Abstract
BACKGROUND AND OBJECTIVES:
Polysomnography defines the pathophysiology of obstructive sleep apnea syndrome (OSAS) but does not predict some important comorbidities or their response to adenotonsillectomy. We assessed whether OSAS symptoms, as reflected on the Sleep-Related Breathing Disorders Scale of the Pediatric Sleep Questionnaire (PSQ), may offer clinical predictive value.
METHODS:
Baseline and 7-month follow-up data were analyzed from 185 participants (aged 5–9 years with polysomnographically confirmed OSAS) in the surgical treatment arm of the multicenter Childhood Adenotonsillectomy Trial. Associations were assessed between baseline PSQ or polysomnographic data and baseline morbidity (executive dysfunction, behavior, quality of life, sleepiness) or postsurgical improvement.
RESULTS:
At baseline, each 1-SD increase in baseline PSQ score was associated with an adjusted odds ratio that was ∼3 to 4 times higher for behavioral morbidity, 2 times higher for reduced global quality of life, 6 times higher for reduced disease-specific quality of life, and 2 times higher for sleepiness. Higher baseline PSQ scores (greater symptom burden) also predicted postsurgical improvement in parent ratings of executive functioning, behavior, quality of life, and sleepiness. In contrast, baseline polysomnographic data did not independently predict these morbidities or their postsurgical improvement. Neither PSQ nor polysomnographic data were associated with objectively assessed executive dysfunction or improvement at follow-up.
CONCLUSIONS:
PSQ symptom items, in contrast to polysomnographic results, reflect subjective measures of OSAS-related impairment of behavior, quality of life, and sleepiness and predict their improvement after adenotonsillectomy. Although objective polysomnography is needed to diagnose OSAS, the symptoms obtained during an office visit can offer adjunctive insight into important comorbidities and likely surgical responses.
Keywords: adenotonsillectomy, apnea hypopnea index, clinical prediction, obstructive sleep apnea, polysomnography, questionnaires
What’s Known on This Subject:
Obstructive sleep apnea syndrome (OSAS) is associated with significant comorbidity: behavioral problems, sleepiness, and impaired quality of life. However, the utility of OSAS symptoms versus polysomnography in the prediction of comorbidities or response to treatment is not well known.
What This Study Adds:
Among children with OSAS, the Pediatric Sleep Questionnaire, a well-validated, simple 1-page symptom inventory, predicts key adenotonsillectomy-responsive OSAS comorbidities and their improvement after adenotonsillectomy. In contrast, polysomnographic results do not offer similar predictive value.
Obstructive sleep apnea syndrome (OSAS) is a common disorder in childhood and can result in a range of adverse health outcomes. In children, adenotonsillar hypertrophy is the most common cause of OSAS, and adenotonsillectomy is the most common treatment.1,2 In clinical research, OSAS is defined in large part by objective sleep laboratory–based polysomnography (PSG) measurements. However, multiple previous efforts to show clinically useful associations between PSG findings and important comorbidities, or their response to adenotonsillectomy, have been disappointing.3–10 In clinical practice, differences exist between medical professional societies as to whether PSG should be obtained to assess OSAS before adenotonsillectomy. Practice guidelines from the American Academy of Sleep Medicine and American Academy of Pediatrics recommend PSG to confirm and characterize OSAS before adenotonsillectomy,1,2,11 whereas the American Academy of Otolaryngology–Head and Neck Surgery recommends PSG for children with certain complex medical conditions or when there is discordance between tonsil size and reported severity of OSAS symptoms.12
Less work has been done to define what adjunctive measures, in addition to PSG, may assist pediatricians, otolaryngologists, and families who are considering adenotonsillectomy to understand what adverse impact OSAS is having for the child and what the probability is that this adverse impact will be ameliorated by surgery. For example, few studies have examined whether baseline OSAS symptoms can predict postoperative changes in parent-perceived behavior, sleepiness, and quality of life, outcomes that assume key importance to families, regardless of PSG results. One study reported that preoperative OSAS symptoms predicted OSAS-related neurobehavioral morbidity, and its response to adenotonsillectomy, as well as or better than did PSG findings.13 Since then, a large multicenter, randomized clinical trial, the Childhood Adenotonsillectomy (CHAT) study, found that OSAS treatment improves key subjectively assessed health outcomes (behavior, sleepiness, symptoms, and quality of life) but not objective cognitive measures.10 Data from the surgical arm of this study offer an ideal opportunity to assess more definitively whether OSAS symptoms, as reflected by a well-validated symptom inventory, the Sleep-Related Breathing Disorders Scale of the Pediatric Sleep Questionnaire (PSQ),14 provide clinical utility beyond the PSG in assessment of concurrent OSAS-related comorbidities, specifically neurobehavioral problems, sleepiness, and quality of life. We also assess the extent to which the PSQ scale may provide incremental value in prediction of OSAS-related, treatment-responsive outcomes after adenotonsillectomy.
Methods
Study Design, Setting, and Population
Details of the CHAT study design have been published.10,15 In brief, habitually snoring 5- to 9-year-old children who were candidates for adenotonsillectomy were recruited from otolaryngology practices, pediatric sleep clinics, and other sources at 6 US medical centers for this prospective, randomized, controlled, single-blind trial. The key inclusion criterion was PSG-confirmed OSAS with an obstructive apnea/hypopnea index (AHI) ≥2 events per hour or an obstructive apnea index (OAI) ≥1. Key exclusion criteria were AHI >30, OAI >20, oxygen saturation <90% for ≥2% of total sleep time, recurrent tonsillitis, BMI z score ≥3, or medication for attention-deficit/hyperactivity disorder. Institutional review board approval was obtained at each site. Written informed consent from 1 parent and assent from each child age ≥7 years were obtained.
