Abstract
Fibronectin (FN) is an extracellular matrix (ECM) protein including numerous fibronectin type III (FNIII) repeats with different functions. The alternatively spliced FN variant containing the extra domain A (FNIII EDA), located between FNIII 11 and FNIII 12, is expressed in sites of injury, chronic inflammation, and solid tumors. Although its function is not well understood, FNIII EDA is known to agonize Toll-like receptor 4 (TLR4). Here, by producing various FN fragments containing FNIII EDA, we found that FNIII EDA's immunological activity depends upon its local intramolecular context within the FN chain. N-terminal extension of the isolated FNIII EDA with its neighboring FNIII repeats (FNIII 9-10-11) enhanced its activity in agonizing TLR4, while C-terminal extension with the native FNIII 12-13-14 heparin-binding domain abrogated it. In addition, we reveal that an elastase 2 cleavage site is present between FNIII EDA and FNIII 12. Activity of the C-terminally extended FNIII EDA could be restored after cleavage of the FNIII 12-13-14 domain by elastase 2. FN being naturally bound to the ECM, we immobilized FNIII EDA-containing FN fragments within a fibrin matrix model along with antigenic peptides. Such matrices were shown to stimulate cytotoxic CD8+ T cell responses in two murine cancer models.
Fibronectin (FN) is a ubiquitous multidomain extracellular matrix (ECM) component and is critically important in numerous ECM-dependent processes such as cell adhesion, migration, growth, and differentiation1. Notably, certain functions of FN depend on the presence of the alternatively spliced type III repeats extra domains A (FNIII EDA) and B (FNIII EDB). These extra domains are expressed in the vicinity of developing vessels during embryogenesis2,3 and later in particular cases such as after tissue injury4,5,6, within tumors7,8,9, and at sites of chronic inflammation such as psoriatic lesions10,11,12. Although expression of FNIII EDA and FNIII EDB is crucial for embryonic development13, their postnatal functions remain incompletely elucidated.
One of the most interesting functions of FNIII EDA is its ability to act as an endogenous ligand for the pattern recognition receptor Toll-like receptor 4 (TLR4)14 and activate its signaling pathway in a MyD88-dependent manner, which leads to the activation of NF-κB. Importantly, this TLR4-agonizing activity has been shown to contribute to disease progression, specifically in driving fibrosis in scleroderma15 and stimulating the inflammatory cascade in psoriatic lesions10.
Here, we sought to explore the importance of the local molecular context of FNIII EDA in its immunological activities, as the domain resides in the vicinity of other active FNIII repeats in the natural sequence FNIII 9-10-11-EDA-12-13-14 (Fig. 1a). FNIII 10 contains the well-studied RGD sequence that binds integrins such as αvβ316 and FNIII 9 contains the synergy site PHSRN that is critical for α5β1 integrin activation17. FNIII 11 has been shown to bind anastellin18, a small FN type I fragment, increasing its thermolytic digestion. The FNIII 12-13-14 domain is one of the major heparin-binding sites of FN19 and has been shown to promiscuously bind growth factors20,21. In some of FN's biological activities, this molecular context has been shown to be important; for example, the proximal location of the integrin-binding domain and the growth factor binding domain has been shown to lead to synergistic signaling between the ligated integrin and the ligated growth factor receptor, potentiating growth factor signaling21,22,23. Based on these recent findings, we were motivated to investigate the potential importance of the molecular context of FNIII EDA's placement within the FN chain upon its activity in inducing immune responses.
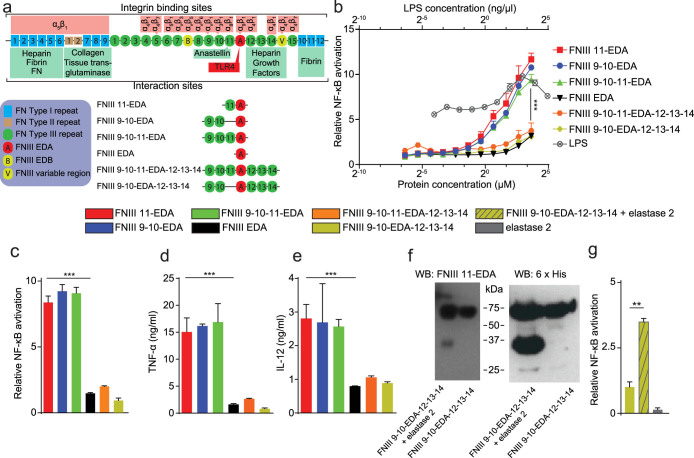
Figure 1. TLR4 activation by FNIII EDA is modulated by the context of its neighboring FNIII domains.
(a) FNIII EDA-containing FN fragments produced and of full length FN with some of its interaction sites displayed. (b) Activation of NF-κB in THP1-Blue cells as an indication of TLR agonization in response to various FNIII EDA-containing FN fragments, compared to LPS. The presence of a domain N-terminal to the FNIII EDA domain enhances activation, and the neighboring C-terminal domain abrogates activation. In the absence of the FNIII 12-13-14 domain, activation is comparable to that achieved by LPS. (c) Activation of NF-κB in HEK-Blue TLR4 cells as a cellular bioassay of TLR4 agonization. The presence of a domain N-terminal to the FNIII EDA domain enhances TLR4 agonization, and the neighboring C-terminal domain abrogates TLR4 agonization. In the absence of the FNIII 12-13-14 domain, TLR4 agonization is strong. (d, e) Production of TNF-α (d) or IL-12p70 (e) from murine bone marrow-derived DCs upon stimulation with various FNIII EDA-containing FN fragments. In DCs, the same pattern of activation was observed as with the previous cell lines: the presence of a domain N-terminal to the FNIII EDA domain enhances activation, and the neighboring FNIII 12-13-14 domain abrogates activation. (f) Western blots of the digestion product of FNIII 9-10-EDA-12-13-14 by elastase 2 and undigested control, blotted against the FNIII EDA domain (N-terminal to a predicted elastase 2 cleavage site) and a 6xHis tag (C-terminal). Cleavage was observed to yield two fragments of similar molecular weight, consistent with the presence of elastase 2 cleavage site at position 87 of the 94 amino acid-long FNIII EDA domain. (g) Activation of HEK-Blue TLR4 cells with the digested and undigested FNIII 9-10-EDA-12-13-14. Digestion of FNIII 9-10-EDA-12-13-14 with elastase 2 recovers activation, consistent with elastase 2-dependent cryptic agonization of TLR4. Cellular experiments in c, d, e and f were done in the presence of 10 μg/ml polymixin B, to avoid any influence of potentially contaminating LPS. In c, d, e and f, bars and curves represent mean ± SEM of triplicate cultures from two independent experiments. **P < 0.01; ***P < 0.001.
Moreover, FNIII EDA is known to induce the expression of inflammatory cytokines and matrix metalloproteinases (MMPs)24, and these activities have been shown to be dependent on the presence of the neighboring domains FNIII11 and FNIII1224. For such exposure to occur in vivo, it would require FN to be cleaved by a protease, but only the bacterial protease thermolysin from Bacillus thermoproteolyticus has been shown to cleave FN close to the C-terminus of FNIII EDA25. Because no mammalian protease had been shown to carry out the same function, we were thus also motivated to search for mammalian proteases that might modulate the activity of FNIII EDA by inducing such cleavage.
Finally, because FNIII EDA can induce cellular immune responses including activation of CD8+ T cell responses26,27,28,29,30,31, the domain has also been explored as a cancer vaccine adjuvant in mouse models28,30,31. However, FNIII EDA showed potency mostly in combination with other TLR agonists28,31. Here, because FNIII EDA is naturally displayed bound to the ECM, we hypothesized that its relatively weak adjuvancy could be related to the fact that the domain was delivered as a soluble protein. Therefore, we also explored whether FNIII EDA variants could have better adjuvant potency when bound to the ECM.
