serves to protect the underlying zones and provides resistance to shear forces. The middle zone is a transitional layer composed of proteoglycans and thick, obliquely oriented collagen fibrils. The chondrocytes in this zone are more spherical and less abundant. The oblique orientation of the collagen fibrils marks a transition from a resistance to shear forces to resistance to compressive forces. The deep zone contains collagen fibrils and chondrocytes aligned perpendicular to the joint surface. This arrangement allows for the greatest resistance to compressive forces. The highest proteoglycan content and lowest water concentration are found in this zone.9 The calcified zone is separated from the deep zone by the tidemark. The primary role of the calcified zone is to firmly secure articular cartilage to the subchondral bone. This highly organized structure is responsible for the unique mechanical properties of articular cartilage.
The essential functions of articular cartilage are to provide a smooth, lubricated surface for low-friction articulation and to facilitate the transmission of loads to the underlying subchondral bone.9 It possesses a distinct capacity to withstand high cyclic loads. The high water content and low permeability of articular cartilage allow for efficient load transmission, while the electrostatic forces between water and proteoglycans provide resistance to compression.
Synovium
The synovium lines the joint cavity, producing synovial fluid that lubricates the joint surfaces and provides nutrition to the articular cartilage.12 It is composed of two distinct layers, the synovial lining (or intima) and the subintimal stroma. The synovial intima lies between the subintimal stroma and the synovial cavity. It is composed of highly specialized mesenchymal cells and ECM. Two distinct synoviocytes have been identified in the synovial intima. Type A synoviocytes are phagocytic cells derived from macrophages that are responsible for clearing particulate matter from the joint cavity. Type B synoviocytes are fibroblast-derived cells responsible for the synthesis of hyaluronon and lubrican, two important components of synovial fluid. The subintimal stroma lies between the synovial intima and the joint capsule. It is composed of loose connective tissue, with the primary cell types being fibroblasts and macrophages. Blood vessels, lymphatics, and nerves are all found within this subintimal layer.
Joint capsule
The joint capsule is essential for the proper functioning of synovial joints. It forms the seal that contains the synovial fluid within the joint, imparts passive stability by limiting joint movement, and provides active stability via its proprioceptive nerve endings.13 It is composed of collagen fibers that are firmly adhered to bone through a fibrocartilaginous attachment. Localized thickenings of the capsule form capsular ligaments that provide strong points of fixation to bone. Tendons commonly attach to the joint capsule and occasionally replace it, as is the case with the quadriceps and patellar tendons in the anterior knee. Blood vessels and nerves pass through the joint capsule, supplying it and the underlying synovium. Nerve endings found in the joint capsule are thought to be proprioceptive and play an important role in active protection of the capsule and associated ligaments by reflex control of the appropriate musculature.13
Tendon
Tendons are dense, regularly arranged connective tissues that serve as functional and anatomic bridges between muscle and bone.12,14 The primary function of tendons is to transfer force from muscle to bone. They consist of an abundant ECM and relatively few cells.14 The primary cell found in tendon is the tenocyte, a fibroblast-type cell responsible for the synthesis of the matrix components. The primary component of the ECM is collagen (primarily type I), with smaller amounts of elastin, ground substance, and water being present. These collagen fibers are aligned parallel to the tendon long axis, giving tendons one of the highest tensile strengths among all soft tissues. Elastin is found in very small quantities, accounting for <1% of the dry weight of tendons. This small quantity allows for large changes in connective tissue geometry with relatively little energy expenditure.14
The structural organization of tendons begins with collagen fibrils and elastin fibers bound together by the surrounding endotenon. The endotenon is a thin layer of connective tissue that contains blood vessels, nerves, and lymphatics. This layer is continuous with the epitenon, a synovial-like membrane that envelops the tendon.14 In some tendons, this epitenon is covered by loose areolar tissue known as the paratenon, whereas in other tendons, this paratenon is replaced by a true synovial sheath known as the tenosynovium. The epitenon combined with the paratenon is known as the peritenon. At the tendon– bone interface, the endotenon becomes continuous with the periosteum.14 There are two different types of tendon attachments to bone. The first type is known as direct insertion and involves tendon insertion through fibrocartilage, then mineralized fibrocartilage, and finally into bone.15 The second type of insertion is known as indirect insertion and involves attachment of the superficial fibrils to the periosteum and attachment of deeper fibrils directly onto bone.
While the primary function of tendons is the transmission of force from muscle to bone, several recent studies have demonstrated the importance of certain periarticular tendons in joint stability. Alexander et al.16 showed that loading the long head of the biceps tendon significantly decreased humeral head translation in a cadaveric model. In another cadaveric model, Ziai et al.17 found that the peroneus longus tendon had a substantial effect on the passive stability of the ankle joint in the setting of lateral ligament injury. Furthermore, Giles et al.18 demonstrated increased glenohumeral stiffness following loading of the conjoined tendon of the short head of the biceps and coracobrachialis. These studies highlight the role of certain periarticular tendons in joint stability.
Ligament
Ligaments can be defined as dense bands of collagenous fibers that span a joint and are anchored to bone at either end.19 The primary function of ligaments is to provide passive stability to a joint through a normal range of motions under an applied load. Bundles of collagen fibrils form the majority of the ligament substance.14 These fibrils are typically aligned in the direction of tension applied to the ligament during normal joint motion. On a microscopic level, these fibrils are noted to have a wave or crimp pattern, which allows for slight elongation of the ligament under physiologic loads without failure. This is in contrast to the collagen organization seen in tendons, which have a lower propensity to stretch. In addition, ligaments typically have a higher ratio of elastin, larger quantities of reducible cross-links, more type III collagen, less total collagen, and a higher glycosaminoglycan content when compared to tendons.12
Fibroblasts are the primary cell type found in ligaments; endothelial cells of small vessels and nerve cells are also present.14 These spindle-shaped fibroblastic cells extend between the collagen fibrils and are responsible for synthesizing and maintaining the ECM. Collagen is the major matrix component accounting for 70%–80% of the dry weight of the ligament. Type I collagen accounts for approximately 90% of the total collagen and type III for approximately 10%, while other types are present in very small amounts. Although proteoglycans account for <1% of the dry weight of ligaments, they play an important role in organizing the ECM and interacting with tissue fluid.14 This organization and fluid interaction contribute to the mechanical properties of ligaments and allow them to perform their function.