Outcomes
Outcomes in these analyses were selected to reflect the primary outcome variable in CHAT (the Developmental Neuropsychological Assessment [NEPSY-A/E], a cognitive measure that was shown not to be responsive to surgery) and the other key outcome measures that did prove responsive to adenotonsillectomy. Selected measures included an objective measure of attention and executive functioning (NEPSY-A/E), validated neurobehavioral rating scales, global and disease-specific measures of quality of life, and sleepiness. The NEPSY-A/E generates scores ranging from 50 to 150, with 100 representing the population mean and higher scores indicating better functioning.16 Neurobehavioral assessments included caregiver ratings of executive functioning on the Behavior Rating Inventory of Executive Function (BRIEF) global executive composite score (scores range from 28 to 101), with higher scores indicating worse functioning17; attention on the Conners’ Rating Scale Revised: Long Version Global Index (T scores range from 38 to 90), with higher scores indicating worse functioning18; and behavior on the Child Behavior Checklist (CBCL) total score (mean T-scores = 50, SD = 10), with higher scores indicating worse behaviors.19 We assessed global quality of life with both parent and child ratings on the Pediatric Quality of Life Inventory (PedsQL), in which scores range from 0 to 100 and higher scores indicate better quality of life.20 We assessed disease-specific quality of life by using the 18-item Obstructive Sleep Apnea-18 (OSAS-18) assessment tool, a composite of OSAS-related symptoms and quality of life in which scores range from 18 to 126, with higher scores indicating worse quality of life.21 We assessed sleepiness by using the Epworth Sleepiness Scale (ESS), modified for children, in which scores range from 0 to 24, with higher scores indicating greater daytime sleepiness.7
Predictors
PSG
Details about the PSG protocol are available in the online supplement that accompanied the primary outcome paper.10 In brief, children underwent full, in-laboratory PSG performed by study-certified technicians according to a standardized protocol, with similar sensors, and according to American Academy of Sleep Medicine guidelines for acquisition and pediatric scoring.22 PSG data indicating greater OSAS severity include higher AHI values, 3% oxygen desaturation index (ODI3%), percentage sleep time with end-tidal CO2 (ETco2) values >50 mm Hg, arousal index, percentage of non–rapid eye movement sleep stages N1 and N2, or lower values for percentage of stage N3 or rapid eye movement (REM) stage sleep. Studies were scored centrally, and scoring reliability was assessed throughout the study; intraclass correlation coefficients (between and within scorers) exceeded 0.9 for most sleep parameters.
Sleep-Related Breathing Disorders Scale of the PSQ
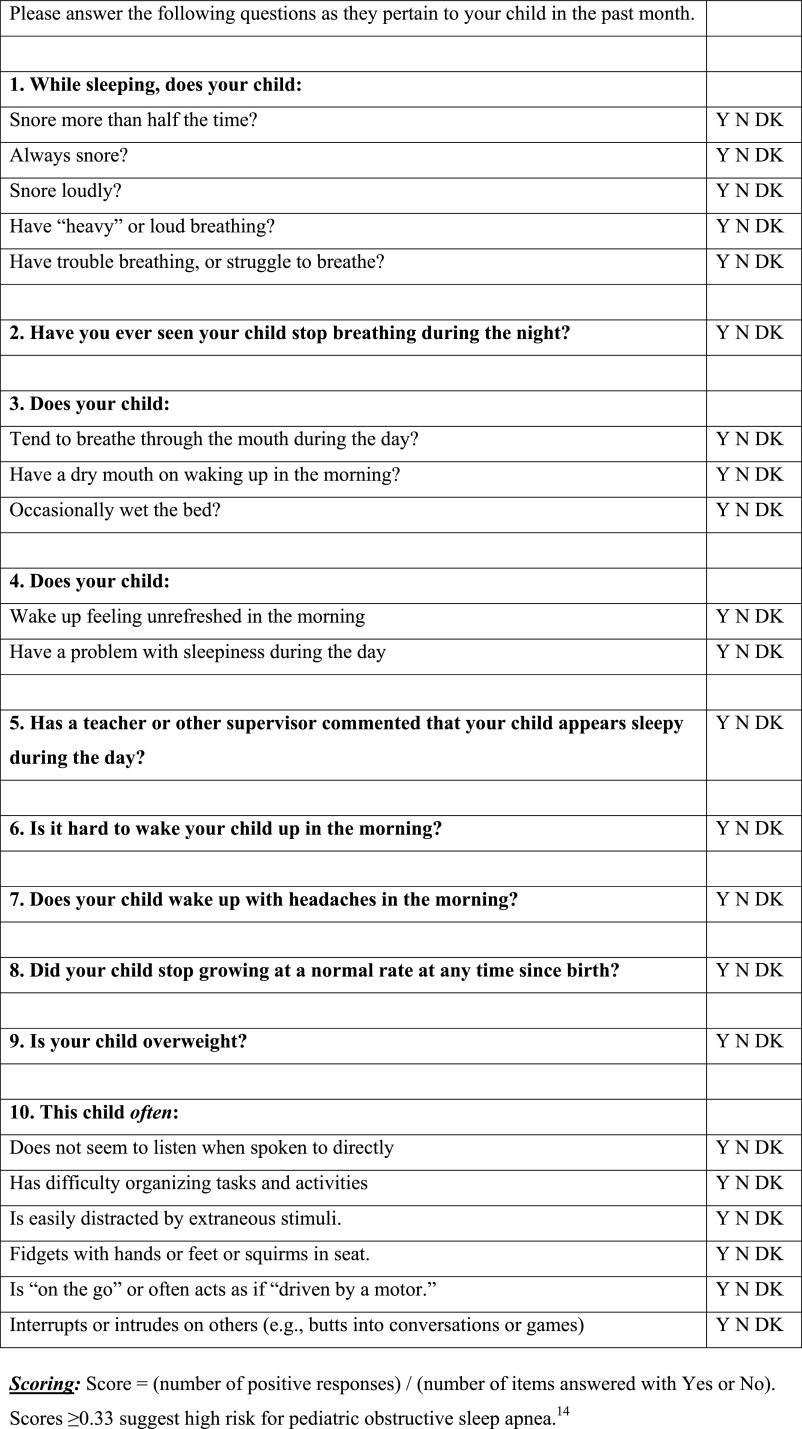
The PSQ scale contains 22 items that ask about snoring frequency, loud snoring, observed apneas, difficulty breathing during sleep, daytime sleepiness, inattentive or hyperactive behavior, and other pediatric OSAS features, each shown to correlate (area under the receiver operating curve ≥0.8) with PSG-confirmed OSAS in referred children.14 Responses are yes = 1, no = 0, and don’t know = missing. The mean response on nonmissing items is the score, which can vary from 0 to 1. Higher scores indicate more OSAS-related symptoms. Previous data indicated that a cutoff value of 0.33 was most effective in identifying pediatric OSAS.14 Subscales within the Sleep-Related Breathing Disorder Scale include a 4-item sleepiness scale, 4-item snoring scale, and 6-item inattention and hyperactivity scale derived from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria for attention-deficit/hyperactivity disorder.23 PSQ items and response options are shown in Fig 1.