Results
Production of FN fragments
To investigate the importance of the biomolecular context of FNIII EDA's activity as a TLR4 agonist, we expressed various FN fragments containing FNIII EDA (Fig. 1a). As the simplest construct, FNIII EDA was produced as a single FNIII repeat. Extending N-terminally, FNIII 11 was incorporated to produce FNIII 11-EDA. To further explore N-terminal extensions, FNIII 9-10-EDA and FNIII 9-10-11-EDA were expressed, thus comprising the cell-binding domain with the synergy site1. To explore C-terminal extensions, FNIII 9-10-EDA-12-13-14 and FNIII 9-10-11-EDA-12-13-14 were expressed, thus comprising the FNIII 12-13-14 domain, which binds heparin1 and growth factors20. All FN fragments were successfully produced, and endotoxin levels were verified as being under 0.2 EU/μg using a limulus amebocyte lysate assay. Purity of the FN fragments is shown by SDS-PAGE in Fig. S1a.
The immunostimulatory activity of FNIII EDA can be modulated by addition or deletion of neighboring domains
To explore the dependence of TLR4 agonization upon the presence of various neighboring domains in the FN protein chain, we characterized TLR agonization using THP1-Blue cells (derived from the human monocytic THP1 cell line and modified to contain a reporter of agonization of several TLRs) and HEK-Blue TLR4 cells (derived from the human embryonic kidney 293 cell line and modified to contain a reporter of agonization of TLR4) as well as cytokine expression in dendritic cells (DCs). As expected, FNIII EDA agonized TLR4 in THP1-Blue cells in a dose-dependent manner (Fig. 1b). Interestingly, addition of FNIII 11 at the N-terminus, i.e. FNIII 11-EDA, agonized TLR4 up to 4-fold more potently (P < 0.001) in THP1-Blue cells and up to 5-fold more potently (P < 0.001) in HEK-Blue TLR4 cells, compared to FNIII EDA (Fig. 1b, c). To explore if this gain of activity derived from intrinsic activity within FNIII 11 or from an effect of N-terminal extension, which could for example influence the stability of the FNIII EDA domain, we produced the alternative construct FNIII 9-10-EDA, i.e. containing a neighboring N-terminal extension with FN's cell-binding domain and synergy site yet not the N-terminal FNIII 11 domain. Surprisingly, FNIII 9-10-EDA agonized TLR4 to the same extent as FNIII 11-EDA in both cell lines. Moreover, the complete N-terminal construct, FNIII 9-10-11-EDA, showed statistically equivalent behavior (Fig. 1b, c). We further explored the impact of neighboring FNIII repeats by extending FNIII EDA to the C-terminus with the native structure FNIII 9-10-11-EDA-12-13-14, as well as FNIII 9-10-EDA-12-13-14. Unexpectedly, in both THP1-Blue and HEK-Blue TLR4 cells, C-terminal extension with the FNIII 12-13-14 domain abrogated TLR4 agonization (Fig. 1b, c). We then tested the ability of the different FNIII EDA-containing constructs to stimulate the production of inflammatory cytokines in DCs, which are key monitors of innate immune signals such as TLR4 agonists. DC expression of TNF-α and IL-12p70 closely paralleled the TLR4 agonization that was observed in THP1-Blue and HEK-Blue TLR4 cells. The N-terminal extension of FNIII EDA strongly enhanced cytokine response, while C-terminal extension with FNIII 12-13-14 strongly decreased it (Fig. 1d, e).
The immunostimulatory activity of FNIII EDA is cryptic and dependent upon elastase 2 cleavage
We sought to understand the biological significance of the profound diminution of FNIII EDA activity through C-terminal extension with the FNIII 12-13-14 domain, hypothesizing that the FNIII EDA repeat is cryptic in its TLR4 agonizing activity. Using bioinformatics analytical tools32, we suspected the presence of an elastase 2 (a serine protease, also referred to as neutrophil elastase) substrate between FNIII EDA and FNIII 12, after ile87 in the 94 amino acid-long FNIII EDA domain. We verified the presence of such a cleavage site by incubating FNIII 9-10-EDA-12-14 with elastase 2. The digestion products were analyzed using Western blotting, detecting FNIII EDA or the 6xHis tag that is located at the C-terminus of the FN fragment. Therefore, the expected digestion products of the 67 kDa FNIII 9-10-EDA-12-14 protein are a 33 kDa FNIII EDA-positive fragment and a 34 kDa 6xHis tag-positive fragment. Western blotting confirmed the presence of the cleavage site, since both digestion products were detected (Fig. 1f).
To assess if the biological activity of FNIII 9-10-EDA-12-14 could be recovered after digestion by elastase 2, we characterized TLR4 agonization in the HEK-Blue TLR4 cells. Proteolytic enhancement of TLR4 agonization was observed (Fig. 1g), consistent with activity obtained with the recombinant fragments lacking FNIII12-14 although not as high. The elastase 2 enzyme itself did not activate TLR4 in this assay (Fig. 1g). Other proteases tested (MMP-2, MMP-3, MMP-9, thrombin and plasmin) did not show cleavage of the protein as indicated by SDS-PAGE analysis (Fig. S1b).
Fibrin matrices functionalized with FNIII 9-10 + FNIII 11-EDA mediate CD8+ T cell expansion and effector phenotype
Since FNIII 11-EDA showed potent TLR4 activation in vitro, we evaluated its immunological activity in vivo. We selected a model based on the xenoantigen ovalbumin (OVA), in which vaccine-induced weak CD8+ T cell responses could potentially be further boosted (Fig. 2a). To evaluate boosting the CD8+ T cell responses, we co-delivered FNIII 11-EDA and the H2kb MHC I immunodominant peptide OVA257–26433. Because FNIII EDA is naturally present within FN in the ECM, we decided to incorporate the FN fragments bound in matrix to mimic the in vivo situation. Moreover, this strategy allows creating an adjuvant-rich microenvironment, which has proved to be successful for cancer vaccination in other studies34. Here, as a matrix model, we used a fibrin hydrogel resembling a fibrin clot, with a volume of 150 μL and a concentration of 8 mg/mL of fibrinogen. To be incorporated in the matrix, the FN fragments were designed to contain a transglutaminase substrate sequence derived from α2-plasmin inhibitor (NQEQVSPL, denoted herein TG) at the N-terminus. Therefore, the FN fragments could be covalently incorporated in the matrix through the transglutaminase activity of factor XIIIa, which naturally crosslinks fibrin35. In addition, the OVA257–264 peptide was designed to be incorporated in fibrin. The TG sequence was fused to a longer sequence of OVA, namely OVA250–264, so as to comprise the protease substrate site SGLEQLE within the OVA chain that allows processing by APC antigen proteolysis machinery and liberation of the OVA257–264 epitope from TG-OVA250–264 for cross-presentation on MHC I36. Furthermore, because cell migration within fibrin is enhanced by integrin binding, we incorporated the major integrin-binding domain of FN, FNIII 9-1037. Two fibrin matrix compositions were tested: fibrin functionalized with FNIII 9-10 plus FNIII 11-EDA and fibrin functionalized with FNIII 9-10-EDA.
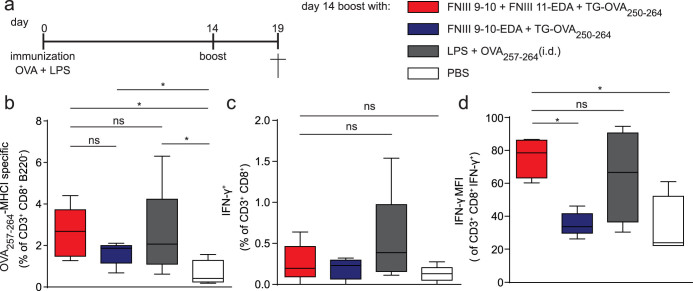
Figure 2. Fibrin matrices functionalized with FNIII 9-10 + FNIII 11-EDA stimulate an antigen-specific CD8+ T cell response.
