Abstract
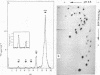
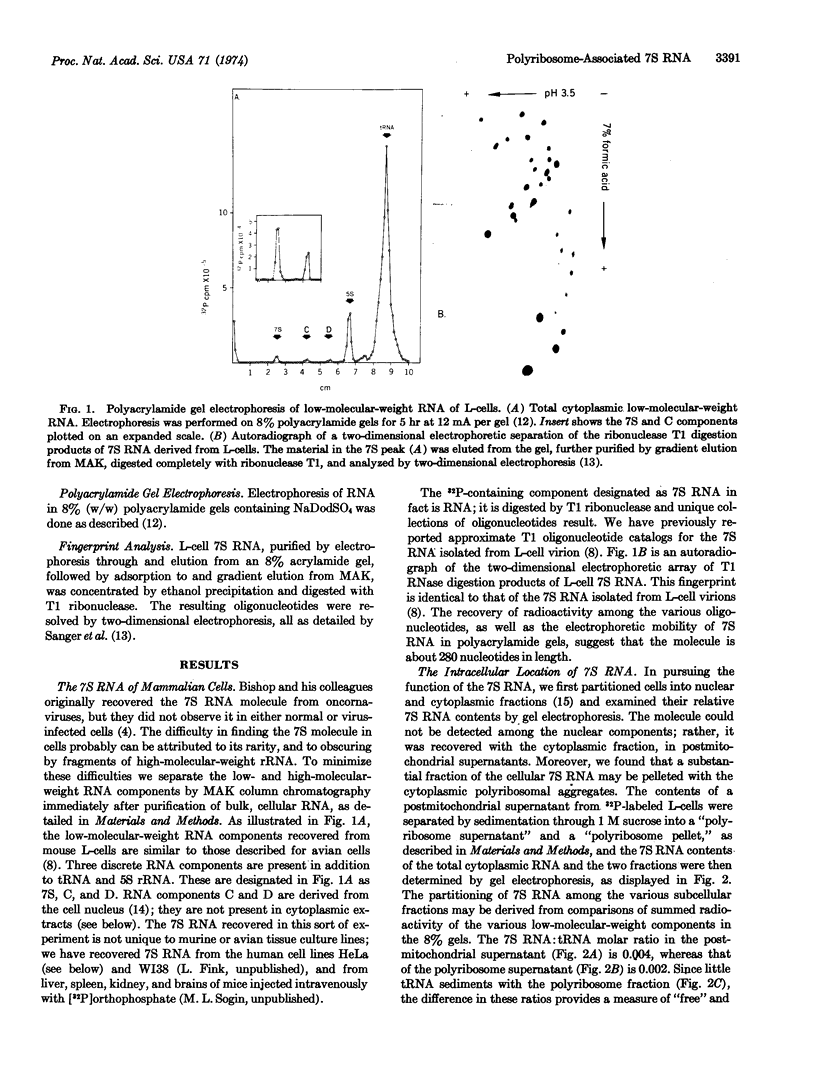
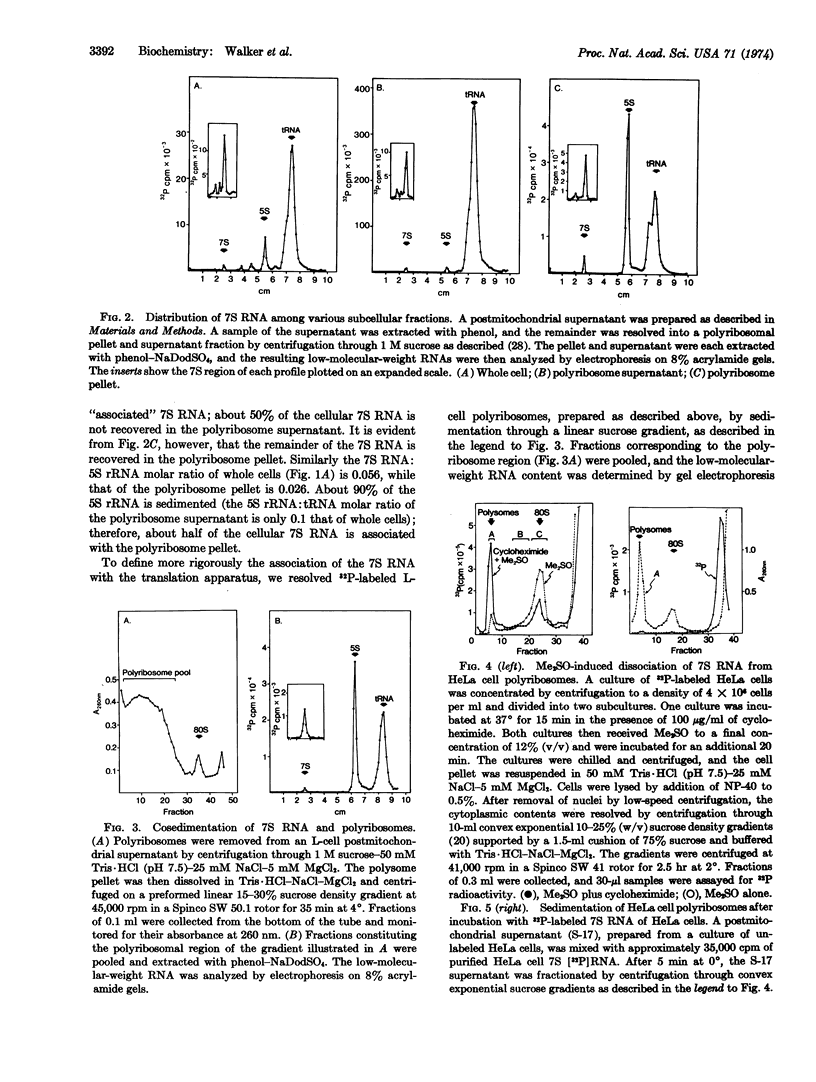
The 7S RNA species first demonstrated in avian and murine oncornaviruses and later in normal, uninfected cells is found associated in part with cellular polyribosomes. A molar ratio of 7S RNA to 5S ribosomal RNA of 0.05 indicates that there is approximately one mole of 7S RNA per mole of messenger RNA. Dissociation of polyribosomes with dimethylsulfoxide results in a marked decrease in the sedimentation rate of the 7S RNA. The dimethylsulfoxide-induced dissociation of polyribosomes and the concomitant movement of the 7S RNA from the polyribosome region into lighter regions of a sucrose gradient are both inhibited by cycloheximide, indicating that the 7S RNA is indeed associated with polyribosomes and not with a ribonucleoprotein particle sedimenting with polyribosomes.
Keywords: L-cells, RNA fingerprinting, gel electrophoresis, dimethylsulfoxide
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M., Levinson W. E., Sullivan D., Fanshier L., Quintrell N., Jackson J. The low molecular weight RNAs of Rous sarcoma virus. II. The 7 S RNA. Virology. 1970 Dec;42(4):927–937. doi: 10.1016/0042-6822(70)90341-7. [DOI] [PubMed] [Google Scholar]
- Blobel G., Sabatini D. Dissociation of mammalian polyribosomes into subunits by puromycin. Proc Natl Acad Sci U S A. 1971 Feb;68(2):390–394. doi: 10.1073/pnas.68.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonar R. A., Sverak L., Bolognesi D. P., Langlois A. J., Beard D., Beard J. W. Ribonucleic acid components of BAI strain A (myeloblastosis) avian tumor virus. Cancer Res. 1967 Jun;27(6):1138–1157. [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Association of 4S ribonucleic acid with oncornavirus ribonucleic acids. J Virol. 1971 Aug;8(2):254–256. doi: 10.1128/jvi.8.2.254-256.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Erikson R. L., Henry B., Pace N. R. Comparison of oligonucleotides produced by RNase T1 digestion of 7 S RNA from avian and murine oncornaviruses and from uninfected cells. Virology. 1973 May;53(1):40–46. doi: 10.1016/0042-6822(73)90463-7. [DOI] [PubMed] [Google Scholar]
- Fan H., Baltimore D. RNA metabolism of murine leukemia virus: detection of virus-specific RNA sequences in infected and uninfected cells and identification of virus-specific messenger RNA. J Mol Biol. 1973 Oct 15;80(1):93–117. doi: 10.1016/0022-2836(73)90235-0. [DOI] [PubMed] [Google Scholar]