FIGURE 1.
Items and response options for the Sleep-Disordered Breathing Scale of the PSQ.14 Copyright © 2000 The Regents of the University of Michigan. The Sleep-Related Breathing Disorder Scale of the PSQ can be licensed and obtained without charge online at http://inventions.umich.edu/technologies/3773/sleep-related-breathing-disorder-scale-srbd-scale-from-pediatric-sleep-questionnaire-to-identify-symptoms-of-obstructive-sleep-apnea-in-children.
Statistical Analyses
Spearman’s correlation coefficients were used to summarize the unadjusted associations between baseline PSG or PSQ measures and baseline comorbidity measures or their improvement after adenotonsillectomy. The strength of the correlations was categorized as large (>0.5), moderate (0.3–0.5), small (0.1–0.3), or insubstantial (<0.1).24 The potential PSQ and PSG predictor variables were reduced to those most strongly correlated with behavioral health measures. Multivariable logistic regression models were used to assess the extent to which the total PSQ scale and AHI at baseline independently predicted morbidity measures at baseline or their improvement across 7 months. Each participant’s morbidity score at baseline was dichotomized based on whether it was ≥0.5 SD worse than the mean. Each participant’s outcome measure change score was dichotomized based on whether improvement was ≥0.5 SD from that participant’s baseline. The predictor variable (PSQ or AHI) was normalized so that the odds ratio (OR) in each model reflected the effect on the given morbidity measure of a 1-SD increase in the predictor variable, equivalent to 0.18 points on the PSQ or 5.8 points in the AHI. The c-statistic was used to assess goodness of fit. Values for this measure range from 0.5 to 1. A value of 0.5 indicates that the model is no better than chance, and a value of 1.0 indicates that the model perfectly identifies those within a group and those not. All models were adjusted for age, race, gender, and BMI z score. The level of significance was set at .05 and not adjusted for multiple comparisons. Analyses were performed with a statistical software program (SAS version 9.2; SAS Institute Inc, Cary, NC).
Results
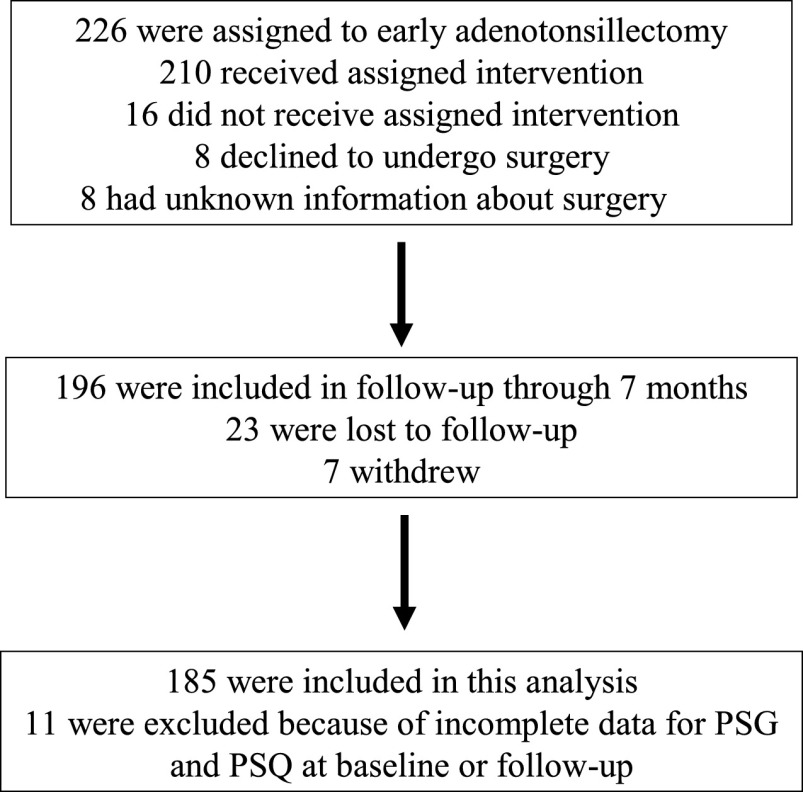
Analyses are restricted to the 185 participants (94% of the 196) with complete PSQ and PSG data at baseline and postadenotonsillectomy visits (Fig 2). Baseline characteristics, PSG data, and PSQ scores are summarized in Table 1. The mean PSQ score was 0.48 ± 0.18, and 142 (77%) had a positive PSQ total score ≥0.33. Table 2 summarizes the OSAS comorbidity measures, all of which improved significantly after adenotonsillectomy. Table 3 summarizes the associations of the baseline PSQ total score with various PSG measures at baseline and at follow-up. After adenotonsillectomy, the median (interquartile range [IQR]) AHI change from baseline to 7 months was −3.5 (6.1), and 79% of the participants “normalized,” that is, had an AHI <2 and OAI <1. The mean PSQ change from baseline to 7 months was −0.3 ± 0.2, and 148 (80%) of the PSQ total scores were <0.33. Baseline PSQ scores were significantly associated with AHI and the ODI3% at both baseline and follow-up but were not associated with higher ETco2 values or other PSG measures.
FIGURE 2.
Detailed information about the study flow.
TABLE 1.