(a) Immunization schedule. C57BL/6J mice were immunized with 50 μg OVA and 20 μg LPS on day 0. The ability of FNIII EDA-containing fibrin-binding FN fragments to boost a CD8+ T cell response was tested on day 14 by implanting s.c. fibrin matrices functionalized with 5 nmol of TG-OVA250–264 (comprising the MHC-I binding immunodominant peptide of ovalbumin, OVA257–264) + 5 nmol of FNIII 11-EDA or FNIII 9-10-EDA (each with an N-terminal TG domain); alternatively mice were injected i.d. with 5 nmol of soluble OVA257–264 + 50 μg of LPS or with PBS only. On day 19, mice were sacrificed and the spleen was harvested. (b) Fibrin gels functionalized with fibrin-binding FNIII 9-10 + FNIII 11-EDA (each with an N-terminal TG domain) + TG-OVA250–264 induced levels of OVA257–264-MHC-I-specific CD8+ T cells in the spleen comparable to that induced by i.d. injections of LPS + OVA257–264. (c, d) IFN-γ production from splenocytes restimulated for 6 h ex vivo with OVA257–264 were evaluated by flow cytometry. The proportion of IFN-γ producing CD8+ T cell was similar among the different groups. However, the production of IFN-γ by CD8+ T cells was greater, as shown by the mean fluorescence intensity (MFI) of IFN-γ intracellular staining, in mice that were boosted with fibrin matrices functionalized with FNIII 9-10 + FNIII 11-EDA (each with an N-terminal TG domain) + TG-OVA250–264 or with LPS and OVA257–264; the group boosted with FNIII 11-EDA yielded statistically similar responses as the group boosted with LPS. Box plots represent median ± 95% confidence interval (n = 5). *P < 0.05; ns, not significant.
First, we gave an intradermal (i.d.) vaccine prime dose of soluble OVA adjuvanted with lipopolysaccaride (LPS), which resulted in strong CD4+ but modest CD8+ T cell responses, as indicated by OVA257–264:H2kb p:MHC I pentamer binding (Fig. 2b). Boosting with the soluble MHC I epitope OVA257–264 + LPS increased the frequency of antigen-specific CD8+ T cells (P < 0.05). Boosting was also achieved by implanting fibrin matrices functionalized with FNIII 9-10 + FNIII 11-EDA + TG-OVA250–264 (P < 0.05) and with FNIII 9-10-EDA + TG-OVA250–264 (P < 0.05), although the boosting was marginally improved with the former (Fig. 2b). As an indication of the effector phenotype of these cells, we performed ex vivo restimulation of splenocytes with OVA257–264: although the frequency of IFN-γ-expressing CD8+ T cells was not enhanced by the FNIII EDA-containing proteins (Fig. 2c), the intensity of IFN-γ expression was enhanced by treatment with fibrin functionalized with FNIII 9-10 + FNIII 11-EDA (P < 0.05) (Fig. 2d).
Fibrin matrices functionalized with FNIII 11-EDA ± FNIII 9-10 mediate functional cytotoxic T lymphocyte responses in the E.G7-OVA tumor model
To explore the extent to which FNIII 11-EDA-generated CD8+ T cells were capable of cell killing, i.e. function as cytotoxic T lymphocytes (CTLs), we employed a thymoma tumor model in which the cells express the xenoantigen OVA (E.G7-OVA). Tumor cells were injected subcutaneously (s.c.) in the back, and resulting tumors were allowed to reach a volume of 50 mm3. At that point, a fibrin matrix containing the FN fragments of interest and TG-OVA250–264 was implanted at a distant site at the level of the shoulder girdle close to the brachial lymph nodes or soluble OVA257–264 controls were injected i.d. in the four footpads, and delays in tumor growth were measured. Soluble controls were injected i.d. in the four footpads.
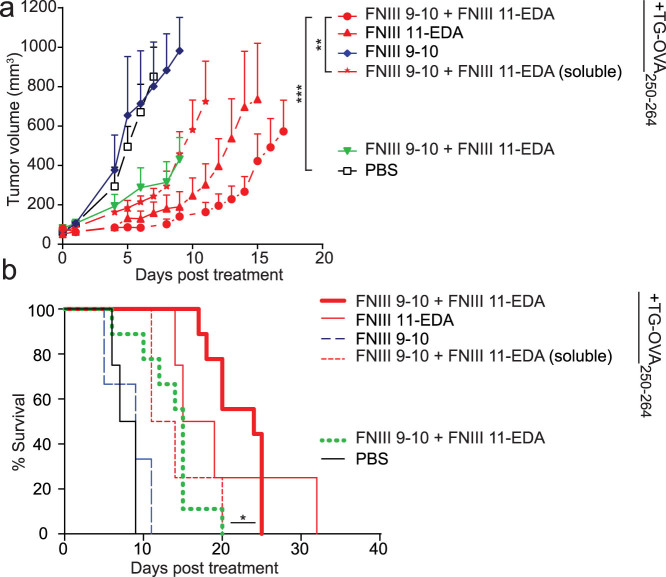
When the fibrin matrix was implanted only once, strong delays in tumor growth were observed with treatments comprising FNIII 11-EDA, especially in conjunction with FNIII 9-10 (Fig. 3a). Omission of FNIII 11-EDA from the fibrin matrix resulted in no delay of tumor growth. Although the TG-OVA250–264 peptide-free FNIII-EDA-containing matrix slightly reduced the tumor growth, the absence of antigen significantly reduced the response, demonstrating that the response is not mainly based on a general upregulation of immunity but is rather specific to the antigen co-incorporated in the fibrin matrix. Moreover, FNIII 9-10 + FNIII 11-EDA was not as effective when administered not bound to fibrin matrix, demonstrating functionality in the matrix-immobilized form. The Kaplan-Meier survival curves shown in Fig. 3b illustrate the benefits of co-delivery of FNIII 9-10 and FNIII 11-EDA with the tumor cell-specific peptide epitope. Although all tumors eventually reached 1000 mm3, the substantial delay in approaching this volume induced by a fibrin matrix with FNIII 11-EDA, especially in conjunction with FNIII 9-10, clearly indicates induction of functional CTL responses.
Figure 3. Single implantation of fibrin matrices functionalized with FNIII 9-10 + FNIII 11-EDA in E.G7-OVA tumor bearing mice reduces tumor growth rate.
C57BL/6J mice were injected s.c. with 106 E.G7-OVA cells on the back. When tumors reached 50 ± 5 mm3, the ability of fibrin-binding FNIII EDA-containing FN fragments to induce a functional CD8+ T cell response was tested by treating mice with fibrin matrices functionalized with various matrix formulations implanted s.c. or with soluble formulations injected i.d. Animals were sacrificed either when the tumor reached 1000 mm3 or for humane reasons. (a, b) Tumor growth was significantly delayed in animals vaccinated using a fibrin matrix functionalized with FNIII 11-EDA ± FNIII 9-10 (each with an N-terminal TG domain) + TG-OVA250–264 both in terms of tumor volume (a) and animal survival (b). TG-OVA250–264-free matrices also proved slightly effective in delaying tumor growth (a) and extending animal survival (b). Growth curves were stopped when the second animal of the corresponding group died, the value corresponding to the first animal which died was kept as a constant until the curve was stopped. Growth curves represent mean ± SEM; Kaplan-Meier survival-curves (n = 4–9). *P < 0.05; **P < 0.01; ***P < 0.001.
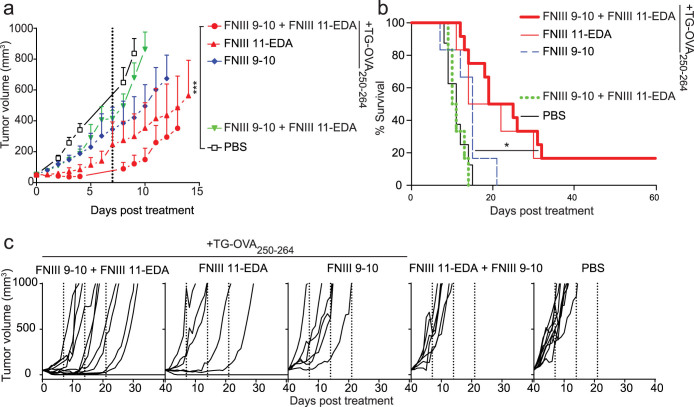
In the experiments described above, a single administration of a functionalized fibrin matrix was performed and the fibrin was slowly resorbed, whereas physiologically the FNIII EDA-containing FN proteolytic fragments would be present chronically. Thus, to further explore the ability of its chronic presence to induce CTL activity, we administered the functional fibrin matrices weekly after the tumors reached 50 mm3. In this model also, tumor growth was significantly delayed in animals treated with fibrin matrices functionalized with FNIII 11-EDA ± FNIII 9-10 + TG-OVA250–264 both in terms of tumor volume (Fig. 4a) and survival (Fig. 4b). Furthermore, none of the mice treated with PBS or with fibrin matrices lacking either FNIII 11-EDA or TG-OVA250–264 survived more than 21 days post treatment, whereas half of the mice treated with fibrin matrices functionalized with FNIII 11-EDA + TG-OVA250–264 ± FNIII 9-10 survived longer than 21 days and 20% even showed complete regression of the tumor, as shown in survival curves (Fig. 4b) and the individual growth curves (Fig. 4c). The efficacy of multiple injections of the soluble formulation was not assessed here as that approach already proved to be significantly less effective than functionalized fibrin matrix forms with a single administration. In this model of chronic exposure, co-treatment with FNIII 9-10 and FNIII 11-EDA yielded stronger CTL responses than mono-treatment with FNIII 11-EDA, both with the TG-OVA250–264 peptide antigen comprising the OVA257–264 epitope.