The ligament–bone interface is a complex structure that has been described as two distinct insertion types: direct and indirect. Direct insertion involves passage of a ligament directly into cortical bone. The superficial ligament collagen fibers merge with the fibrous layer of the periosteum, while the majority of the insertion consists of deeper fibers directly penetrating the cortex.14 These deep fibers pass through ligament substance, fibrocartilage, mineralized fibrocartilage, and finally into bone. This direct insertion typically occurs at a right angle to the bone. In contrast, indirect insertions typically occur more obliquely. This type of insertion is less common and usually involves a wide surface area of insertion along the bone surface as opposed to insertion directly into the cortex.14 These insertions are believed to allow gradual transmission of force between ligament and bone.12
Meniscus
Menisci are fibrocartilaginous, wedge-shaped structures that are most developed in the knee but are also found in the acromioclavicular, sternoclavicular, and temporomandibular joints.12 Though previously thought of as an unnecessary appendage, the menisci of the knee are now recognized as integral components of the knee joint. They have been shown to play important roles in joint stability, load distribution, shock absorption, and lubrication.12 Following meniscectomy, the tibiofemoral contact area decreases by 50%–70%, resulting in increased contact stresses and, ultimately, degenerative changes.20 When a compressive load is applied to a meniscus, fluid flows out of the meniscus and into the joint space lubricating the joint surfaces. This also distributes synovial fluid throughout the joint, which provides nutrition to articular cartilage.20
The adult meniscus is approximately 72% water by wet weight and 28% organic material (ECM and cells).21 Collagen types I, II, III, IV, VI, and XVIII make up the majority of the organic component, with the remainder represented by glycosaminoglycans, glycoproteins, and elastin. The composition, structure, and vascularity of the menisci vary with location and age. During prenatal development, the entire meniscus is well vascularized and highly cellular. With aging, the peripheral portion of the meniscus remains well vascularized. The ECM in this zone is composed predominantly of circumferentially oriented type I collagen fibrils. In contrast, the inner portion of the meniscus becomes avascular, with a predominance of radially oriented type II collagen. In the young individual, decorin is the predominant proteoglycan synthesized in the menisci. The relative proportion of aggrecan synthesized increases with age and eventually becomes more abundant than decorin.12
There is some controversy in the literature regarding the cell types found within menisci. In general, there appear to be three distinct cell types present.12,21 Cells found in the outer portion of the meniscus are typically fusiform, with branch-like cytoplasmic extensions allowing for communication with other cells and the ECM. Due to their similar appearance, these cells are often referred to as fibroblast-like cells. In contrast, cells in the inner portion of the meniscus are round and embedded in an abundant ECM similar to articular cartilage. As such, these cells are referred to as fibrochondrocytes or chondrocyte-like cells. The final cell type is found in the superficial meniscus and is fusiform in shape without cytoplasmic extensions. It is postulated that these cells are progenitor cells with some regenerative potential.
Subchondral bone
Subchondral bone, as its name implies, is the bone found immediately below the articular cartilage. The primary function of the subchondral bone is to attenuate the forces on the joints by transferring load from articular cartilage to bone. It is composed of a superficial layer of compact cortical bone and an underlying layer of cancellous trabecular bone.12 A thin layer of calcified cartilage separates the subchondral bone from the overlying articular cartilage. Immediately beneath this calcified cartilage is the subchondral bone plate or cortical end plate. This end plate forms an irregular surface in which cartilage is “keyed,” allowing for transformation of shear forces into tensile and compressive forces.22 Below the end plate, the subchondral bone is trabecular in nature and highly vascular.
Periarticular muscle
Contraction of periarticular muscles has a significant effect on joint stability. Depending on the direction of the summation of forces generated by an individual muscle across a joint, it may act to stabilize or destabilize the joint.23 Generally, dynamic joint stability is achieved by the coordinated contraction and relaxation of multiple muscle groups. For instance, the highly mobile and relatively unstable glenohumeral joint of the shoulder achieves dynamic stability through the forces exerted across the joint by the various rotator cuff muscles. Each of the four rotator cuff muscles has a distinct direction of pull. As such, the force exerted by each muscle as the shoulder is taken through a physiologic range of motion must be coordinated to achieve stability. Periarticular muscles can contribute to joint stability when the summation of the magnitude and direction of their forces creates a centrally located joint force.23
Ligament Injury-Associated Joint Instability
Joint instability following ligament damage creates a clinical and economic burden in the health care system, as well as a physical and emotional burden for affected patients. The knee, ankle, and shoulder joints demonstrate a high incidence of injury and instability. To understand the causes and effects of injuries to these joints, we focus on joint structure, incidence of injury, and major injury risk factors.
Knee
The knee is a complex weight-bearing joint that connects the bones of the upper and lower leg and allows for flexion, extension, and some rotational movement. Anatomically, it is the largest synovial joint in the human body. Ligaments are crucial for the stability of the knee joint because they provide mechanical reinforcement and control the range of motion. Damage to ligaments is the most common form of knee injury.24 The four major stabilizing ligaments of the knee are the medial collateral ligament (MCL), lateral collateral ligament (LCL), anterior cruciate ligament (ACL), and posterior cruciate ligament (PCL). The MCL and LCL are located on the medial and lateral sides of the joint, connecting the femur to the tibia and fibula, respectively. These ligaments stabilize the knee by preventing side-to-side movement. The ACL and PCL are situated in the middle of the joint and attach the tibia to the femur. The two ligaments anchor the tibia and the femur by preventing the bones from anterior or posterior sliding during movement.25 With these ligaments providing stability to the joint, the risk for knee injury is relatively low during normal movement. However, studies of adolescents and young adults show that physical activity, especially participation in sports or exercise, is a major risk factor for knee injuries.24,26,27 Understanding knee injury risk will allow for better design of injury reduction programs.
