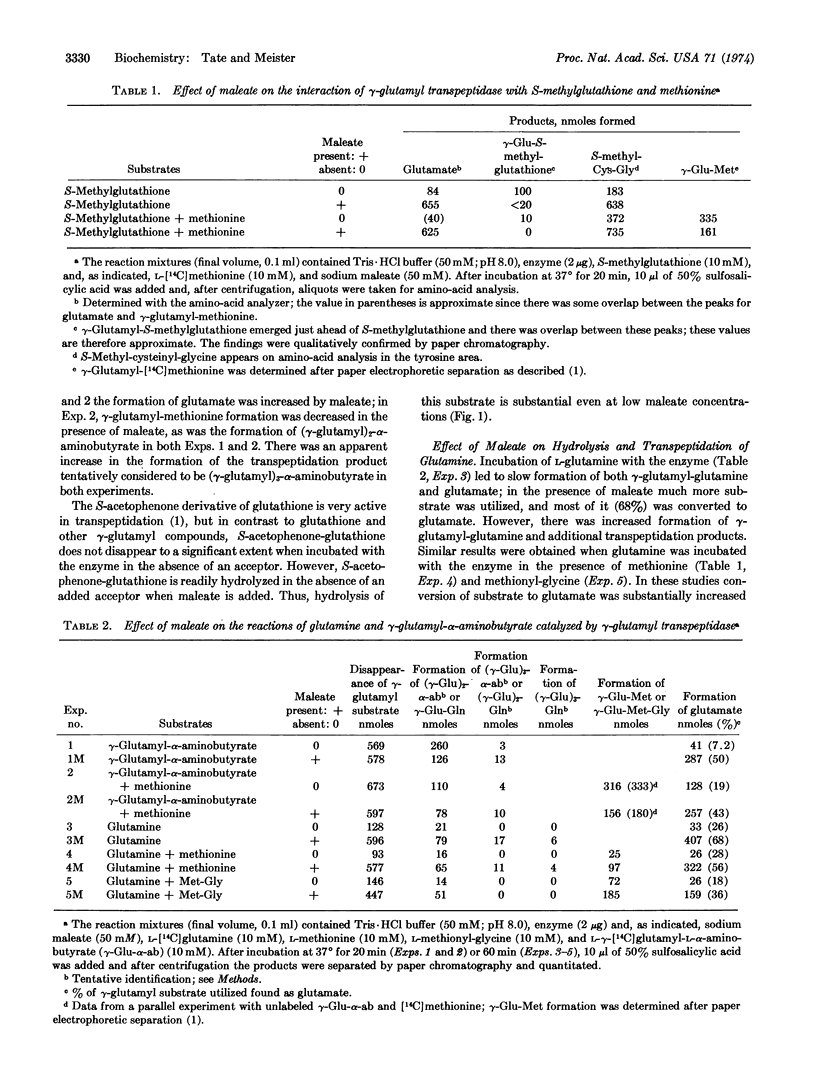
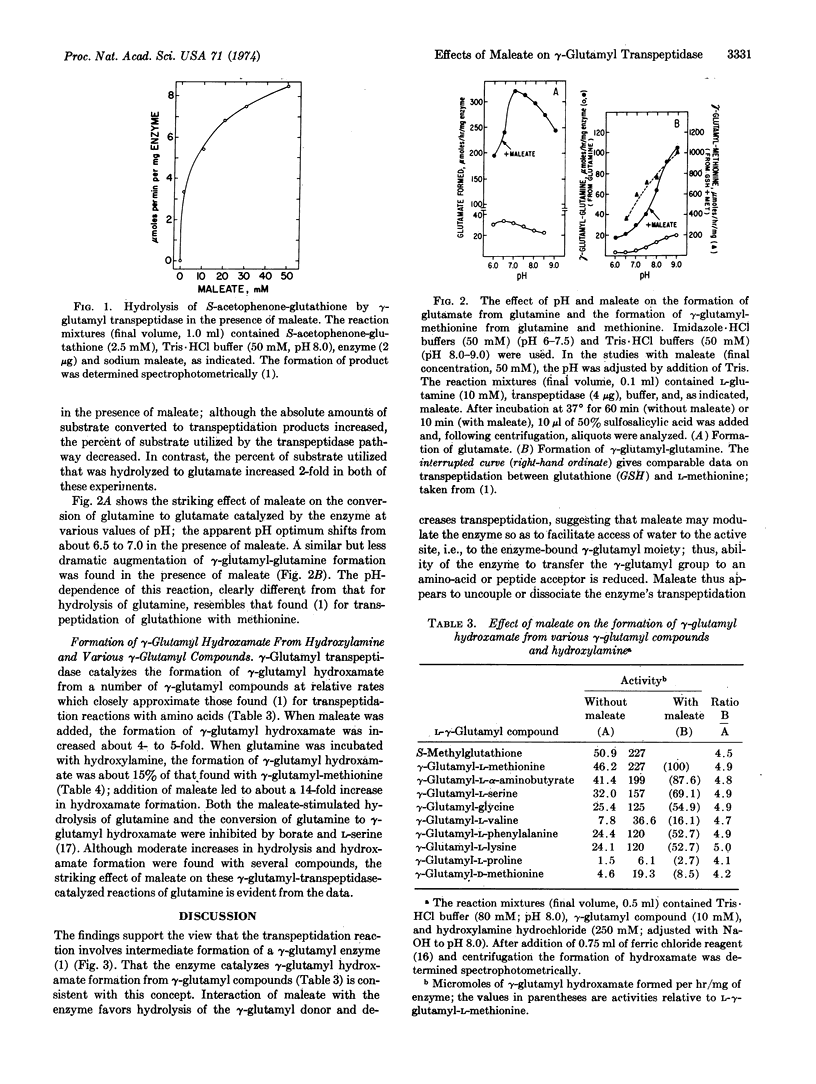
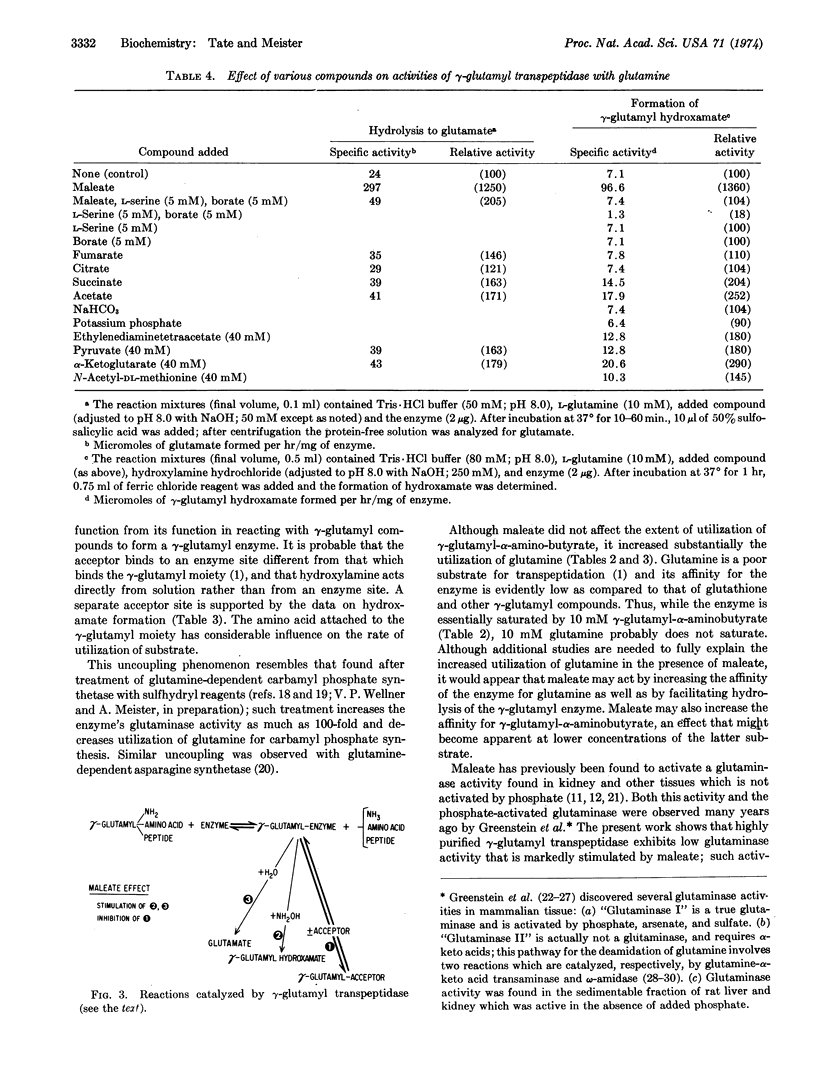
Abstract
γ-Glutamyl transpeptidase catalyzes transfer of the γ-glutamyl moiety of glutathione (and other γ-glutamyl compounds) to amino acid and peptide acceptors; this reaction probably involves (a) formation of a γ-glutamyl enzyme and (b) reaction of the γ-glutamyl-enzyme with an acceptor. Maleate decreases the latter reaction and markedly increases hydrolysis of the γ-glutamyl donor, apparently by affecting the enzyme so as to facilitate reaction of the γglutamyl enzyme with water. Transpeptidase catalyzes γ-glutamyl hydroxamate formation from many γ-glutamyl compounds and hydroxylamine; this reaction is stimulated 4- to 5-fold by maleate. Glutamine, a poor substrate for transpeptidation as compared to glutathione, is slowly hydrolyzed and converted to γ-glutamyl-glutamine by the transpeptidase; in the presence of maleate, hydrolysis of glutamine is markedly (>10-fold) increased, as is also its conversion to γ-glutamyl hydroxamate in the presence of hydroxylamine. The findings suggest that the previously described “maleate-stimulated phosphate-independent glutaminase” is a catalytic function of γ-glutamyl transpeptidase. Transpeptidase-catalyzed glutaminase activity may play a role in renal ammoniagenesis. The ability of maleate to decrease transpeptidation of γ-glutamyl compounds (and to increase their hydrolysis to glutamate), when considered in the light of earlier findings that treatment of animals with maleate produces aminoaciduria, is consistent with function of transpeptidase and the γ-glutamyl cycle in amino-acid transport.