Figure 4. Multiple implantations of fibrin matrices functionalized with FNIII 11-EDA ± FNIII 9-10 reduces E.G7-OVA tumor growth rate and in cases mediates regression.
C57BL/6J mice were injected s.c. with 106 E.G7-OVA cells on the back. When tumors reached 50 ± 5 mm3, mice were treated weekly (day 0 and dashed lines) for three weeks with fibrin matrices functionalized with various formulations implanted s.c. Mice were sacrificed when the tumor reached 1000 mm3 or for humane reasons. Tumor growth was significantly delayed in animals treated using a fibrin matrix functionalized with FNIII 11-EDA ± FNIII 9-10 (each with an N-terminal TG domain) + TG-OVA257–264 both in terms of tumor volume (a) and animal survival (b). Individual growth curves (c) show regression in some of the animals treated with fibrin gels functionalized with TG-OVA250–264 and FNIII 11-EDA with or without FNIII 9-10 (each with an N-terminal TG domain). Growth curves were stopped when the second animal of the corresponding group died, the value corresponding to the first animal which died was kept as a constant until the curve was stopped. Growth curves represent mean ± SEM; Kaplan-Meier survival-curves (n = 6–12). *P < 0.05;***P < 0.001.
Fibrin matrices functionalized with FNIII 9-10 + FNIII 11-EDA mediate functional cytotoxic T lymphocyte responses in the B16–F10 tumor model with an endogenous antigen
In the experiments with E.G7-OVA cells, the surrogate tumor antigen OVA is a xenoantigen, not centrally tolerized in the mouse. To explore the activity of the FNIII 11-EDA domain to induce immunological responses versus a centrally tolerized antigen, we used the B16–F10 melanoma model. Fibrin matrices were functionalized with FNIII EDA-containing FN fragments and with the MHC I immunodominant epitope from tyrosinase-related protein-2 (TRP-2), namely TRP-2180–18838. As with the OVA-derived epitope, we designed the TRP-2-derived epitope to contain a longer peptide sequence from TRP-2 for proteolytic processing of the antigen and an N-terminal TG sequence for immobilization in fibrin, namely TG-TRP-2173–188. Mice were treated starting from the third day after tumor inoculation, when the tumors were palpable, and were further treated weekly for three weeks. Consistent with the results in the E.G7-OVA model, tumor growth was significantly delayed in animals treated with the fibrin matrix functionalized with FNIII 11-EDA + FNIII 9-10 + TG-TRP-2173-188, as shown by the individual (Fig. 5a) and aggregate (Fig. 5b) growth curves, as well as the Kaplan-Meier survival curves (Fig. 5c). As in the experiments with the E.G7-OVA cells, the combination of the antigen with either FNIII 11-EDA or FNIII 11-EDA + FNIII 9-10 yielded similar results; only the latter, which proved slightly more effective at reducing the initial tumor volume, was retained for testing against the aggressive B16–F10 model.
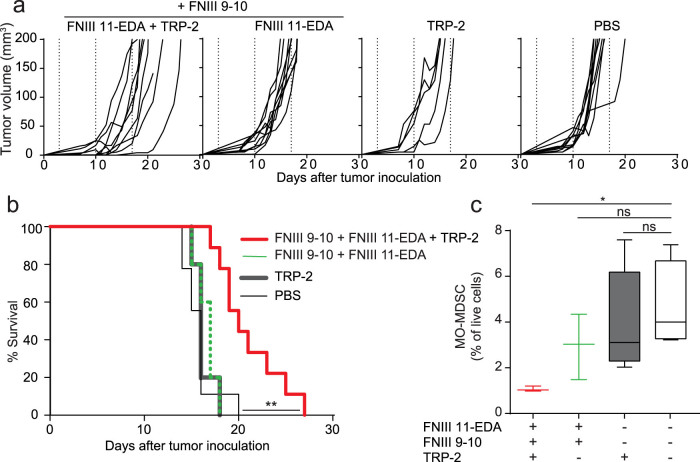
Figure 5. Multiple implantations of fibrin matrices functionalized with FNIII 9-10 + FNIII 11-EDA reduces B16-F10 melanoma tumor growth rate and modulates anti-tumor immune suppression.
C57BL/6J mice were injected s.c. with 2.5 × 105 B16–F10 cells on the back on day 0. On day 3, mice were treated weekly (dashed lines) for three weeks with fibrin gels functionalized with various formulations implanted s.c. Animal were sacrificed either when the tumor reached 200 mm3 or for humane reasons. Individual growth curves (a) and survival curves (b) show that tumor growth was significantly delayed in animals treated with fibrin matrices functionalized with FNIII 11-EDA + FNIII 9-10 (each with an N-terminal TG domain) + TG-TRP-2173–188, comprising the MHC-I immunodominant peptide epitope from the melanocyte-specific protein TRP-2 (TRP-2180–188). (c) The percentage of monocytic myeloid-derived suppressor cells (MO-MDSC, flow-cytometry gated as being CD11b+CD11c-MHC-II-Ly6ChiLy6G-) in the spleen was significantly reduced in the mice treated with the fibrin gel containing and FNIII 9-10 + FNIII 11-EDA (each with an N-terminal TG domain) + TG-TRP-2173–188 compared to the naïve control. Graphs show Kaplan-Meier survival-curves (n = 6–12). Box plots represent median ± 95% confidence interval (n = 3–5). *P < 0.05; **P < 0.01.
The B16–F10 melanoma model is known as an immune suppressive model, owing in part to the infiltration of monocytic myeloid-derived suppressor cells (MO-MDSCs) in the tumor as well as their proliferation in the spleen39,40. Therefore, we examined MO-MDSC numbers in the spleens of the B16-F10-bearing mice. Mice receiving the fibrin matrices containing TG-TRP-2173–188 but no FNIII fragments showed the same number of splenic MO-MDSCs compared to untreated mice or mice treated with fibrin matrices functionalized with FNIII 11-EDA + FNIII 9-10 without TG-TRP-2173–188. However, mice treated with fibrin matrices functionalized with FNIII 11-EDA + FNIII 9-10 + TG-TRP-2173–188 showed a substantial reduction in the number of splenic MO-MDSCs, demonstrating the ability of the FNIII 11-EDA domain to induce local (delay of tumor growth) and systemic (reduction in splenic MO-MDSC numbers) immune responses to an endogenous antigen, here the melanocyte-specific antigen TRP-2.
Discussion
Although roles of FN in processes such as embryogenesis and wound healing have been described41,42, FN's interaction with the immune system is not well understood. The FNIII EDA domain, present in a splice variant of FN, which is found in sites of transient inflammation as in tissue damage4,5,6 and of chronic inflammation such as psoriatic lesions10,11,12 and scleroderma lesions15, has been shown to agonize TLR414. In the case of scleroderma, for example, agonization of TLR4 was shown to drive a cycle of fibrosis, leading to further FNIII EDA-containing FN expression, increasing TLR4 agonization and promoting continued fibrosis15; as such, TLR4 agonization via FNIII EDA is central to the pathophysiology of the disease. Moreover, because of its ability to activate TLR4, FNIII EDA has been explored in cancer vaccinology, utilizing FNIII EDA-antigen fusion proteins as DC-targeting adjuvants to induce anti-tumor CTLs28,30,31. In this work, at least part of the effect of the FNIII EDA domain has been attributed to targeting the antigen for DC uptake through binding to TLR428,30. Nevertheless, FNIII EDA showed anti-tumor potency only in combination with other TLR ligands.