As greater emphasis is placed on exercise and physical activity to promote health in both adolescents and adults, the burden caused by knee injuries will continue to mount. A 10-year study estimated that >650,000 patients annually present with knee injuries to emergency departments in the United States, accounting for a rate of injury of 2.29 per 1,000 people across all age groups. Furthermore, the true incidence of knee injury is probably higher when considering the number of patients who seek treatment outside of emergency settings. Of these knee injuries, 42% are the result of sprains to ligaments, by far the most common type of knee injury.24 Differences in age, gender, and cause of injury have been examined in relation to ligament injury, most often focusing on sports-related injuries in younger individuals. A study on an adolescent population looked specifically at cruciate ligament injury and found an incidence of 5.7 per 1,000 people over a 9-year follow-up. For subjects who participated in organized sports, females were 8.5 times and males 4.0 times more likely to experience cruciate ligament injury than nonparticipating individuals.27 A meta-analysis of ACL tear studies revealed that female soccer and basketball participants are three times more susceptible to injury than male counterparts. However, some sports such as lacrosse and downhill skiing demonstrate no gender difference.26 Training programs that have shown success in preventing injuries, such as neuromuscular training for female athletes, should be increasingly implemented and continually examined for best practices.28,29
Ankle
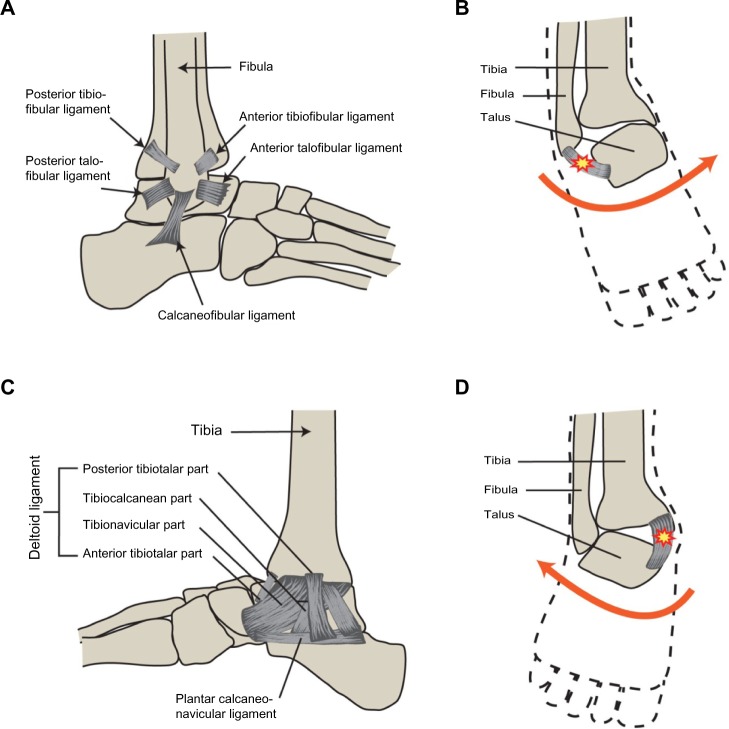
The ankle is the second joint that demonstrates a high susceptibility to injury. It is a synovial joint that connects the tibia and fibula bones of the leg to the talus of the foot, allowing for dorsal and plantar flexion. The subtalar joint is located just beneath the ankle. It connects the talus bone to the calcaneus and allows for side-to-side movement of the foot. The ligaments of the ankle involved in joint stability are the deltoid, anterior talofibular ligament (ATFL), posterior talofibular ligament (PTFL), and calcaneofibular ligament (CFL). They help control the ankle’s range of motion, with the deltoid providing medial stability and the ATFL, PTFL, and CFL ensuring lateral stability. When the ligaments of the ankle are damaged, the potential for recurrent injury is high. A severe injury of major ligaments of the ankle may cause instability of the joint (Fig. 1).
Figure 1.
(A) A representation of primary lateral ligaments of the ankle and the tibiofibular ligaments. (B) A typical inversion injury of the ankle that leads to damage of the lateral ankle ligaments. (C) The deltoid ligament, which is the primary medial ankle ligament complex. (D) A typical eversion injury of the ankle, which results in damage to the medial ligaments of the ankle.
Similar to knee injuries, ankle injuries often occur during participation in sports or exercise; consequently, populations of athletes are often used in incidence studies. For example, ankle injuries are estimated to account for 14% of all athletic injuries,30 with sprains to ankle ligaments accounting for >75% of ankle injuries.31,32 The ATFL is the most commonly injured ankle ligament, involved in an estimated 85% of sprains sustained during high school sports in the United States.33 A major problem accompanying ankle injury is the high rate of recurrence associated with chronic ankle instability. Approximately 15% of all ankle sprains occur in ankles with previous ligament injury.33 Further characterization of patients with chronic ankle instability by specific impairment, activity limitations, and participation restrictions could help in the design of both targeted treatments and injury reduction programs.34
Shoulder
The major joint of the shoulder is the ball glenohumeral joint. This synovial joint consists of the connection between the humerus and the lateral scapula. In addition to ligaments, the articular capsule is stabilized by the muscles of the rotator cuff, surrounding tendons, and the cartilaginous glenoid labrum. One of the stabilizing tendons is the long head of the biceps that travels inside the joint cavity and coordinates movement between the shoulder and the elbow. Structurally, the shallow glenoid cavity of the scapula acts as the shoulder socket and allows considerable rotational movement. Shoulder ligaments, including the coracohumeral, transverse humeral, and three glenohumeral ligaments, act mainly to control the range of motion of the joint. Overall, the arrangement of the articular capsule is remarkably loose, allowing large separation between the bones of the joint, which also contributes to the large freedom of motion.25 The stability of the shoulder is low as a result of the significant mobility of the joint. Therefore, a high incidence of injury is observed in the shoulder.