Keywords: amino-acid transport, ammoniagenesis, γ-glutamyl cycle, glutathione, glutamine
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERT Z., ORLOWSKI M., SZEWCZUK A. Histochemical demonstration of gamma-glutamyl transpeptidase. Nature. 1961 Aug 19;191:767–768. doi: 10.1038/191767a0. [DOI] [PubMed] [Google Scholar]
- BERLINER R. W., KENNEDY T. J., HILTON J. G. Effect of maleic acid on renal function. Proc Soc Exp Biol Med. 1950 Dec;75(3):791–794. doi: 10.3181/00379727-75-18344. [DOI] [PubMed] [Google Scholar]
- Bergeron M., Vadeboncoeur M. Microinjections of L-leucine into tubules and peritubular capillaries of the rat. II. The maleic acid model. Nephron. 1971;8(4):367–374. doi: 10.1159/000179939. [DOI] [PubMed] [Google Scholar]
- GLENNER G. G., FOLK J. E. Glutamyl peptidases in rat and guinea pig kidney slices. Nature. 1961 Oct 28;192:338–340. doi: 10.1038/192338a0. [DOI] [PubMed] [Google Scholar]
- George S. G., Kenny J. Studies on the enzymology of purified preparations of brush border from rabbit kidney. Biochem J. 1973 May;134(1):43–57. doi: 10.1042/bj1340043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glossmann H., Neville D. M. gamma-Glutamyltransferase in kidney brush border membranes. FEBS Lett. 1972 Jan 1;19(4):340–344. doi: 10.1016/0014-5793(72)80075-9. [DOI] [PubMed] [Google Scholar]
- HARRISON H. E., HARRISON H. C. Experimental production of renal glycosuria, phosphaturia, and aminoaciduria by injection of maleic acid. Science. 1954 Oct 15;120(3120):606–608. doi: 10.1126/science.120.3120.606. [DOI] [PubMed] [Google Scholar]