In our study, we first sought to explore the dependence of FNIII EDA's agonization of TLR4 on its intramolecular context, addressing roles played by the N-terminally neighboring FNIII 11 domain and FNIII 9-10 domain, which binds cell-surface integrins α5β1 and αvβ31,16,17,43, and by the C-terminally neighboring domain FNIII 12-13-14, which binds heparin and heparan sulfate19 and growth factors21. Moreover, we hypothesized that the agonization of TLR4 by FNIII EDA could be cryptic, because some of the physiological mechanisms involving FN have been shown to be carried out by cryptic sites44,45,46,47 requiring conformational modification or proteolytic cleavage to exert their functions, including stimulation of MMP expression by FNIII EDA24. Thus, we were also motivated to search for proteases that might enhance FNIII EDA activity.
We expressed a family of FN fragments (Fig. 1a) comprising the FNIII EDA repeat with various N-terminal and C-terminal extensions, so as to include FNIII 9-10, FNIII 11, and FNIII 12-13-14. Then, we characterized the ability of these recombinant FN fragments to agonize TLR4, activate DCs, stimulate CD8+ T cell expansion, and induce functional CTLs, where functionality was judged by cytotoxicity in a tumor vaccine model. Our in vitro results show that extension of the FNIII EDA domain to the N-terminus enhances its ability to agonize TLR4 (Fig. 1b, c) and induce inflammatory cytokine expression from DCs (Fig. 1d, e). This phenomenon was not sensitive to the details of the FNIII domain to the N-terminus, as extensions with FNIII 11, FNIII 9-10, and FNIII 9-10-11 yielded equivalent results (Fig. 1b, e). Thus, we conclude that co-binding of integrins α5β1 and αvβ3 is likely not involved in the effect. Rather, the observed 4–5 fold enhancement of TLR4-agonization activity may be due to structural stabilization of the FNIII EDA repeat, which, like other FNIII domains, is not stabilized by disulfide bonding. It is noteworthy that agonization of TLR4 by N-terminally extended FNIII EDA is very potent, comparable to that achieved by the prototypical microbial TLR4 agonist LPS (Fig. 1b), yet without the cytotoxicity that is accompanied by high doses of LPS, which leads to lower NF-κB signaling48 (Fig. 1b).
The possibility that our fragments could be contaminated with LPS was excluded by conducting these experiments in the presence of polymyxin B, which binds and inactivates LPS49,50; moreover, the expression and purification protocol for FNIII EDA (observed to be a poor agonist) and FNIII 11-EDA, FNIII 9-10-EDA, and FNIII 9-10-11 EDA (good agonists) were very similar. Finally, LPS endotoxin levels were tested in all the recombinant FN fragments and were found to be below 0.2 EU/μg in each.
Considering the C-terminal context of FNIII EDA, the existence of a protease substrate was suggested between the FNIII EDA and FNIII 12 domains using a bioinformatic analysis32, specifically at the C-terminal end of FNIII EDA (position 87 of 94 total). Experimentally, elastase 2 (neutrophil elastase) was observed to cleave between FNIII EDA and FNIII 12 (Fig. 1f), and cleavage was shown to enhance TLR4 agonization compared to the intact control (Fig. 1g). Returning to the observation of the role of FNIII EDA and TLR4 in lesions such as in psoriasis10,11,12 and scleroderma15, which are characterized by neutrophil infiltration51,52, it may be that neutrophil-derived elastase-2-mediated proteolysis of FN to potentiate a cryptic TLR4-agonizing site is involved in the pathophysiological mechanism.
Although FN is present in the serum, its main biological activities are carried out in immobilized form as a component of the ECM. Thus, we also sought to investigate FNIII EDA's immunological activity when immobilized in a matrix. As previously shown by our laboratory, recombinant proteins bearing an enzymatic substrate for the coagulation transglutaminase Factor XIIIa can be covalently incorporated into fibrin matrices53, a method that has proven to be an efficient delivery method for integrin ligands37 and growth factors in the context of tissue repair20,22,37. To determine whether FNIII EDA would induce similar reactions while presented in a more natural environment, we produced ECM-mimicking fibrin matrices functionalized with FNIII EDA-containing FN fragments and with the MHC I immunodominant peptide from OVA as a model antigen. In a first experiment aiming to test antigen-specific CD8+ T cell expansion, exposure to fibrin matrices functionalized with FNIII 11-EDA and TG-OVA250–264 induced expansion (Fig. 2b) with more intense IFN-γ production upon re-stimulation with antigen (Fig. 2d). Interestingly, the effect of the engineered matrix was comparable to that achieved by administration of OVA257–264 (which does not require antigen processing) adjuvanted with LPS (Fig. 2d).
Surprisingly, fibrin matrices functionalized with FNIII 9-10-EDA induced significantly lower responses than matrices functionalized with both FNIII 11-EDA and FNIII 9-10. Such a difference may be due to FNIII 9-10-EDA's tendency to precipitate at the concentrations used for the preparation of the fibrin matrices. These results show that FNIII EDA-rich ECM analogs can enhance a pre-established immune response at the level of antigen-specific CD8+ T cell expansion.
Because FNIII EDA has been shown to induce cellular immune responses including activation of CD8+ T cell responses, the domain in soluble form26,27,28,29,30,31 has been explored extensively as a cancer vaccine adjuvant in mouse models including when antigens are fused to it28,30,31, although most potently in combination with other TLR agonists28,31. Notably, when FNIII EDA-antigen fusions have been used as cancer vaccines, in conjugation with other TLR agonists, FNIII EDA has been used without N-terminal extension and as a soluble protein (i.e. not linked to the ECM)28,30,31. Here, we employed tumor cell-killing assays to characterize the functionality of ECM-bound FNIII EDA's effect upon CD8+ T cells to act as CTLs. Two models were utilized, one with a xenogeneic model tumor antigen, OVA (the E.G7-OVA thymoma model; Fig. 3 and 4), where the antigen is not centrally tolerized, and one with an endogenous tumor antigen TRP-2 (B16-F10 melanoma; Fig. 5), in which central tolerance must be overcome. The B16F10 melanoma model progresses very quickly, and as such the rate of tumor growth may outpace the rate of the adaptive immune response. In both models, administration of matrix-bound antigen (TG-OVA250–264 or TG-TRP-2173–188) with matrix-bound FNIII 11-EDA induced substantial delays in tumor growth, without any additional TLR agonists. Furthermore, while the addition of FNIII 9-10 did not improve the survival time of the mice, it nonetheless helped reduce the average tumor volume. Moreover, in the case of the E.G7-OVA model with repeated matrix implantation, complete tumor remission was observed in some instances (Fig. 4), however it is not known if the eventual escape in other animals from immune killing occurs via a bona fide immunological mechanism or due to selective pressure of vaccination toward antigenic selection leading to elimination of the OVA-encoding plasmid54,55.
To demonstrate that a prolonged presence of the FNIII EDA domain bound to the fibrin matrix was beneficial, the same molecules in soluble form were injected i.d (Fig. 3). This control route was chosen, because it allows for an efficient delivery of the vaccine to the same skin-draining lymph node as the tumor (in addition to other non-affected lymph nodes) and thus maximizes the effect via a high percentage of targeted APCs being in contact with both the tumor antigens and the vaccine56. However, i.d. injection was much less effective in delaying tumor growth (Fig. 3) compared to the fibrin matrix, clearly demonstrating that matrix binding of FNIII EDA fragment is beneficial. Furthermore, we judged the role of the inflammation from the surgical implantation of the matrix as being negligible, as FNIII-11-EDA-free matrices failed to provide protection against the tumor despite the presence of TG-OVA250–264 (Fig. 3). Thus, using two tumor models, one with an exogenous model antigen and one with an endogenous antigen, N-terminally-extended FNIII EDA without C-terminal extension with the native FNIII 12-13-14 domain was shown to induce potent immunity as characterized by the cytotoxic functionality of induced CTLs when the FN domains were co-administered in a matrix with antigen. Moreover, the ability to kill the syngeneic melanoma demonstrates the ability to induce functional autoimmunity.