By understanding shoulder instability and injury risk factors, better injury reduction strategies can be developed for at-risk populations. In the United States, the estimated incidence of shoulder dislocation is 23.9 per 100,000 person-years.35 Risk factors for shoulder injury include athletic participation, male gender, and young or old age.35,36 In prospective cohort studies of young military populations, 3%–6% sustained shoulder dislocations or partial-dislocations.37,38 The recurrence of shoulder injury is a significant problem. Studies of young and adult patients have evaluated the chance of recurrent shoulder instability after standard nonoperative treatment as 55%–67%, with a young male population presenting recurring injuries at an 87% rate during a 5-year follow-up.37,39 To combat the high incidence of recurrence, arthroscopic or open repair surgeries have been shown to be effective. Randomized clinical trials demonstrate that surgical stabilization of the shoulder is more effective at preventing recurrence of injury than immobilization and rehabilitation alone.40–42 Identifying specific structural risks associated with shoulder instability is another way to combat recurrence. It has been shown in a population of professional rugby players that shoulder translation or anterior laxity is associated with increased risk of dislocation.43 Additionally, baseline testing for shoulder instability, strength, range of motion, and structural measurements demonstrate that the most significant structural risk for posterior shoulder injury is increased glenoid retroversion.38 Screening processes and targeted injury prevention strategies should continue to be developed using identifiers for individuals at risk for shoulder injury.
Joint Instability-Induced OA
Many types of joint injury increase the risk of developing OA, including injuries associated with joint instability such as ligament tears and dislocations.44 Currently, effective treatments to prevent or stop the progression of OA are limited by our lack of mechanistic knowledge. Joint replacement or joint fusion is often the treatment used as a last resort for late-stage OA. Understanding the mechanical and biological mechanisms underlying the progression of OA may lead to new opportunities for clinical intervention.
Injuries that directly damage articular cartilage or lead to joint instability are highly associated with PTOA.45 However, the mechanisms that underlie the progression of PTOA as a result of injury-induced mechanical changes are not fully understood. Abnormal loading such as changes in contact stress, distribution, and directional gradients have been studied in association with PTOA. It has been demonstrated in a study of human cadaveric ankles that joint instability significantly increased contact stress directional gradients in conjunction with articular surface incongruity.46 Additionally, joint mechanics in subjects with OA and self-reported instability have been compared to the same in stable OA patients and non-OA patients. It was shown that instability is associated with altered knee joint movement patterns and contact mechanics.47 These studies suggest that joint instability-associated articular surface incongruity may lead to altered joint contact stresses, displaying a greater area with high contact stress exposure than intact joints. This may cause articular cartilage damage.
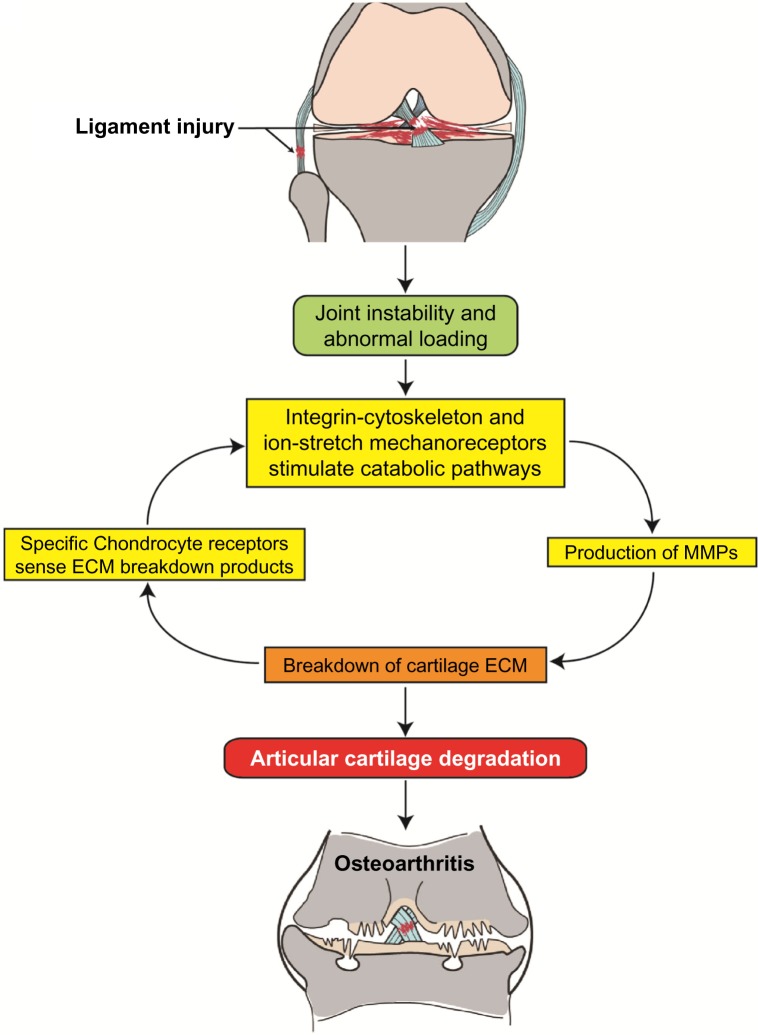
Joint instability may result in abnormal mechanical loading on the affected joint and subsequent disruption of the ECM. ECM damage may lead to release of glycosylated aminoglycans and collagen molecules, which are sensed by the surrounding chondrocytes through mechanoreceptors and cell surface receptors.48–50 This leads to changes in gene expression and cartilage metabolism, including increased expression of catabolic factors (eg, matrix-degrading enzymes) and decreased expression of cartilage structural proteins (eg, type-II collagen and aggrecan), which could set up a cascade of events leading to degradation of the articular cartilage.51 Although much remains to be elucidated about how chondrocytes sense strain and damage to the matrix around them, it is clear that abnormal mechanical stimulations may cause dysfunction of articular chondrocytes and breakdown of cartilage ECM, leading to articular cartilage degradation. In addition, abnormal loading can lead to chondrocyte death mediated by oxidative stress52,53 or integrin–cytoskeleton interactions.54 Chondrocyte death robs cartilage of the ability to produce and maintain its ECM. If articular cartilage lesions are not successfully repaired, the affected joint will progress to PTOA. The possible mechanisms that trigger the progression to PTOA are illustrated in Figure 2.