- Horowitz B., Meister A. Glutamine-dependent asparagine synthetase from leukemia cells. Chloride dependence, mechanism of action, and inhibition. J Biol Chem. 1972 Oct 25;247(20):6708–6719. [PubMed] [Google Scholar]
- Katunuma N., Katsunuma T., Tomino I., Matsuda Y. Regulation of glutaminase activity and differentiation of the isozyme during development. Adv Enzyme Regul. 1968;6:227–242. doi: 10.1016/0065-2571(68)90015-0. [DOI] [PubMed] [Google Scholar]
- Katunuma N., Tomino I., Nishino H. Glutaminase isozymes in rat kidney. Biochem Biophys Res Commun. 1966 Feb 3;22(3):321–328. doi: 10.1016/0006-291x(66)90485-2. [DOI] [PubMed] [Google Scholar]
- MEISTER A. Enzymatic transfer of alpha-amino groups. Science. 1954 Jul 9;120(3106):43–50. doi: 10.1126/science.120.3106.43. [DOI] [PubMed] [Google Scholar]
- MEISTER A. Studies on the mechanism and specificity of the glutamine-alpha-keto acid transamination-deamidation reaction. J Biol Chem. 1954 Sep;210(1):17–35. [PubMed] [Google Scholar]
- MEISTER A., TICE S. V. Transamination from glutamine to alpha-keto acids. J Biol Chem. 1950 Nov;187(1):173–187. [PubMed] [Google Scholar]
- Meister A. On the enzymology of amino acid transport. Science. 1973 Apr 6;180(4081):33–39. doi: 10.1126/science.180.4081.33. [DOI] [PubMed] [Google Scholar]
- Morgan E. J., Friedmann E. Interaction of maleic acid with thiol compounds. Biochem J. 1938 Apr;32(4):733–742. doi: 10.1042/bj0320733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTEY M. O., BIRNBAUM S. M., GREENSTEIN J. P. Solubilized kidney glutaminase I. Arch Biochem Biophys. 