Participant Characteristics
| Variable | Total Sample (N = 185) |
|---|---|
| Demographics | |
| Age, y | 7.0 ± 1.4 |
| Male gender, n (%) | 84 (45.4%) |
| Race, n (%) | |
| African American | 97 (52.4%) |
| Caucasian | 67 (36.2%) |
| Asian | 4 (2.2%) |
| Other | 17 (9.2%) |
| Hispanic ethnicity, n (%) | 12 (6.5%) |
| Maternal education less than high school, n (%) | 16 (8.8%) |
| Household income <$30 000,a n (%) | 69 (45.4%) |
| Obese, BMI ≥95th percentile, n (%) | 75 (40.5%) |
| PSG findings | |
| Respiratory | |
| AHI, median (IQR); range | 4.8 (6.4); 1.2–27.7 |
| ODI3%, median (IQR); range | 4.9 (8); 0.0–32.8 |
| ETco2 >50 mm Hg,b % total sleep time, median (IQR); range | 2.0 (13.5); 0–86.8 |
| Obstructive hypoventilation,b n (%) | 25 (16.1%) |
| Sleep | |
| Arousal index | 8.7 ± 3.2 |
| Total sleep time, min | 457.7 ± 54.5 |
| % Sleep time in stage N1 | 8.7 ± 4.3 |
| % Sleep time in stage N2 | 41.3 ± 7.5 |
| % Sleep time in stage N3 | 31.2 ± 7.2 |
| % Sleep time in stage REM | 18.8 ± 4.3 |
| PSQ scale | |
| Total score,c range | 0.49 ± 0.18; 0.05–0.95 |
| Snoring subscale, range | 0.77 ± 0.27; 0.00–1.00 |
| Sleepiness subscale, range | 0.44 ± 0.34; 0.00–1.00 |
| Behavioral subscale | 0.44 ± 0.33; 0.00–1.00 |
Categorical data are reported as number and percentage. Plus–minus values are means ± SD, and nonnormally distributed data are reported as medians and IQRs. N1, non-REM stage 1 sleep; N2, non-REM stage 2 sleep; N3, non-REM stage 3 sleep.
Data for household income are missing for 33 participants.
Number of participants with obstructive hypoventilation defined as ETco2 value >50 mm Hg for ≥25% of total sleep time. Data are missing on ETco2 and hypoventilation for 30 participants. Available participants all have quality CO2 signals for ≥50% of the sleep time.
PSQ total score ≥0.33 indicates high risk for OSAS.
TABLE 2.
Summary of Behavioral, Sleepiness, and Quality of Life Measures at Baseline and at 7-Month Follow-up After Adenotonsillectomy
| Neurobehavioral Measure | No. With Available Data (baseline/follow-up) | Baseline | Follow-up | Change From Baselinea | Pb | Effect Size |
|---|---|---|---|---|---|---|
| NEPSY-A/E score | (184/183) | 30.9 ± 6.2 | 33.5 ± 5.9 | 2.7 ± 5.2, N = 183 | <.001 | −0.43 |
| BRIEF score | (185/184) | 49.5 ± 10.8 | 46.2 ± 11.3 | −3.3 ± 8.3, N = 184 | <.001 | 0.30 |
| Conners’ Rating Scale | (185/182) | 52.1 ± 11.4 | 49.2 ± 10.8 | −2.9 ± 9.9, N = 182 | <.001 | 0.26 |
| CBCL score | (180/181) | 52.1 ± 10.9 | 48.2 ± 12.0 | −3.9 ± 8.0, N = 178 | <.001 | 0.34 |
| PedsQL (Child) | (184/184) | 68.8 ± 15.4 | 72.3 ± 15.2 | 3.6 ± 17.2, N = 183 | .006 | −0.23 |
| PedsQL (Parent) | (185/184) | 78.8 ± 15.4 | 84.5 ± 14.9 | 5.7 ± 14.6, N = 184 | <.001 | −0.37 |
| OSAS-18 score | (184/183) | 52.8 ± 17.7 | 30.7 ± 13.8 | −21.9 ± 15.9, N = 182 | <.001 | 1.39 |
| ESS | (185/185) | 7.1 ± 4.7 | 5.0 ± 4.4 | −2.0 ± 4.3, N = 185 | <.001 | 0.44 |
Data are summarized as mean ± SD.
Number of participants with available change data (baseline minus follow-up) differs slightly from the number with available data at follow-up: CBCL (N = 178), PedsQL Child (N = 183), OSAS-18 (N = 182).
P values were based on paired t test of baseline and follow-up.
TABLE 3.
Correlations (Spearman ρ) of the Baseline PSQ Total Scale Score With PSG Measures at Baseline and 7-Month Follow-up After Adenotonsillectomy
| PSG Variables | Baseline | Follow-up |
|---|---|---|
| Spearman ρ | Spearman ρ | |
| Obstructive AHI | .148* | .262*** |
| ODI3% | .150* | .236*** |
| Sleep time (%) with ETco2 values >50 mm Hga | .053 | .051 |
| Arousal index | .054 | .071 |
| Total sleep time, min | −.045 | −.079 |
| % Sleep time, stage N1 | .011 | −.101 |
| % Sleep time, stage N2 | .067 | −.054 |
| % Sleep time, stage N3 | .006 | .075 |
| % Sleep time, stage REM | −.131 | .066 |
N1, non-REM stage 1 sleep; N2, non-REM stage 2 sleep; N3, non-REM stage 3 sleep.
Data missing on ETco2 for 30 participants at baseline and 32 participants at follow-up.
P < .05; *** P ≤ .001.
Correlations Between Baseline PSQ or PSG and Behavioral Health Measures, Sleepiness, and Quality of Life at Baseline and Follow-up
Overall, these bivariate comparisons indicate that the strongest PSG correlate of impairment of baseline scores and improvement after adenotonsillectomy was the AHI, and the best PSQ predictor was the total score. Table 4 shows correlations between the baseline PSQ scales or PSG variables and baseline morbidity measures. Of the various PSQ scale scores, the PSQ total score showed the most consistent and strongest correlations with the study’s behavioral, sleepiness, and quality of life metrics. The largest correlations were seen for parent ratings of executive function, attention, behavior, and disease-specific quality of life. Comparing the correlation coefficients for different PSQ subscales (snoring, sleepiness, inattention and hyperactivity) helped identify which components of the PSQ scale contributed most to the associations between the full PSQ scale and various health outcomes. For example, the PSQ inattention and hyperactivity subscale showed large and significant correlations with parent rating scales for executive function, attention, and behavior. In contrast to the PSQ, PSG measures had fewer significant correlations with these symptom and behavioral measures. The AHI and ODI3% showed a small correlation with disease-specific quality of life. Of the other sleep measures, a higher percentage of stage N3 showed a small correlation with parent ratings of more problems in executive functioning, attention, and behavior, the inverse of what might be expected. Less REM sleep showed a small correlation with worse disease-specific quality of life. Table 5 shows the correlations between baseline PSG or PSQ results and health outcomes after adenotonsillectomy. Outcome measures showed associations that were similar in direction and magnitude to those generated by the baseline health measures: many moderate to high correlations between PSQ and study outcomes, with fewer and lower-magnitude correlations between PSG and the outcomes.
TABLE 4.