Figure 2.
A diagram shows ligament injury-induced knee joint instability and abnormal mechanical loading, which activates chondrocyte mechanoreceptors and catabolic pathways, leading to articular cartilage degradation through a mechanoreceptor–MMP–ECM breakdown cycle. Failed repair of damaged joint tissues results in the progression of PTOA.
There are a multitude of options available for the prevention and treatment of joint instability-induced PTOA, including both nonsurgical and surgical options (Table 1). Nonsurgical options include, but are not limited to, bracing/splinting, physical therapy, activity modification, prolotherapy, and anti-inflammatory medication. The primary goals of these therapies are to increase joint stability, improve function, reduce pain, and prevent progression of OA. Bracing or splinting may provide external stability to the unstable joint and prevent recurrent joint injury. Physical therapy may restore joint stability by strengthening periarticular muscles and increasing proprioceptive awareness. Similarly, low-impact exercises such as swimming and cycling may strengthen periarticular muscles while limiting the forces across the joint. Prolotherapy, a regenerative injection therapy, may relieve joint pain and enhance the healing of ligaments and tendons.55 Anti-inflammatory medicines such as nonsteroidal anti-inflammatory drugs and intra-articular steroid injections may be useful in symptomatic treatment; however, these therapies have not been shown to slow the natural progression of PTOA and may have deteriorative effects on articular cartilage and other joint tissues.56,57
Table 1.
Treatment options for joint instability-associated OA.
| NONSURGICAL | SURGICAL |
|---|---|
| Bracing/splinting | Ligament repair/reconstruction |
| Physical therapy | Meniscus repair |
| Activity modification | Labrum repair |
| Anti-inflammatory medicines | Corrective osteotomy |
| Intra-articular injection | Joint arthroplasty (replacement) |
| Prolotherapy | Joint arthrodesis (fusion) |
When conservative management fails, there are many surgical options for the treatment and prevention of PTOA. These include, but are not limited to, ligament repair/reconstruction, meniscus/labrum repair, corrective osteotomy, joint arthroplasty (replacement), and joint arthrodesis (fusion). Primary repair or reconstruction of a torn ligament is often implemented to restore stability to a joint and, in theory, prevent recurrent injury to the articular cartilage. However, the effectiveness of ligamentous repair or reconstruction at limiting the progression of PTOA is not well supported. For instance, while ACL reconstruction has been shown to significantly improve patient satisfaction, symptoms, function, activity level, and stability, it has not been shown to decrease the rate of development of OA.58,59 Similarly, meniscal and labral repairs are often performed to reduce pain and instability about the knee and shoulder, respectively. Meniscal repair in particular has been shown to be successful in reducing pain and restoring function, with one study reporting 91% good/excellent overall clinical results.60 Despite these promising results, the ability of meniscal repair to alter the progression of PTOA has not been borne out in the literature.
While early surgical intervention plays an important role in reducing pain and instability, disease progression often necessitates more invasive procedures. Joint malalignment resulting from trauma can increase the rate of progression of PTOA by increasing intra-articular stress. A corrective osteotomy can be used under the right circumstances to correct malalignment and reduce joint forces. Favorable outcomes following this procedure have been shown for multiple joints, including the knee, ankle, and wrist.61–63 Reduction of intra-articular stress (eg, joint distraction) has been performed for decades and remains a reasonable treatment for patients in whom PTOA is initiated from mechanical factors with increased intra-articular stress.64
Once severe joint destruction has developed, joint replacement (arthroplasty) or joint fusion (arthrodesis) becomes the primary surgical option. Total joint arthroplasty is a very effective treatment option for end-stage OA. While total knee arthoplasty (TKA) for PTOA has been shown to significantly improve pain and function, the complication rate is higher than that of TKA performed for idiopathic OA.65 While arthroplasty is a well-established treatment option for OA of the hip and knee, total ankle arthroplasty has only recently been utilized. More commonly, PTOA of the ankle has traditionally been treated with arthrodesis. This has been shown to consistently reduce pain and improve patient satisfaction, with one recent meta-analysis reporting 68% patients achieving good/excellent results.66 However, joint arthrodesis will inevitably reduce motion and increase stress across adjoining joints. Given the long-term implications of these treatment options and the fact that PTOA often affects younger patients for whom total joint replacement or arthrodesis is not a desirable treatment, emphasis should be placed on developing more effective strategies for management of joint instability and prevention of PTOA.
Acknowledgments
The authors thank Mr. Brian Egan for his graphic and editorial assistance.