- Faras A. J., Garapin A. C., Levinson W. E., Bishop J. M., Goodman H. M. Characterization of the low-molecular-weight RNAs associated with the 70S RNA of Rous sarcoma virus. J Virol. 1973 Aug;12(2):334–342. doi: 10.1128/jvi.12.2.334-342.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost J. P., Sahib M. K. Role of cyclic adenosine 3',5'-monophosphate in the induction of hepatic enzymes. II. Effect of N6,O2'-dibutyryl cyclic adenosine 3'-5'-monophosphate on the kinetics of ribonucleic acid synthesis in purified rat liver nuclei. J Biol Chem. 1971 Mar 25;246(6):1623–1629. [PubMed] [Google Scholar]
- Kaempfer R., Kaufman J. Translational control of hemoglobin synthesis by an initiation factor required for recycling of ribosomes and for their binding to messenger RNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3317–3321. doi: 10.1073/pnas.69.11.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham H., Darnell J. E. Entrance of mRNA into HeLa cell cytoplasm in puromycin-treated cells. J Mol Biol. 1965 Nov;14(1):13–22. doi: 10.1016/s0022-2836(65)80225-x. [DOI] [PubMed] [Google Scholar]
- Lodish H. G., Housman D., Jacobsen M. Initiation of hemoglobin synthesis. Specific inhibition by antibiotics and bacteriophage ribonucleic acid. Biochemistry. 1971 Jun 8;10(12):2348–2356. doi: 10.1021/bi00788a027. [DOI] [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- Pace N. R., Bishop D. H., Spiegelman S. The kinetics of product appearance and template involvement in the in vitro replication of viral RNA. Proc Natl Acad Sci U S A. 1967 Aug;58(2):711–718. doi: 10.1073/pnas.58.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pene J. J., Knight E., Jr, Darnell J. E., Jr Characterization of a new low molecular weight RNA in HeLa cell ribosomes. J Mol Biol. 1968 May 14;33(3):609–623. doi: 10.1016/0022-2836(68)90309-4. [DOI] [PubMed] [Google Scholar]
- Robinson W. S., Pitkanen A., Rubin H. The nucleic acid of the Bryan strain of Rous sarcoma virus: purification of the virus and isolation of the nucleic acid. Proc Natl Acad Sci U S A. 1965 Jul;54(1):137–144. doi: 10.1073/pnas.54.