Spearman Correlations (ρ) Between Baseline PSQ or PSG and Baseline Comorbidity Measures
| OSAS Assessments | NEPSY-A/E | BRIEF | Conners | CBCL | PedsQL Child | PedsQL Parent | OSAS-18 | ESS |
|---|---|---|---|---|---|---|---|---|
| PSQ scale | ||||||||
| Total score | −.063 | .500*** | .530*** | .537*** | −.204** | −.471*** | .697*** | .396*** |
| Snoring subscale | −.001 | .018 | .083 | .091 | −.039 | −.044 | .350*** | .159* |
| Sleepiness subscale | .047 | .136 | .179** | .225** | −.086 | −.331*** | .464*** | .291*** |
| Inattention and hyperactivity subscale | −.114 | .635*** | .635*** | .586*** | −.175* | −.399*** | .383*** | .244** |
| PSG variables | ||||||||
| AHI | −.012 | −.038 | −.069 | −.012 | −.024 | −.046 | .150* | .148 |
| ODI3% | −.057 | .021 | −.034 | .024 | −.034 | −.044 | .148* | .139 |
| ETco2 values >50 mm Hg | .149 | −.087 | −.069 | −.052 | −.005 | .050 | .021 | .095 |
| Arousal index | −.020 | −.040 | −.069 | −.083 | .044 | .027 | .081 | −.003 |
| Total sleep time, min | −.023 | −.132 | −.061 | −.072 | −.004 | .016 | −.098 | .047 |
| Percentage N1 sleep | −.052 | −.002 | −.097 | −.046 | .048 | .038 | .109 | .072 |
| Percentage N2 sleep | −.060 | −.141 | −.125 | −.116 | −.061 | .101 | .015 | .085 |
| Percentage N3 sleep | .093 | .178* | .213* | .161* | .038 | −.127 | .033 | −.079 |
| Percentage REM sleep | .047 | −.041 | −.064 | −.063 | .042 | .023 | −.209** | −.093 |
N1, non-REM stage 1 sleep; N2, non-REM stage 2 sleep; N3, non-REM stage 3 sleep.
P < .05; ** P ≤ .01; *** P ≤ .001.
TABLE 5.
Spearman Correlations (ρ) Between Baseline PSQ or PSG and Outcomes (7-mo Change Scores) After Adenotonsillectomy
| NEPSY-A/E | BRIEF | Conners | CBCL | PedsQL Child | PedsQL Parent | OSAS-18 | ESS | |
|---|---|---|---|---|---|---|---|---|
| OSAS assessments | ||||||||
| PSQ scale | ||||||||
| Total score | −.052 | .329*** | .446*** | .386*** | −.181** | −.379*** | .338*** | .185** |
| Snoring subscale | .047 | −.036 | .047 | .056 | .025 | .038 | .015 | .056 |
| Sleepiness subscale | −.081 | .037 | .094 | .079 | −.062 | −.125 | .215** | .077 |
| Inattention and hyperactivity subscale | −.079 | .495*** | .573*** | .471*** | −.232** | −.384*** | .279*** | .117 |
| PSG variables | ||||||||
| AHI | .014 | −.023 | −.014 | −.002 | −.165* | −.036 | .048 | .115 |
| ODI3% | −.011 | .011 | .034 | .021 | −.121 | −.071 | .088 | .124 |
| ETco2 values >50 mm Hg | .089 | −.054 | −.072 | −.050 | −.067 | .080 | −.149 | .102 |
| Arousal index | .046 | −.083 | −.084 | −.122 | −.068 | .121 | −.055 | −.093 |
| Total sleep time, min | .095 | −.118 | −.132 | −.092 | .008 | .028 | −.150* | .095 |
| Percentage N1 sleep | −.043 | .032 | .046 | −.024 | −.093 | .062 | .032 | −.038 |
| Percentage N2 sleep | −.067 | −.129 | −.064 | −.135 | .135 | .029 | −.025 | .131 |
| Percentage N3 sleep | .154* | .107 | .068 | .144 | −.028 | −.094 | .016 | −.155* |
| Percentage REM sleep | .004 | .123 | .094 | .089 | −.050 | −.015 | −.057 | .165* |
N1, non-REM stage 1 sleep; N2, non-REM stage 2 sleep; N3, non-REM stage 3 sleep.
P < .05; ** P ≤ .01; *** P ≤ .001.
Logistic Regression of Baseline Morbidity Measures or Their Improvement at Follow-up on Baseline PSG or PSG Results
Table 6 shows the results of multivariable logistic regression models in which baseline AHI and PSQ total score, adjusted for age, race, gender, and BMI z score, were evaluated as predictors of impaired health status at baseline. Even after adjustment for baseline AHI, the PSQ total score was strongly predictive of impaired behavioral health, including parent ratings of more problems in executive function (OR 3.2), attention (OR 4.2), and behavior (OR 3.1); lower parent-rated global quality of life (OR 2.5) and disease-specific quality of life (OR 5.7); lower child-rated global quality of life (1.6); and more sleepiness (OR 1.8). In contrast, the AHI showed no independent associations with these study metrics after adjustment for baseline PSQ total score. Neither the PSQ total score nor AHI predicted lower objectively measured attention and executive function.
TABLE 6.
Logistic Regression of Baseline Morbidity on Baseline PSQ Scale and AHI
| No. (%) of Participants With Impairmenta | OR (95% CI) | P | |
|---|---|---|---|
| Low NEPSY-A/E | 43 (23%) | ||
| PSQ | 0.81 (0.55–1.19) | .28 | |
| AHI | 1.07 (0.74–1.53) | .73 | |
| High BRIEF | 51 (28%) | ||
| PSQ | 3.15 (2.02–4.91) | <.0001 | |
| AHI | 0.68 (0.44–1.06) | .09 | |
| High Connors | 47 (25%) | ||
| PSQ | 4.23 (2.54–7.05) | <.0001 | |
| AHI | 0.80 (0.52–1.22) | .30 | |
| High CBCL | 50 (28%) | ||
| PSQ | 3.13 (2.00–4.91) | <.0001 | |
| AHI | 0.87 (0.58–1.29) | .48 | |
| Low PedsQL (child) | 61 (33%) | ||
| PSQ | 1.58 (1.13–2.22) | .008 | |
| AHI | 0.89 (0.63–1.24) | .48 | |
| Low PedsQL (parent) | 56 (30%) | ||
| PSQ | 2.52 (1.69–3.75) | <.0001 | |
| AHI | 1.10 (0.77–1.55) | .61 | |
| High OSAS-18 | 49 (27%) | ||
| PSQ | 5.70 (3.24–10.05) | <.0001 | |
| AHI | 1.29 (0.86–1.94) | .22 | |
| High ESS | 48 (26%) | ||
| PSQ | 1.84 (1.26–2.68) | .002 | |
| AHI | 0.96 (0.68–1.38) | .84 |
Logistic regression adjusted for age, gender, race, and BMI z score. PSQ score and AHI normalized to model the effect of a 1-SD increase (equivalent to 0.18 points on the PSQ or 5.8 points for the AHI), after adjustment for the baseline value of the other predictor and other covariates in the model.