Footnotes
Author Contributions
Conceived and designed the experiments: JW, DB, AM. Analyzed the data: DB, AM, MT, JW. Wrote the first draft of the manuscript: DB, AM. Contributed to the writing of the manuscript: DB, AM, MT, JW. Agree with manuscript results and conclusions: DB, AM, MT, JW. Jointly developed the structure and arguments for the paper: JW, DB, AM, MT. Made critical revisions and approved final version: DB, JW, AM, MT. All authors reviewed and approved of the final manuscript.
FUNDING: This work was supported in part by the U.S. National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases grant R01 AR059088 (to J. Wang), Department of Orthopedic Surgery at the University of Kansas Medical Center, the U.S. Department of Defense medical research grant W81XWH-12–1-0304 (to J. Wang), the Mary A. and Paul R. Harrington Distinguished Professorship Endowment (to J. Wang), and the Asher Orthopedic Research Endowment. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
ACADEMIC EDITOR: Chuanju Liu, Editor in Chief
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE). Published by Libertas Academica. Learn more about this journal.
REFERENCES
- 1.Pereira D, Peleteiro B, Araujo J, Branco J, Santos RA, Ramos E. The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthritis Cartilage. 2011;19(11):1270–85. doi: 10.1016/j.joca.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:185–99. doi: 10.1093/bmb/lds038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson DD, Chubinskaya S, Guilak F, et al. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res. 2011;29(6):802–9. doi: 10.1002/jor.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20(10):739–44. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 5.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756–69. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 6.Onur TS, Wu R, Chu S, Chang W, Kim HT, Dang AB. Joint instability and cartilage compression in a mouse model of posttraumatic osteoarthritis. J Orthop Res. 2014;32(2):318–23. doi: 10.1002/jor.22509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivera JC, Wenke JC, Buckwalter JA, Ficke JR, Johnson AE. Posttraumatic osteoarthritis caused by battlefield injuries: the primary source of disability in warriors. J Am Acad Orthop Surg. 2012;20(suppl 1):S64–9. doi: 10.5435/JAAOS-20-08-S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuijt MT, Inklaar H, Gouttebarge V, Frings-Dresen MH. Knee and ankle osteoarthritis in former elite soccer players: a systematic review of the recent literature. J Sci Med Sport. 2012;15(6):480–7. doi: 10.1016/j.jsams.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1(6):461–8. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33(2):295–306. doi: 10.1177/0363546504273510. [DOI] [PubMed] [Google Scholar]
- 11.Hunter W. Of the structure and disease of articulating cartilages. 1743. Clin Orthop Relat Res. 1995;(317):3–6. [PubMed] [Google Scholar]
- 12.Firestein GS, Kelley WN. Kelley’s Textbook of Rheumatology. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2013. [Google Scholar]
- 13.Ralphs JR, Benjamin M. The joint capsule: structure, composition, ageing and disease. J Anat. 1994;184(pt 3):503–9. [PMC free article] [PubMed] [Google Scholar]
- 14.DeLee J, Drez D, Miller MD. DeLee and Drez’s Orthopaedic Sports Medicine: Principles and Practice. 3rd ed. Philadelphia: Saunders/Elsevier; 2010. [Google Scholar]
- 15.Cooper RR, Misol S. Tendon and ligament insertion. A light and electron microscopic study. J Bone Joint Surg Am. 1970;52(1):1–20. [PubMed] [Google Scholar]
- 16.Alexander S, Southgate DF, Bull AM, Wallace AL. The role of negative intraarticular pressure and the long head of biceps tendon on passive stability of the glenohumeral joint. J Shoulder Elbow Surg. 2013;22(1):94–101. doi: 10.1016/j.jse.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Ziai P, Benca E, von Skrbensky G, et al. The role of the peroneal tendons in passive stabilisation of the ankle joint: an in vitro study. Knee Surg Sports Traumatol Arthrosc. 2013;21(6):1404–8. doi: 10.1007/s00167-012-2273-2. [DOI] [PubMed] [Google Scholar]
- 18.Giles JW, Boons HW, Ferreira LM, Johnson JA, Athwal GS. The effect of the conjoined tendon of the short head of the biceps and coracobrachialis on shoulder stability and kinematics during in-vitro simulation. J Biomech. 2011;44(6):1192–5. doi: 10.1016/j.jbiomech.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Frank CB. Ligament structure, physiology and function. J Musculoskelet Neuronal Interact. 2004;4(2):199–201. [PubMed] [Google Scholar]
- 20.Rath E, Richmond JC. The menisci: basic science and advances in treatment. Br J Sports Med. 2000;34(4):252–7. doi: 10.1136/bjsm.34.4.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makris EA, Hadidi P, Athanasiou KA. The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32(30):7411–31. doi: 10.1016/j.biomaterials.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imhof H, Sulzbacher I, Grampp S, Czerny C, Youssefzadeh S, Kainberger F. Subchondral bone and cartilage disease: a rediscovered functional unit. Invest Radiol. 2000;35(10):581–8. doi: 10.1097/00004424-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 23.An KN. Muscle force and its role in joint dynamic stability. Clin Orthop Relat Res. 2002;403(suppl):S37–42. doi: 10.1097/00003086-200210001-00005. [DOI] [PubMed] [Google Scholar]