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saborio J. L., Koch G. Reversible inhibition of protein synthesis in HeLa cells by dimethylsulfoxide. J Biol Chem. 1973 Dec 25;248(24):8343–8347. [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Sawyer R. C., Dahlberg J. E. Small RNAs of Rous sarcoma virus: characterization by two-dimensional polyacrylamide gel electrophoresis and fingerprint analysis. J Virol. 1973 Dec;12(6):1226–1237. doi: 10.1128/jvi.12.6.1226-1237.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schincariol A. L., Joklik W. K. Early synthesis of virus-specific RNA and DNA in cells rapidly transformed with Rous sarcoma virus. Virology. 1973 Dec;56(2):532–548. doi: 10.1016/0042-6822(73)90056-1. [DOI] [PubMed] [Google Scholar]
- Siegert W., Konings R. N., Bauer H., Hofschneider P. H. Translation of avian myeloblastosis virus RNA in a cell-free lysate of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Apr;69(4):888–891. doi: 10.1073/pnas.69.4.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Messenger RNA in HeLa cells: kinetics of formation and decay. J Mol Biol. 1973 Aug 5;78(2):321–334. doi: 10.1016/0022-2836(73)90119-8. [DOI] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Stability of HeLa cell mRNA in actinomycin. Nature. 1972 Nov 10;240(5376):100–102. doi: 10.1038/240100a0. [DOI] [PubMed] [Google Scholar]
- Stephenson M. L., Scott J. F., Zamecnik P. C. Evidence that the polyadenylic acid segment of "35S" RNA of avian myeloblastosis virus is located at the 3'-OH terminus. Biochem Biophys Res Commun. 1973 Nov 1;55(1):8–16. doi: 10.1016/s0006-291x(73)80052-x. [DOI] [PubMed] [Google Scholar]
- Trávnícek M. RNA with amino acid-acceptor activity isolated from an oncogenic virus. Biochim Biophys Acta. 1968 Oct 29;166(3):757–759. [PubMed] [Google Scholar]
- Vecchio G., Tsuchida N., Shanmugam G., Green M. Virus-specific messenger RNA and nascent polypeptides in polyribosomes of cells replicating murine sarcoma-leukemia viruses. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2064–2068. doi: 10.1073/pnas.70.7.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WETTSTEIN F. O., NOLL H., PENMAN S. EFFECT OF CYCLOHEXIMIDE ON RIBOSOMAL AGGREGATES ENGAGED IN PROTEIN SYNTHESIS IN VITRO. Biochim Biophys Acta. 1964 Jul 22;87:525–528. doi: 10.1016/0926-6550(64)90131-8. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A., Penman S. Small molecular weight monodisperse nuclear RNA. J Mol Biol. 1968 Dec;38(3):289–304. doi: 10.1016/0022-2836(68)90387-2. [DOI] [PubMed] [Google Scholar]