Clinically meaningful impairment defined for this analysis as a morbidity score ≥0.5 SD worse than baseline mean.
Table 7 shows results from adjusted multivariable logistic regressions of improved health outcomes (by at least 0.5 SD) at 7 months on baseline PSQ and AHI. The baseline PSQ total score remained independently predictive of improvement in parent ratings of executive function, attention, behavior, global quality of life, disease-specific quality of life, and sleepiness. In contrast, no behavioral, sleepiness, or quality of life outcomes were predicted independently by baseline AHI. Each of the logistic regression models that considered AHI and PSQ, either jointly as shown or individually (models not shown), had low to moderate c-statistics (0.55 to 0.82), indicating that no model provided highly accurate and reliable classification of the likelihood of improved outcomes after adenotonsillectomy.
TABLE 7.
Logistic Regression of Outcome Improvement Across 7 Months on Baseline PSQ Scale and AHI
| No. (%) of Participants With Improvement From Baselinea | OR (95% CI) | P | |
|---|---|---|---|
| Higher NEPSY-A/E | 80 (44%) | ||
| PSQ | 0.98 (0.72–1.34) | .92 | |
| AHI | 0.99 (0.73–1.35) | .95 | |
| Lower BRIEF | 64 (35%) | ||
| PSQ | 1.45 (1.04–2.02) | .03 | |
| AHI | 1.14 (0.82–1.57) | .44 | |
| Lower Connors | 54(30%) | ||
| PSQ | 1.57 (1.11–2.23) | .01 | |
| AHI | 1.01 (0.71–1.42) | .97 | |
| Lower CBCL | 79 (44%) | ||
| PSQ | 1.58 (1.13–2.21) | .008 | |
| AHI | 1.01 (0.73–1.40) | .95 | |
| Higher PedsQL (child) | 73 (40%) | ||
| PSQ | 1.27 (0.82–1.97) | .29 | |
| AHI | 0.66 (0.39–1.11) | .11 | |
| Higher PedsQL (parent) | 66 (36%) | ||
| PSQ | 1.69 (1.09–2.60) | .02 | |
| AHI | 0.89 (0.57–1.41) | .63 | |
| Lower OSAS-18 | 147 (81%) | ||
| PSQ | 2.16 (1.38–3.38) | .0008 | |
| AHI | 1.10 (0.72–1.67) | .67 | |
| Lower ESS | 82 (44%) | ||
| PSQ | 1.72 (1.24–2.38) | .001 | |
| AHI | 1.05 (0.77–1.43) | .77 |
Logistic regression adjusted for age, gender, race, and BMI z score. PSG and AHI normalized to reflect the effect of a 1-SD increase (equivalent to 0.18 points on the PSQ or 5.8 points in the AHI), after adjustment for the baseline value of the other predictor and other covariates in the model.
Clinically meaningful improvement defined as an improvement in the morbidity measure by ≥0.5 SD from its baseline mean.
Discussion
This large, multicenter, prospective study of children with sleep laboratory–confirmed OSAS shows that results from the PSQ, a simple, 1-page symptom inventory for childhood OSAS, were clearly associated with baseline behavioral impairment, sleepiness, and reduced quality of life and predictive of their improvement after adenotonsillectomy. Like the PSQ, the standard assessments of behavior, quality of life, and daytime sleepiness are all subjective and parent rated. Therefore, informant bias cannot be excluded as an explanation for their stronger associations compared with objective PSG measures. Nonetheless, each of these outcome measures reflects an area of critical importance to children and their families. The current findings are also important to clinicians, because the PSQ may assist in their efforts to identify candidates for adenotonsillectomy who are most likely to experience improvement in the key areas identified by CHAT study to be responsive to adenotonsillectomy. Whereas replication of comprehensive CHAT assessments for behavior, sleepiness, and quality of life may not be realistic in routine clinical practice, the 22 question items of the PSQ fit on 1 page and take ∼5 minutes to complete and ∼1 minute to score.
Our study confirms the findings from a single-center study13 that compared the PSQ scale score to PSG data for prediction of OSAS-related sleepiness and neurobehavioral outcomes and extends those findings by including a broader range of health outcomes in children at multiple clinical sites. Our findings are consistent with other studies showing that OSAS severity on PSG does not show strong associations with OSAS-related neurobehavioral morbidity or predict its response to treatment.3–10 Our findings and previous results confirm that PSQ and PSG data assess different, complementary aspects of health burden in OSAS. Whereas the PSG reveals the underlying pathophysiology that defines OSAS, the PSQ tabulation of OSAS symptoms may better reflect or summarize the behavioral, quality of life, and sleepiness complaints that concern families and stand to improve with adenotonsillectomy. Of note, neither the PSQ nor the AHI predicted objectively measured attention or executive impairment at baseline or change after adenotonsillectomy. Our objective measure of attention or executive dysfunction, made in a controlled experimental setting, may be a less robust marker of OSAS disease burden or response to adenotonsillectomy than parent rating measures, as suggested by previous analyses from the CHAT study.10
The major strengths of this study are that data were prospectively collected from a large, well-characterized, multicenter sample of children with PSG-proven OSAS, and selected study outcomes represented a range of important OSAS-related health outcomes (neurobehavioral morbidity, sleepiness, and quality of life) that proved responsive to adenotonsillectomy.10 Data collection was rigorously standardized with high levels of quality control.