Furthermore, in addition to exploring the ability of FNIII EDA to modulate immunity at the site of its presence, we explored effects in the spleen. The B16–F10 model is known to be immune suppressive through induction of MO-MDSCs, which traffic between the tumor and the spleen57. Administration of fibrin matrixes presenting FNIII 11-EDA, FNIII 9-10 and TG-TRP2173–188 resulted in a substantial diminution of the frequency of MO-MDSCs in the spleen, demonstrating an ability to alter this immune suppressive mechanism (Fig. 5c).
In conclusion, these results bring insight into the immunological function of the FNIII EDA-containing FN splice variant in vivo. We revealed that the TLR4 agonizing potential of FNIII EDA is cryptic in FN, becoming exposed by cleavage between FNIII EDA and FNIII 12 by the neutrophil elastase 2. Lacking the C-terminal FNIII 12-13-14 domain, TLR4 agonization was potentiated up to 5 fold. Moreover, by using two tumor growth models, we demonstrated that induction of CTL responses to a xeno- and an auto-antigen by FNIII EDA activity is enhanced when the domain is bound to the an ECM analog. Therefore, because the FNIII EDA-containing splice variant is expressed in sites of chronic inflammation, autoimmunity, and in many tumors10,11,12, this ECM protein may contribute to the pathology and as well to an anti-tumoral benefit to the immune microenvironment by agonizing TLR4 especially after elastase-2-mediated proteolysis. In addition, delivering ECM-bound FNIII EDA fragments in combination with antigens could be an attractive option for anti-tumoral immunotherapies.
Methods
Recombinant proteins
All FN fragments were engineered to bear at their N-terminus the coagulation transglutaminase Factor XIIIa peptide substrate NQEQVSPL (TG) and were cloned in a pET-22b (+) (Novagen) plasmid between the restriction sites NdeI and NotI. This plasmid contains a 6xHis-tag being added at the 3′ end of the inserted genes. E. coli BL21DE3 were transformed and used for protein production. The recombinant proteins were purified using a HIStrap column (GE Healthcare) and washed using 0.1% Triton X-100 in PBS for LPS removal and an ATP solution (2 mM ATP, 50 mM Tris-HCl, 10 mM MgSO4, pH 7.4) for DnaK removal. An imidazole (1 M) buffer was used for elution. The proteins were then stored in TBS at −80°C. Endotoxin levels were tested by Quantitative Chromogenic Limulus Amebocyte Lysate and were below 0.2 EU/μg.
Reagents
Ultrapure LPS Escherichia coli 0111:B4 was purchased from InvivoGen. Low endotoxin grade OVA (<0.01 EU/μg protein) was used for immunization (Hyglos). MHC-I H-2Kb immunodominant peptides OVA257–264 (SIINFEKL) and TRP-2180–188 (SVYDFFVWL) were ordered from GenScript with the TG-sequence at their N-termini followed with N-terminal extension from the native protein OVA (SGLEQLE)36 allowing proteolytic processing to liberate the epitopes (NQEQVSPLSGLEQLESIINFEKL, NQEQVSPLSGLEQLESVYDFFVWL). PE-labeled H-2Kb/OVA257–264 pentamer was purchased from ProImmune. Pentamer staining was performed according to the manufacturer's instructions.
Western blotting
FNIII 9-10-EDA-12-13-14 was first incubated with elastase 2 (Merck) at a 50:1 ratio for 3 h at 37°C in PBS. SDS-PAGE (12% polyacrylamide gels) was then performed, followed by transfer to a nitrocellulose membrane. The membrane was then incubated either with an HRP-conjugated anti-6xHis (abcam) or with an anti-FNIII EDA (abcam, IST-9) antibody followed by an HRP-conjugated goat anti-mouse IgG1 secondary antibody (SouthernBiotech). After incubation, the blot was processed with SuperSignal West Pico Chemiluminescent Substrate (Pierce).
In vitro stimulation of bone marrow-derived dendritic cells
Murine bone marrow-derived DCs were generated as described elsewhere58. On day 8, cells were plated at a density of 5 × 105 cells/well in 96-well plates and incubated overnight with 0.5 μM of recombinant proteins pre-incubated with 10 μg/ml of polymixin B to block interactions with any potentially contaminating LPS. Cytokines released in the supernatant were measured by ELISA (eBioscience).
In vitro stimulation of HEK-Blue TLR4 cells
HEK-Blue TLR4 LPS Detection Kit (Invivogen) containing HEK293 cells are engineered to express TLR4, MD2 and CD14 as well as a secreted embryonic alkaline phosphatase (SEAP) reporter gene controlled by an NF-κB-inducible promoter. HEK-Blue TLR4 cells were plated at a density of 104 cells/well in 48-well plates and were cultured for 48 h until they were 80% confluent. They were then incubated overnight with 0.5 μM of recombinant proteins pre-incubated with 10 μg/ml of polymixin B. All protein solutions and an elastase 2 control were incubated 3 h at 37°C before stimulation. FNIII 9-10-EDA-12-13-14 + elastase 2 was prepared in a 50:1 ratio. After incubation, the supernatant was diluted 1:10 in HEKBlue Detection Medium (Invivogen) and the expression of SEAP was measured by light absorption at 620 nm. Cell culture medium was DMEM + 10% FBS and assay medium was DMEM + 2% FBS.
In vitro stimulation of THP1-Blue cells
THP1-Blue cells (Invivogen) are human THP-1 monocyte cell line engineered to express an NF-κB-inducible SEAP reporter construct. THP1-Blue cells were plated at a density of 5 × 105 cells/well in 96-well plates and incubated overnight with two-fold serial dilutions of recombinant proteins pre-incubated with 10 μg/ml of polymixin B or LPS ranging from 0.012 μM to 12.5 μM and 0.06 ng/μl to 30 ng/μl, respectively. After incubation, the supernatant was diluted 1:10 in HEKBlue Detection Medium (Invivogen) and the expression of SEAP was measured by light absorption at 620 nm. Cell culture medium was RPMI 1640 (2 mM L-glutamine, 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, 10 mM HEPES and 1.0 mM sodium pyruvate) with 10% heat inactivated FBS supplemented with 100 μg/ml of Zeocin and assay medium was RPMI 1640 + 2% FBS.
Mice and tumor cells
C57BL/6J mice (aged 7–8 wk) were purchased from Harlan Laboratories and kept under pathogen-free conditions at the animal facility of Ecole Polytechnique Fédérale de Lausanne. All experiments were performed in accordance with Swiss law and with approval from the Cantonal Veterinary Office of Canton de Vaud, Switzerland. E.G7-OVA thymoma cells (i.e., EL4 cells (a thymoma line) expressing OVA as a surrogate tumor antigen, ATCC TIB-39) were obtained from American Type Culture Collection (ATCC) and grown in RPMI medium 1640 (Invitrogen) supplemented with 10% heat-inactivated FBS, 1 mM sodium pyruvate, 30 mM Hepes, 50 μM 2-mercaptoethanol, and 0.4 mg/mL G418 antibiotic (Sigma). B16–F10 (ATCC CRL-2539) melanoma cells were obtained from American Type Culture Collection (ATCC) and were maintained in DMEM supplemented with 10% (vol/vol) FBS and penicillin/streptomycin/amphotericin B.
Preparation of functionalized fibrin matrices
Fibrin matrices (150 μL) were formed by mixing a fibrinogen solution with a thrombin solution of equal volume in 1.5 mL Eppendorf tubes. The fibrin solution contained 16 mg/mL fibrinogen (plasminogen-, von Willebrand factor- and FN-depleted; Enzyme Research Laboratories) in HEPES buffer (20 mM HEPES, 150 mM NaCl, pH 7.4). The thrombin solution contained, 2 U/mL human thrombin (Sigma–Aldrich), 5 nmol of TG-OVA250–264 and/or 5 nmol of FNIII 11-EDA or FNIII 9-10-EDA, and 5 mM Ca2+ in HEPES buffer.