- 24.Gage BE, McIlvain NM, Collins CL, Fields SK, Comstock RD. Epidemiology of 6.6 million knee injuries presenting to United States emergency departments from 1999 through 2008. Acad Emerg Med. 2012;19(4):378–85. doi: 10.1111/j.1553-2712.2012.01315.x. [DOI] [PubMed] [Google Scholar]
- 25.Gray H. Gray’s Anatomy. Edinburgh: Churchill Livingstone; 1989. [Google Scholar]
- 26.Prodromos CC, Han Y, Rogowski J, Joyce B, Shi K. A meta-analysis of the incidence of anterior cruciate ligament tears as a function of gender, sport, and a knee injury-reduction regimen. Arthroscopy. 2007;23(12):1320–1325e1326. doi: 10.1016/j.arthro.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Parkkari J, Pasanen K, Mattila VM, Kannus P, Rimpela A. The risk for a cruciate ligament injury of the knee in adolescents and young adults: a population-based cohort study of 46 500 people with a 9 year follow-up. Br J Sports Med. 2008;42(6):422–6. doi: 10.1136/bjsm.2008.046185. [DOI] [PubMed] [Google Scholar]
- 28.Mandelbaum BR, Silvers HJ, Watanabe DS, et al. Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. Am J Sports Med. 2005;33(7):1003–10. doi: 10.1177/0363546504272261. [DOI] [PubMed] [Google Scholar]
- 29.Hewett TE, Myer GD, Ford KR. Reducing knee and anterior cruciate ligament injuries among female athletes: a systematic review of neuromuscular training interventions. J Knee Surg. 2005;18(1):82–8. doi: 10.1055/s-0030-1248163. [DOI] [PubMed] [Google Scholar]
- 30.Fong DT, Man CY, Yung PS, Cheung SY, Chan KM. Sport-related ankle injuries attending an accident and emergency department. Injury. 2008;39(10):1222–7. doi: 10.1016/j.injury.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 31.Fong DT, Hong Y, Chan LK, Yung PS, Chan KM. A systematic review on ankle injury and ankle sprain in sports. Sports Med. 2007;37(1):73–94. doi: 10.2165/00007256-200737010-00006. [DOI] [PubMed] [Google Scholar]
- 32.Nelson AJ, Collins CL, Yard EE, Fields SK, Comstock RD. Ankle injuries among United States high school sports athletes, 2005–6. J Athl Train. 2007;42(3):381–7. [PMC free article] [PubMed] [Google Scholar]
- 33.Swenson DM, Collins CL, Fields SK, Comstock RD. Epidemiology of U.S. high school sports-related ligamentous ankle injuries, 2005/06–2010/11. Clin J Sport Med. 2013;23(3):190–6. doi: 10.1097/JSM.0b013e31827d21fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hiller CE, Kilbreath SL, Refshauge KM. Chronic ankle instability: evolution of the model. J Athl Train. 2011;46(2):133–41. doi: 10.4085/1062-6050-46.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zacchilli MA, Owens BD. Epidemiology of shoulder dislocations presenting to emergency departments in the United States. J Bone Joint Surg Am. 2010;92(3):542–9. doi: 10.2106/JBJS.I.00450. [DOI] [PubMed] [Google Scholar]
- 36.Owens BD, Agel J, Mountcastle SB, Cameron KL, Nelson BJ. Incidence of glenohumeral instability in collegiate athletics. Am J Sports Med. 2009;37(9):1750–4. doi: 10.1177/0363546509334591. [DOI] [PubMed] [Google Scholar]
- 37.Owens BD, Duffey ML, Nelson BJ, DeBerardino TM, Taylor DC, Mountcastle SB. The incidence and characteristics of shoulder instability at the United States Military Academy. Am J Sports Med. 2007;35(7):1168–73. doi: 10.1177/0363546506295179. [DOI] [PubMed] [Google Scholar]
- 38.Owens BD, Campbell SE, Cameron KL. Risk factors for posterior shoulder instability in young athletes. Am J Sports Med. 2013;41(11):2645–9. doi: 10.1177/0363546513501508. [DOI] [PubMed] [Google Scholar]
- 39.Robinson CM, Howes J, Murdoch H, Will E, Graham C. Functional outcome and risk of recurrent instability after primary traumatic anterior shoulder dislocation in young patients. J Bone Joint Surg Am. 2006;88(11):2326–36. doi: 10.2106/JBJS.E.01327. [DOI] [PubMed] [Google Scholar]
- 40.Kirkley A, Griffin S, Richards C, Miniaci A, Mohtadi N. Prospective randomized clinical trial comparing the effectiveness of immediate arthroscopic stabilization versus immobilization and rehabilitation in first traumatic anterior dislocations of the shoulder. Arthroscopy. 1999;15(5):507–14. doi: 10.1053/ar.1999.v15.015050. [DOI] [PubMed] [Google Scholar]
- 41.Kirkley A, Werstine R, Ratjek A, Griffin S. Prospective randomized clinical trial comparing the effectiveness of immediate arthroscopic stabilization versus immobilization and rehabilitation in first traumatic anterior dislocations of the shoulder: long-term evaluation. Arthroscopy. 2005;21(1):55–63. doi: 10.1016/j.arthro.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 42.Jakobsen BW, Johannsen HV, Suder P, Sojbjerg JO. Primary repair versus conservative treatment of first-time traumatic anterior dislocation of the shoulder: a randomized study with 10-year follow-up. Arthroscopy. 2007;23(2):118–23. doi: 10.1016/j.arthro.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Ranalletta M, Bongiovanni S, Suarez F, Ovenza JM, Maignon G. Do patients with traumatic recurrent anterior shoulder instability have generalized joint laxity? Clin Orthop Relat Res. 2012;470(4):957–60. doi: 10.1007/s11999-011-1992-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buckwalter JA, Brown TD. Joint injury, repair, and remodeling: roles in post-traumatic osteoarthritis. Clin Orthop Relat Res. 2004;423:7–16. [PubMed] [Google Scholar]