Limitations include a study design that excluded more severely affected children (3.5% of those screened) with higher apnea hypopnea indices or sustained hypoxemia, in whom PSG data may have been a better predictor of health outcomes.10 Conversely, the CHAT study also excluded 49% of otherwise eligible children who proved to have very low AHI values. The PSQ range was also restricted because all participants had to snore and be operative candidates, so the low range of scores was not captured. The study included a restricted age range that could have limited capture of the full disease burden of OSAS. Because parents in the adenotonsillectomy arm of CHAT were not blinded, the experience of the surgery may have influenced their ratings of improved outcomes. However, the stronger associations of the PSQ versus PSG data with baseline OSAS-related morbidities before randomization would not have been affected. Analyses were not adjusted for multiple comparisons, and there was no intention to provide a prediction equation for clinical use. Finally, we did not look at outcomes outside the very broad metric of neurologic issues, such as growth, cardiovascular, metabolic, and operative risks, which are all very important.
Our data do not support the use of the PSQ as a replacement for the PSG, nor do they support the sole use of either the PSG or PSQ for making individual treatment decisions. Notably, despite the significant associations of PSQ total scores with tested comorbidities and postsurgical improvements, the c-statistics for all models (PSQ or PSG or combined) were low to moderate. In this study, only 77% of habitually snoring children with PSG-confirmed OSAS had “positive” PSQ scores. In a previous study of children scheduled for adenotonsillectomy, the PSQ had a sensitivity of 78% and a specificity of 72% for PSG-defined OSAS,13 with 74% of participants classified accurately. To the extent that the PSQ does not show results identical to those of the PSG, our data suggest that the discrepancy may offer an opportunity to improve some aspects of clinical care rather than an invalid assessment to be disregarded when PSG data are available.
Conclusions
The PSQ provides valuable information with respect to behavior, sleepiness, and quality of life and is likely to be useful as an adjunct to PSG in assessment of children with OSAS who may be surgical candidates. This simple, quick, and inexpensive measure of symptom burden could help clinicians and families assess, in a standardized manner, whether OSAS in any given child is likely to be accompanied by behavioral, sleepiness, and quality of life consequences or, perhaps more importantly, whether those consequences are likely to improve after contemplated surgical intervention. More broadly, current results highlight the important, outcome-defined value of a careful, traditional, office-based clinical assessment, even if that assessment does not correspond well with objective laboratory measures that define pediatric OSAS.2,25,26
Acknowledgments
We acknowledge the support of the CHAT research staff: Jean Arnold, Mary Ellen Carroll, Beth Ann Compton, Mary Anne Cornaglia, Casey Critchlow, Judith Emancipator, Melissa Fernando, Theresa Friederich, Amanda Goodman, Elise Hodges, Xiaoling Hou, Laurie Karamessinis, Kim Lacy, Megan McDougall, Daniel Mobley, Michelle Nicholson, Angela Orlando, Deborah L. Ruzicka, Gauri Sathe, Nancy Scott, Susan Surovec, Omarya Vega, Xingmei Wang, and Catherine Williams. We also appreciate the generous participation of the families enrolled in the study. We are grateful for the helpful guidance during the study of the CHAT Data and Safety Monitoring Board: Lynn Taussig, MD (Chair); Thomas Anders, MD; Julie Buring, ScD; Karina Davidson, PhD; Estelle Gauda, MD; Steven Piantadosi, MD, PhD; Bennett Shaywitz, MD; Benjamin Wilfond, MD; Tucker Woodson, MD; and Robert Zeiger, MD.
For the CHAT: Boston Children’s Hospital, Harvard University, Boston, MA (Eliot Katz, MD; Janice Ware, PhD; Dwight Jones, MD); Brigham and Women’s Hospital and Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA (Susan Redline, MD, MPH; Rui Wang, PhD); Cardinal Glennon Children’s Hospital, Saint Louis University, St Louis, MO (Ron Mitchell, MD; Shalini Paruthi, MD; Karen Snyder, MS); University of Pennsylvania/Children’s Hospital of Philadelphia (Carole Marcus, MBBCh; Nina H. Thomas, PhD; Lisa Elden, MD); Cincinnati Children’s Medical Center, University of Cincinnati, Cincinnati, OH (Raouf Amin, MD; Dean Beebe, PhD; Paul Willging, MD); Montefiore Children’s Hospital, Albert Einstein College of Medicine, Yeshiva University, Bronx, NY (Raanan Arens, MD; Hiren Muzumdar, MD; Shelby Harris, PsyD, CBSM); Rainbow Babies and Children’s Hospital, University Hospitals Case Medical Center, Case Western Reserve University School of Medicine, Cleveland, OH (Carol Rosen, MD; H. Gerry Taylor, PhD; Robert Sprecher, MD; James Arnold, MD); University of Kentucky, Louisville, Kentucky (David Gozal, MD); University of Michigan, Ann Arbor, MI (Ronald Chervin, MD; Susan Garetz, MD, Bruno Giordani, PhD; Tim Hoban, MD); University of Pennsylvania, Philadelphia, PA (Susan Ellenberg, PhD; Renée H. Moore, PhD; Kim Lacy, RN, BSN).
Footnotes
Dr Rosen participated in the study design, data collection, and data interpretation and drafted and edited the manuscript; Dr Wang was the primary statistician who analyzed and interpreted the data and helped edit the manuscript; Dr Taylor participated in the study design, provided oversight of neurobehavioral data collection and interpretation, and helped edit the manuscript; Dr Marcus participated in the study design, data collection, and interpretation and helped edit the manuscript; Drs Katz, Paruthi, Arens, Muzumdar, Mitchell, and Jones participated in data collection and interpretation and helped edit the manuscript; Dr Garetz participated in the study design, oversight of the surgical quality assurance core, and data interpretation and helped edit the manuscript; Mr Weng assisted with data collection, statistical programming, and analysis and reviewed and revised the manuscript; Dr Ellenberg helped design the study, oversaw the data management center, participated in data interpretation, and reviewed and revised the manuscript; Dr Redline designed the study, designed data collection tools, oversaw the conduct of the study, oversaw the PSG reading center, participated in data interpretation, and reviewed and revised the manuscript; Dr Chervin participated in the study design, oversight of neurobehavioral and surgical quality assurance cores, and interpretation of the data, developed the proposal for this manuscript, and edited the manuscript; and all authors approved the final manuscript as submitted.
This trial has been registered at www.clinicaltrials.gov (identifier NCT00560859).