Stimulation of antigen-specific CD8+ response
C57BL/6J mice were immunized by injection in the four footpads with a total dose of 50 μg OVA and 20 μg LPS on day 0. On day 14, fibrin gels functionalized with 5 nmol of TG-OVA250–264 and 5 nmol of FNIII 11-EDA or FNIII 9-10-EDA were implanted s.c. on the back at the level of the shoulder girdle; alternatively mice were injected i.d. with 5 nmol of OVA257–264 and 50 μg of LPS or plain PBS. On day 19 mice were sacrificed and the spleen was harvested.
Single-cell preparation and ex vivo antigen-specific cell restimulation
Splenocytes were obtained by squeezing the spleen with a razor blade and disrupting the cell aggregates on a cell sieve, washing thoroughly, and lysing the red blood cells. For CD8+ T cell antigen-specific restimulation and intracellular cytokine staining, immune cells were cultured at 37°C for 6 h in the presence of 1 μg/mL OVA257–264 peptide. All cells were cultured in IMDM medium supplemented with 10% FBS and 1% penicillin/streptomycin (all from Invitrogen).
Flow cytometry and ELISA
Before antibody staining for flow cytometry, all cells were labeled with live/dead fixable cell viability reagent in PBS (Invitrogen). For surface staining, cells were incubated for 15 min with the antibodies diluted in HBSS (Invitrogen)/0.5% BSA (PAA Laboratories). For antigen specificity, cells were stained with a SIINFEKL:H2Kb MHC I pentamer (ProImmune). For intracellular cytokine staining, cells were fixed in 2% paraformaldehyde solution, washed with 0.5% saponin (Sigma-Aldrich) in HBSS/0.5% BSA solution, and incubated with the antibodies diluted in saponin solution for 30 min. After washing, cells were resuspended in HBSS/0.5% BSA solution for analysis. Samples were acquired on CyAn ADP Analyzer (Beckman Coulter) and data were analyzed with FlowJo software (Tree Star). The antibodies anti-mouse CD11c, CD62L, B220, CD44 and IFN-γ were purchased from BioLegend; anti-mouse CD3ε, CD8, CD11b, Ly6c, Ly6g, TNF-α and MHC-II antibodies were purchased from eBioscience (San Diego, CA, USA). Ready-SET-go! ELISA kits for cytokine detection were purchased from eBioscience and used according to manufacturer's instructions.
Tumor growth assays
Tumor cells were implanted s.c. in the back at the level of the junction between the thoracic and lumbar vertebrae of C57BL/6J mice with inoculation of 106 E.G7-OVA cells or 2.5 × 105 B16–F10 cells in 30 μL PBS (Invitrogen). The mice were then treated once (E.G7-OVA) or weekly for three weeks (E.G7-OVA and B16–F10) when their tumor reached 50 mm3 (E.G7-OVA) or when the tumor became visible (B16–F10). Fibrin gels (150 μl total, fibrinogen (8 mg/ml)) were functionalized with 5 nmol of FNIII domain recombinant proteins and with or without 5 nmol of MHC I immunodominant peptide bound to the fibrin, as described above. Tumors were measured 5 times per week and volumes were calculated as ellipsoids based on three orthogonal measures. Animal were killed either when the tumor reached 200 mm3 (B16–F10) or 1000 mm3 (E.G7-OVA) or for humane reasons such as tumor necrosis, excessive loss of weight or evident isolation from the other animals.
Statistical Analyses
Statistical analyses were performed using Analysis of Variance (ANOVA) and Bonferonni posttest using GraphPad Prism.
Author Contributions
Z.J., M.M.M., L.J. and J.A.H. designed research; Z.J. and A.d.T. performed research; Z.J., M.M.M. and A.d.T. analyzed data; J.A.H. directed the project; and Z.J., M.M.M. and J.A.H. wrote the paper.
Supplementary Material
Figure S1
Acknowledgments
We thank E. Simeoni, X. Quaglia, F. Tortelli, A.J. Grimm and P. Corthésy-Henrioud for technical assistance and useful discussions. Assistance from the Protein Production Core Facility and the Animal Facility staff of the Ecole Polytechnique Fédérale de Lausanne is gratefully acknowledged. This work was funded by the Swiss National Science Foundation and the Heinrich-Lohstöter-Stiftung.
References
- Pankov R. & Yamada K. M. Fibronectin at a glance. J. Cell Sci. 115, 3861–3863 (2002). [DOI] [PubMed] [Google Scholar]
- ffrench-Constant C. Alternative splicing of fibronectin--many different proteins but few different functions. Exp. Cell Res. 221, 261–71 (1995). [DOI] [PubMed] [Google Scholar]
- ffrench-Constant C. & Hynes R. O. Alternative splicing of fibronectin is temporally and spatially regulated in the chicken embryo. Development 106, 375–88 (1989). [DOI] [PubMed] [Google Scholar]
- Jarnagin W. R., Rockey D. C., Koteliansky V. E., Wang S. S. & Bissell D. M. Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. J. Cell Biol. 127, 2037–48 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y. F., Gotwals P. J., Koteliansky V. E., Sheppard D. & Van De Water L. The EIIIA segment of fibronectin is a ligand for integrins alpha 9beta 1 and alpha 4beta 1 providing a novel mechanism for regulating cell adhesion by alternative splicing. J. Biol. Chem. 277, 14467–74 (2002). [DOI] [PubMed] [Google Scholar]
- ffrench-Constant C., Van de Water L., Dvorak H. F. & Hynes R. O. Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. J. Cell Biol. 109, 903–14 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak J. N., Roesli C., Kaspar M., Villa A. & Neri D. The extra-domain A of fibronectin is a vascular marker of solid tumors and metastases. Cancer Res. 67, 10948–57 (2007). [DOI] [PubMed] [Google Scholar]
- Villa A. et al. A high-affinity human monoclonal antibody specific to the alternatively spliced EDA domain of fibronectin efficiently targets tumor neo-vasculature in vivo. Int. J. Cancer 122, 2405–13 (2008). [DOI] [PubMed] [Google Scholar]
- Borsi L., Castellani P., Allemanni G., Neri D. & Zardi L. Preparation of phage antibodies to the ED-A domain of human fibronectin. Exp. Cell Res. 240, 244–51 (1998). [DOI] [PubMed] [Google Scholar]
- McFadden J. P., Baker B. S., Powles A. V. & Fry L. Psoriasis and extra domain A fibronectin loops. Br. J. Dermatol. 163, 5–11 (2010). [DOI] [PubMed] [Google Scholar]
- McFadden J. P., Baker B. S., Powles A. V. & Fry L. Psoriasis and streptococci: postscript regarding extra domain A fibronectin. Br. J. Dermatol. 161, 706–7 (2009). [DOI] [PubMed] [Google Scholar]
- McFadden J., Fry L., Powles A. V. & Kimber I. Concepts in psoriasis: psoriasis and the extracellular matrix. Br. J. Dermatol. 167, 980–6 (2012). [DOI] [PubMed] [Google Scholar]
- Astrof S., Crowley D. & Hynes R. O. Multiple cardiovascular defects caused by the absence of alternatively spliced segments of fibronectin. Dev. Biol. 311, 11–24 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura Y. et al. The extra domain A of fibronectin activates Toll-like receptor 4. J. Biol. Chem. 276, 10229–33 (2001). [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S. et al. FibronectinEDA promotes chronic cutaneous fibrosis through Toll-like receptor signaling. Sci. Transl. Med. 6, 232ra50 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charo I. F., Nannizzi L., Smith J. W. & Cheresh D. A. The vitronectin receptor alpha v beta 3 binds fibronectin and acts in concert with alpha 5 beta 1 in promoting cellular attachment and spreading on fibronectin. J. Cell Biol. 111, 2795–800 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redick S. D., Settles D. L., Briscoe G. & Erickson H. P. Defining fibronectin's cell adhesion synergy site by site-directed mutagenesis. J. Cell Biol. 149, 521–7 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi T. & Erickson H. P. Domain unfolding plays a role in superfibronectin formation. J. Biol. Chem. 280, 39143–51 (2005). [DOI] [PubMed] [Google Scholar]
- Mostafavi-Pour Z., Askari J. A., Whittard J. D. & Humphries M. J. Identification of a novel heparin-binding site in the alternatively spliced IIICS region of fibronectin: roles of integrins and proteoglycans in cell adhesion to fibronectin splice variants. Matrix Biol. 20, 63–73 (2001). [DOI] [PubMed] [Google Scholar]