- 45.Schenker ML, Mauck RL, Ahn J, Mehta S. Pathogenesis and prevention of posttraumatic osteoarthritis after intra-articular fracture. J Am Acad Orthop Surg. 2014;22(1):20–8. doi: 10.5435/JAAOS-22-01-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McKinley TO, Tochigi Y, Rudert MJ, Brown TD. The effect of incongruity and instability on contact stress directional gradients in human cadaveric ankles. Osteoarthritis Cartilage. 2008;16(11):1363–9. doi: 10.1016/j.joca.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farrokhi S, Voycheck CA, Klatt BA, Gustafson JA, Tashman S, Fitzgerald GK. Altered tibiofemoral joint contact mechanics and kinematics in patients with knee osteoarthritis and episodic complaints of joint instability. Clin Biomech (Bristol, Avon) 2014;29(6):629–35. doi: 10.1016/j.clinbiomech.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Milentijevic D, Rubel IF, Liew AS, Helfet DL, Torzilli PA. An in vivo rabbit model for cartilage trauma: a preliminary study of the influence of impact stress magnitude on chondrocyte death and matrix damage. J Orthop Trauma. 2005;19(7):466–73. doi: 10.1097/01.bot.0000162768.83772.18. [DOI] [PubMed] [Google Scholar]
- 49.Natoli RM, Athanasiou KA. Traumatic loading of articular cartilage: mechanical and biological responses and post-injury treatment. Biorheology. 2009;46(6):451–85. doi: 10.3233/BIR-2009-0554. [DOI] [PubMed] [Google Scholar]
- 50.Natoli RM, Scott CC, Athanasiou KA. Temporal effects of impact on articular cartilage cell death, gene expression, matrix biochemistry, and biomechanics. Ann Biomed Eng. 2008;36(5):780–92. doi: 10.1007/s10439-008-9472-5. [DOI] [PubMed] [Google Scholar]
- 51.Grodzinsky AJ, Levenston ME, Jin M, Frank EH. Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng. 2000;2:691–713. doi: 10.1146/annurev.bioeng.2.1.691. [DOI] [PubMed] [Google Scholar]
- 52.Martin JA, McCabe D, Walter M, Buckwalter JA, McKinley TO. N-acetyl-cysteine inhibits post-impact chondrocyte death in osteochondral explants. J Bone Joint Surg Am. 2009;91(8):1890–7. doi: 10.2106/JBJS.H.00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lewis JS, Jr, Furman BD, Zeitler E, et al. Genetic and cellular evidence of decreased inflammation associated with reduced incidence of posttraumatic arthritis in MRL/MpJ mice. Arthritis Rheum. 2013;65(3):660–70. doi: 10.1002/art.37796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goodwin W, McCabe D, Sauter E, et al. Rotenone prevents impact-induced chondrocyte death. J Orthop Res. 2010;28(8):1057–63. doi: 10.1002/jor.21091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hauser RA, Blakemore PJ, Wang J, Steilen D. Structural basis of joint instability as cause for chronic musculoskeletal pain and its successful treatment with regenerative injection therapy (prolotherapy) Open Pain J. 2014;7:9–23. [Google Scholar]
- 56.Cheng OT, Souzdalnitski D, Vrooman B, Cheng J. Evidence-based knee injections for the management of arthritis. Pain Med. 2012;13(6):740–53. doi: 10.1111/j.1526-4637.2012.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raynauld JP, Buckland-Wright C, Ward R, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48(2):370–7. doi: 10.1002/art.10777. [DOI] [PubMed] [Google Scholar]
- 58.Barenius B, Ponzer S, Shalabi A, Bujak R, Norlen L, Eriksson K. Increased risk of osteoarthritis after anterior cruciate ligament reconstruction: a 14-year follow-up study of a randomized controlled trial. Am J Sports Med. 2014;42(5):1049–57. doi: 10.1177/0363546514526139. [DOI] [PubMed] [Google Scholar]
- 59.Leys T, Salmon L, Waller A, Linklater J, Pinczewski L. Clinical results and risk factors for reinjury 15 years after anterior cruciate ligament reconstruction: a prospective study of hamstring and patellar tendon grafts. Am J Sports Med. 2012;40(3):595–605. doi: 10.1177/0363546511430375. [DOI] [PubMed] [Google Scholar]
- 60.Perdue PS, Jr, Hummer CD, III, Colosimo AJ, Heidt RS, Jr, Dormer SG. Meniscal repair: outcomes and clinical follow-up. Arthroscopy. 1996;12(6):694–8. doi: 10.1016/s0749-8063(96)90172-3. [DOI] [PubMed] [Google Scholar]
- 61.Colin F, Gaudot F, Odri G, Judet T. Supramalleolar osteotomy: techniques, indications and outcomes in a series of 83 cases. Orthop Traumatol Surg Res. 2014;100(4):413–8. doi: 10.1016/j.otsr.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 62.Lozano-Calderon SA, Brouwer KM, Doornberg JN, Goslings JC, Kloen P, Jupiter JB. Long-term outcomes of corrective osteotomy for the treatment of distal radius malunion. J Hand Surg Eur Vol. 2010;35(5):370–80. doi: 10.1177/1753193409357373. [DOI] [PubMed] [Google Scholar]
- 63.Lustig S, Khiami F, Boyer P, Catonne Y, Deschamps G, Massin P. Post-traumatic knee osteoarthritis treated by osteotomy only. Orthop Traumatol Surg Res. 2010;96(8):856–60. doi: 10.1016/j.otsr.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 64.Brandt KD, Dieppe P, Radin EL. Etiopathogenesis of osteoarthritis. Rheum Dis Clin North Am. 2008;34:531–59. doi: 10.1016/j.rdc.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 65.Lonner JH, Pedlow FX, Siliski JM. Total knee arthroplasty for post-traumatic arthrosis. J Arthroplasty. 1999;14(8):969–75. doi: 10.1016/s0883-5403(99)90012-8. [DOI] [PubMed] [Google Scholar]
- 66.Haddad SL, Coetzee JC, Estok R, Fahrbach K, Banel D, Nalysnyk L. Intermediate and long-term outcomes of total ankle arthroplasty and ankle arthrodesis. A systematic review of the literature. J Bone Joint Surg Am. 2007;89(9):1899–905. doi: 10.2106/JBJS.F.01149. [DOI] [PubMed] [Google Scholar]