FINANCIAL DISCLOSURE: Relevant to this work, Dr Chervin is named in or has developed patented and copyrighted materials owned by the University of Michigan and designed to assist with assessment or treatment of sleep disorders. These materials include the Pediatric Sleep Questionnaire Sleep-Related Breathing Related Disorder scale, used in the research reported here. Dr Chervin serves on the boards of the American Academy of Sleep Medicine and the International Pediatric Sleep Society, is a section editor for UpToDate, has edited a book for Cambridge University Press, has received support for research and education from Philips Respironics, Fisher Paykel, and Jawbone, and has consulted for MC3 and Zansors. The other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded by National Institutes of Health grants HL083075, HL083129, UL1 RR024134, and UL1 RR024989. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Rosen has consulted for Natus, Advance-Medical and is a consultant for Jazz Pharmaceuticals. Dr Chervin is named in or has developed patented and copyrighted materials owned by the University of Michigan and designed to assist with assessment or treatment of sleep disorders; these materials include the Pediatric Sleep Questionnaire Sleep-Related Breathing Disorder scale, used in the research reported here. This questionnaire is licensed online at no charge by the University of Michigan to appropriate users and (for electronic use) to Zansors. Dr Marcus received a loan of research equipment from Philips Respironics and Ventus Medical. The other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Marcus CL, Brooks LJ, Draper KA, et al. American Academy of Pediatrics . Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):576–584 [DOI] [PubMed] [Google Scholar]
- 2.Marcus CL, Brooks LJ, Draper KA, et al. American Academy of Pediatrics . Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3). Available at: www.pediatrics.org/cgi/content/full/130/3/e714 [DOI] [PubMed] [Google Scholar]
- 3.Chervin RD, Archbold KH. Hyperactivity and polysomnographic findings in children evaluated for sleep-disordered breathing. Sleep. 2001;24(3):313–320 [DOI] [PubMed] [Google Scholar]
- 4.O’Brien LM, Ivanenko A, Crabtree VM, et al. Sleep disturbances in children with attention deficit hyperactivity disorder. Pediatr Res. 2003;54(2):237–243 [DOI] [PubMed] [Google Scholar]
- 5.Huang YS, Chen NH, Li HY, Wu YY, Chao CC, Guilleminault C. Sleep disorders in Taiwanese children with attention deficit/hyperactivity disorder. J Sleep Res. 2004;13(3):269–277 [DOI] [PubMed] [Google Scholar]
- 6.O’Brien LM, Mervis CB, Holbrook CR, et al. Neurobehavioral implications of habitual snoring in children. Pediatrics. 2004;114(1):44–49 [DOI] [PubMed] [Google Scholar]
- 7.Melendres MC, Lutz JM, Rubin ED, Marcus CL. Daytime sleepiness and hyperactivity in children with suspected sleep-disordered breathing. Pediatrics. 2004;114(3):768–775 [DOI] [PubMed] [Google Scholar]
- 8.Beebe DW, Wells CT, Jeffries J, Chini B, Kalra M, Amin R. Neuropsychological effects of pediatric obstructive sleep apnea. J Int Neuropsychol Soc. 2004;10(7):962–975 [DOI] [PubMed] [Google Scholar]
- 9.Gottlieb DJ, Chase C, Vezina RM, et al. Sleep-disordered breathing symptoms are associated with poorer cognitive function in 5-year-old children. J Pediatr. 2004;145(4):458–464 [DOI] [PubMed] [Google Scholar]
- 10.Marcus CL, Moore RH, Rosen CL, et al. Childhood Adenotonsillectomy Trial (CHAT) . A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368(25):2366–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aurora RN, Zak RS, Karippot A, et al. American Academy of Sleep Medicine . Practice parameters for the respiratory indications for polysomnography in children. Sleep. 2011;34(3):379–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roland PS, Rosenfeld RM, Brooks LJ, et al. American Academy of Otolaryngology—Head and Neck Surgery Foundation . Clinical practice guideline: polysomnography for sleep-disordered breathing prior to tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;145(1 suppl):s1–s15 [DOI] [PubMed] [Google Scholar]
- 13.Chervin RD, Weatherly RA, Garetz SL, et al. Pediatric sleep questionnaire: prediction of sleep apnea and outcomes. Arch Otolaryngol Head Neck Surg. 2007;133(3):216–222 [DOI] [PubMed] [Google Scholar]
- 14.Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1(1):21–32 [DOI] [PubMed] [Google Scholar]
- 15.Redline S, Amin R, Beebe D, et al. The Childhood Adenotonsillectomy Trial (CHAT): rationale, design, and challenges of a randomized controlled trial evaluating a standard surgical procedure in a pediatric population. Sleep. 2011;34(11):1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korkman N, Kirk U, Kemp S. NEPSY: A Developmental Neuropsychological Assessment Manual. New York, NY: Psychological Corporation; 1998 [Google Scholar]
- 17.Gioia G, Isquith P, Guy S, Kenworthy L. BRIEF: Behavior Rating Inventory of Executive Function. Odessa, FL: Psychological Assessment Resources; 2000 [Google Scholar]
- 18.Conners C. Connors Rating Scales: Revised Technical Manual. 5th ed. North Tonawanda, NY: Multi-Health Systems; 2001 [Google Scholar]
- 19.Achenbach T, Rescorla L. Manual for the ASEBA School-age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth & Families; 2001 [Google Scholar]
- 20.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800–812 [DOI] [PubMed] [Google Scholar]
- 21.Franco RA, Jr, Rosenfeld RM, Rao M. First place—resident clinical science award 1999. Quality of life for children with obstructive sleep apnea. Otolaryngol Head Neck Surg. 2000;123(1 pt 1):9–16 [DOI] [PubMed] [Google Scholar]
- 22.Iber C, Ancoli-Israel S, Chesson A, Quan S, American Academy of Sleep Medicine The AASM Manual for Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specification. Westchester, IL: American Academy of Sleep Medicine; 2007 [Google Scholar]
- 23.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- 24.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Mahwah, NJ: Erlbaum; 1988 [Google Scholar]
- 25.Carroll JL, McColley SA, Marcus CL, Curtis S, Loughlin GM. Inability of clinical history to distinguish primary snoring from obstructive sleep apnea syndrome in children. Chest. 1995;108(3):610–618 [DOI] [PubMed] [Google Scholar]
- 26.Preutthipan A, Chantarojanasiri T, Suwanjutha S, Udomsubpayakul U. Can parents predict the severity of childhood obstructive sleep apnoea? Acta Paediatr. 2000;89(6):708–712 [DOI] [PubMed] [Google Scholar]