- Martino M. M. & Hubbell J. A. The 12th-14th type III repeats of fibronectin function as a highly promiscuous growth factor-binding domain. FASEB J. 24, 4711–21 (2010). [DOI] [PubMed] [Google Scholar]
- Wijelath E. S. et al. Heparin-II domain of fibronectin is a vascular endothelial growth factor-binding domain: enhancement of VEGF biological activity by a singular growth factor/matrix protein synergism. Circ. Res. 99, 853–60 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino M. M. et al. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci. Transl. Med. 3, 100ra89 (2011). [DOI] [PubMed] [Google Scholar]
- Lin F. et al. Fibronectin growth factor-binding domains are required for fibroblast survival. J. Invest. Dermatol. 131, 84–98 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S. et al. The fibronectin extra domain A activates matrix metalloproteinase gene expression by an interleukin-1-dependent mechanism. J. Biol. Chem. 274, 30756–63 (1999). [DOI] [PubMed] [Google Scholar]
- Sekiguchi K. & Hakomori S. Functional domain structure of fibronectin. Proc. Natl. Acad. Sci. U. S. A. 77, 2661–5 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arribillaga L. et al. A fusion protein between streptavidin and the endogenous TLR4 ligand EDA targets biotinylated antigens to dendritic cells and induces T cell responses in vivo. Biomed Res. Int. 2013, 864720 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansilla C. et al. Immunization against hepatitis C virus with a fusion protein containing the extra domain A from fibronectin and the hepatitis C virus NS3 protein. J. Hepatol. 51, 520–7 (2009). [DOI] [PubMed] [Google Scholar]
- Mansilla C. et al. Eradication of large tumors expressing human papillomavirus E7 protein by therapeutic vaccination with E7 fused to the extra domain a from fibronectin. Int. J. Cancer 131, 641–51 (2012). [DOI] [PubMed] [Google Scholar]
- San Román B. et al. The extradomain A of fibronectin (EDA) combined with poly(I:C) enhances the immune response to HIV-1 p24 protein and the protection against recombinant Listeria monocytogenes-Gag infection in the mouse model. Vaccine 30, 2564–9 (2012). [DOI] [PubMed] [Google Scholar]
- Lasarte J. J. et al. The extra domain A from fibronectin targets antigens to TLR4-expressing cells and induces cytotoxic T cell responses in vivo. J. Immunol. 178, 748–56 (2007). [DOI] [PubMed] [Google Scholar]
- Rudilla F. et al. Combination of a TLR4 ligand and anaphylatoxin C5a for the induction of antigen-specific cytotoxic T cell responses. Vaccine 30, 2848–58 (2012). [DOI] [PubMed] [Google Scholar]
- Song J. et al. PROSPER: an integrated feature-based tool for predicting protease substrate cleavage sites. PLoS One 7, e50300 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipford G. B., Bauer S., Wagner H. & Heeg K. In vivo CTL induction with point-substituted ovalbumin peptides: immunogenicity correlates with peptide-induced MHC class I stability. Vaccine 13, 313–20 (1995). [DOI] [PubMed] [Google Scholar]
- Ali O. A., Emerich D., Dranoff G. & Mooney D. J. In situ regulation of DC subsets and T cells mediates tumor regression in mice. Sci. Transl. Med. 1, 8ra19 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schense J. C. & Hubbell J. A. Cross-linking exogenous bifunctional peptides into fibrin gels with factor XIIIa. Bioconjug. Chem. 10, 75–81 (1999). [DOI] [PubMed] [Google Scholar]
- Craiu A., Akopian T., Goldberg A. & Rock K. L. Two distinct proteolytic processes in the generation of a major histocompatibility complex class I-presented peptide. Proc. Natl. Acad. Sci. U. S. A. 94, 10850–5 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino M. M. et al. Controlling integrin specificity and stem cell differentiation in 2D and 3D environments through regulation of fibronectin domain stability. Biomaterials 30, 1089–97 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst M. R. et al. Identification of a shared HLA-A*0201-restricted T-cell epitope from the melanoma antigen tyrosinase-related protein 2 (TRP2). Cancer Res. 58, 4895–901 (1998). [PubMed] [Google Scholar]
- Gabrilovich D. I. & Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–74 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcetti L. et al. Myeloid-derived suppressor cell role in tumor-related inflammation. Cancer Lett. 267, 216–25 (2008). [DOI] [PubMed] [Google Scholar]
- George E. L., Georges-Labouesse E. N., Patel-King R. S., Rayburn H. & Hynes R. O. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development 119, 1079–91 (1993). [DOI] [PubMed] [Google Scholar]
- Valenick L. V., Hsia H. C. & Schwarzbauer J. E. Fibronectin fragmentation promotes alpha4beta1 integrin-mediated contraction of a fibrin-fibronectin provisional matrix. Exp. Cell Res. 309, 48–55 (2005). [DOI] [PubMed] [Google Scholar]
- Plow E. F., Haas T. A., Zhang L., Loftus J. & Smith J. W. Ligand binding to integrins. J. Biol. Chem. 275, 21785–8 (2000). [DOI] [PubMed] [Google Scholar]
- Zhong C. et al. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J. Cell Biol. 141, 539–51 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel V. & Sheetz M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 7, 265–75 (2006). [DOI] [PubMed] [Google Scholar]
- Davis G. E., Bayless K. J., Davis M. J. & Meininger G. A. Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. Am. J. Pathol. 156, 1489–98 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinde A. V. et al. Identification of the peptide sequences within the EIIIA (EDA) segment of fibronectin that mediate integrin alpha9beta1-dependent cellular activities. J. Biol. Chem. 283, 2858–70 (2008). [DOI] [PubMed] [Google Scholar]
- Xaus J. et al. LPS induces apoptosis in macrophages mostly through the autocrine production of TNF-alpha. Blood 95, 3823–31 (2000). [PubMed] [Google Scholar]
- Baldwin G. et al. Effect of polymyxin B on experimental shock from meningococcal and Escherichia coli endotoxins. J. Infect. Dis. 164, 542–9 (1991). [DOI] [PubMed] [Google Scholar]
- Karplus T. E., Ulevitch R. J. & Wilson C. B. A new method for reduction of endotoxin contamination from protein solutions. J. Immunol. Methods 105, 211–20 (1987). [DOI] [PubMed] [Google Scholar]
- Toichi E., Tachibana T. & Furukawa F. Rapid improvement of psoriasis vulgaris during drug-induced agranulocytosis. J. Am. Acad. Dermatol. 43, 391–5 (2000). [DOI] [PubMed] [Google Scholar]
- Wetzel A. et al. Increased neutrophil adherence in psoriasis: role of the human endothelial cell receptor Thy-1 (CD90). J. Invest. Dermatol. 126, 441–52 (2006). [DOI] [PubMed] [Google Scholar]
- Schense J. C., Bloch J., Aebischer P. & Hubbell J. A. Enzymatic incorporation of bioactive peptides into fibrin matrices enhances neurite extension. Nat. Biotechnol. 18, 415–9 (2000). [DOI] [PubMed] [Google Scholar]
- Beaudette T. T. et al. In vivo studies on the effect of co-encapsulation of CpG DNA and antigen in acid-degradable microparticle vaccines. Mol. Pharm. 6, 1160–9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckpot K. et al. Lentivirally transduced dendritic cells as a tool for cancer immunotherapy. J. Gene Med. 5, 654–67 (2003). [DOI] [PubMed] [Google Scholar]
- Jeanbart L. et al. Enhancing efficacy of anticancer vaccines by targeted delivery to tumor-draining lymph nodes. Cancer Immunol. Res. 2, 436–47 (2014). [DOI] [PubMed] [Google Scholar]
- Lindau D., Gielen P., Kroesen M., Wesseling P. & Adema G. J. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology 138, 105–15 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz M. B. et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223, 77–92 (1999). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1