1954 Mar;49(1):245–247. doi: 10.1016/0003-9861(54)90186-1. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Meister A. The gamma-glutamyl cycle: a possible transport system for amino acids. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1248–1255. doi: 10.1073/pnas.67.3.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REVEL J. P., BALL E. G. The reaction of glutathione with amino acids and related compounds as catalyzed by gamma-glutamyl transpeptidase. J Biol Chem. 1959 Mar;234(3):577–582. [PubMed] [Google Scholar]
- Rosenberg L. E., Segal S. Maleic acid-induced inhibition of amino acid transport in rat kidney. Biochem J. 1964 Aug;92(2):345–352. doi: 10.1042/bj0920345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta P. P., Wellner V. P., Pinkus L. M., Meister A. Observations on the pH dependence of the glutaminase activity of a glutamine amidotransferase, carbamylphosphate synthetase. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2717–2721. doi: 10.1073/pnas.70.10.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Werf P., Orlowski M., Meister A. Enzymatic conversion of 5-oxo-L-proline (L-pyrrolidone carboxylate) to L-glutamate coupled with cleavage of adenosine triphosphate to adenosine diphosphate, a reaction in the -glutamyl cycle. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2982–2985. doi: 10.1073/pnas.68.12.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellner V. P., Meister A. Binding of adenosine triphosphate and adenosine diphosphate by glutamine synthetase. Biochemistry. 1966 Mar;5(3):872–879. doi: 10.1021/bi00867a010. [DOI] [PubMed] [Google Scholar]


